Cordyceps sinensis sustained and controlled release capsule and preparation method thereof
A Cordyceps Suspension and Capsule Technology, which is applied in pharmaceutical formulations, medical preparations containing active ingredients, plant raw materials, etc., can solve problems such as adverse overall health level, low dissolution rate, and long dissolution time limit to enhance human immunity. , the effect of reducing toxic and side effects and reducing the frequency of medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
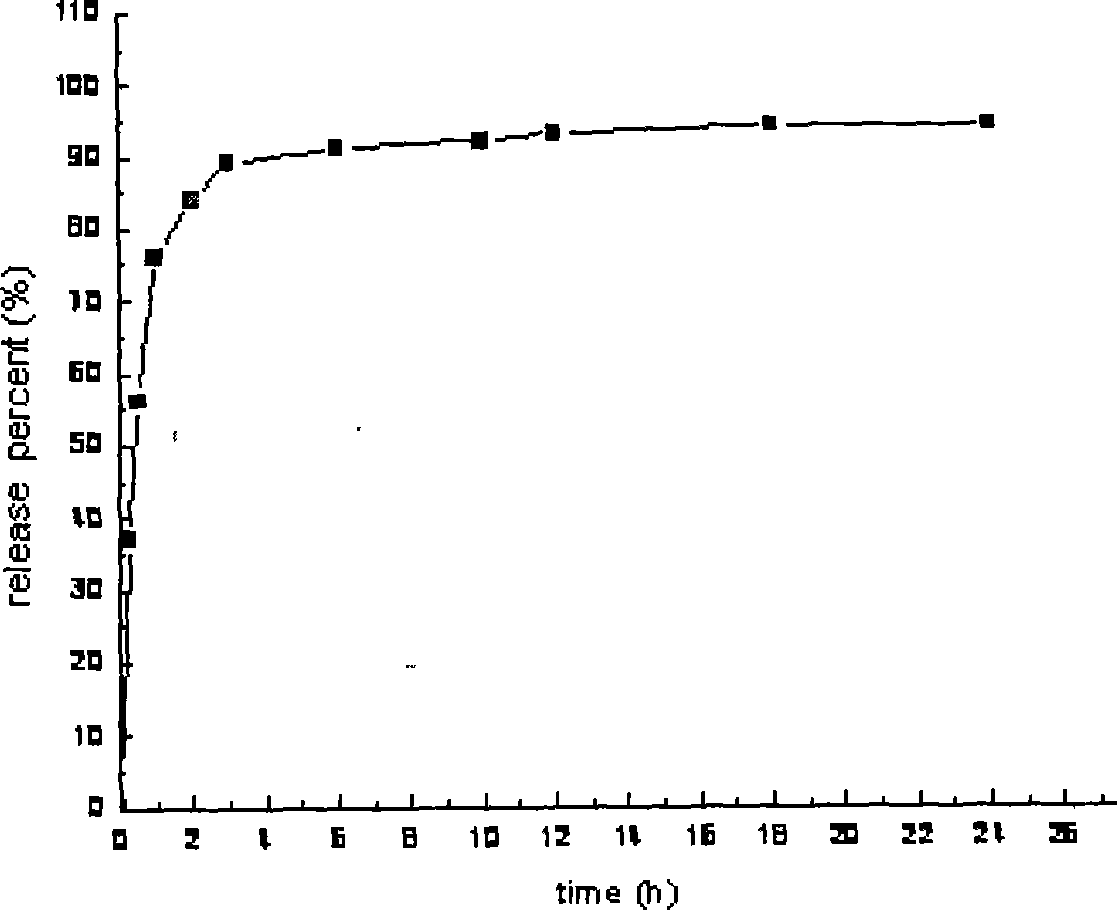
Embodiment 1
[0029] Preparation of Cordyceps sustained-release capsules (specification: 10mg)
[0030] 1 Prescription: (1000 tablets for 10mg)
[0031] (1) Prepare drug-loaded pellets according to the following prescription:
[0032] Cordyceps active extract 80g, polyethylene glycol-4000 15g, polyvinylpyrrolidone 3g, 50% ethanol 10g, magnesium stearate 0.5g.
[0033] Immediate-release pellets were prepared according to the following formulation:
[0034] Drug-loaded pellets 100g, carboxypropylmethylcellulose 2.5g, polyethylene glycol-4000 15g;
[0035] Sustained-release pellets were prepared according to the following prescription:
[0036] Drug-loaded pellets 100g, carboxyethyl cellulose 20g, polyethylene glycol-6000 15g;
[0037] 2 Preparation method:
[0038] (1) Weigh the active extract of Cordyceps sinensis of prescription quantity, put polyethylene glycol-4000, polyvinylpyrrolidone in the mixer and mix evenly, add 50% ethanol to make soft material, sieve through 20-60 mesh, carr...
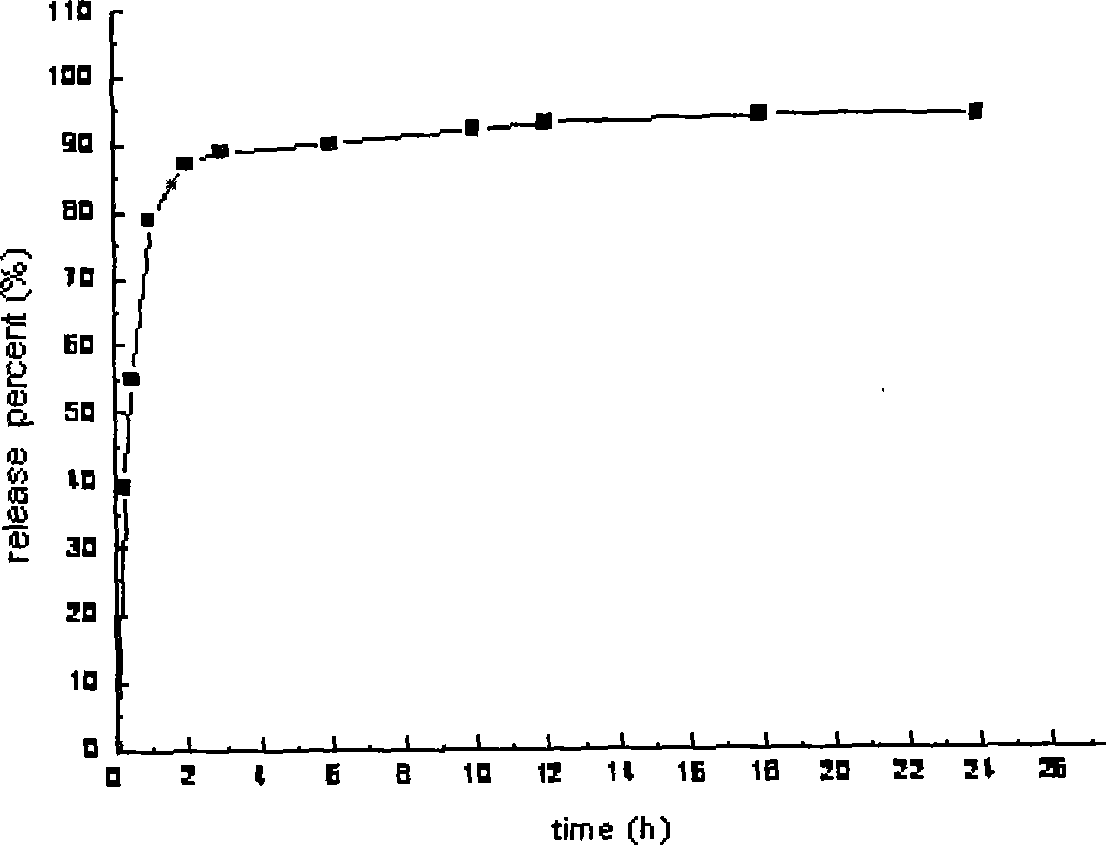
Embodiment 2
[0044] Preparation of Cordyceps capsules (specification: 10mg)
[0045] 1. Prescription (1000 tablets for 10 mg)
[0046] (1) Prepare drug-loaded pellets according to the following prescription:
[0047] Cordyceps active extract 80g, mannitol 37g, crospovidone 15g, PEG-2000 30g, 50% ethanol 10g, talcum powder 0.5g.
[0048] Immediate-release pellets were prepared according to the following formulation:
[0049] Drug-loaded pellets 100g, croscarmellose sodium 15g, PEG-2000 30g;
[0050] Sustained-release pellets were prepared according to the following prescription:
[0051] Drug-loaded pellets 100g, ethyl cellulose 15g, PEG-2000 30g;
[0052] 2 Preparation method:
[0053] (1) The active extract of Cordyceps sinensis, mannitol, crospovidone, and PEG-4000 are placed in a mixer to mix evenly, and an appropriate amount of 50% ethanol is added to make a soft material, and 20-60 meshes are sieved. Perform wet granulation.
[0054] (2) Dry at 40-60°C, and sieve the granules w...
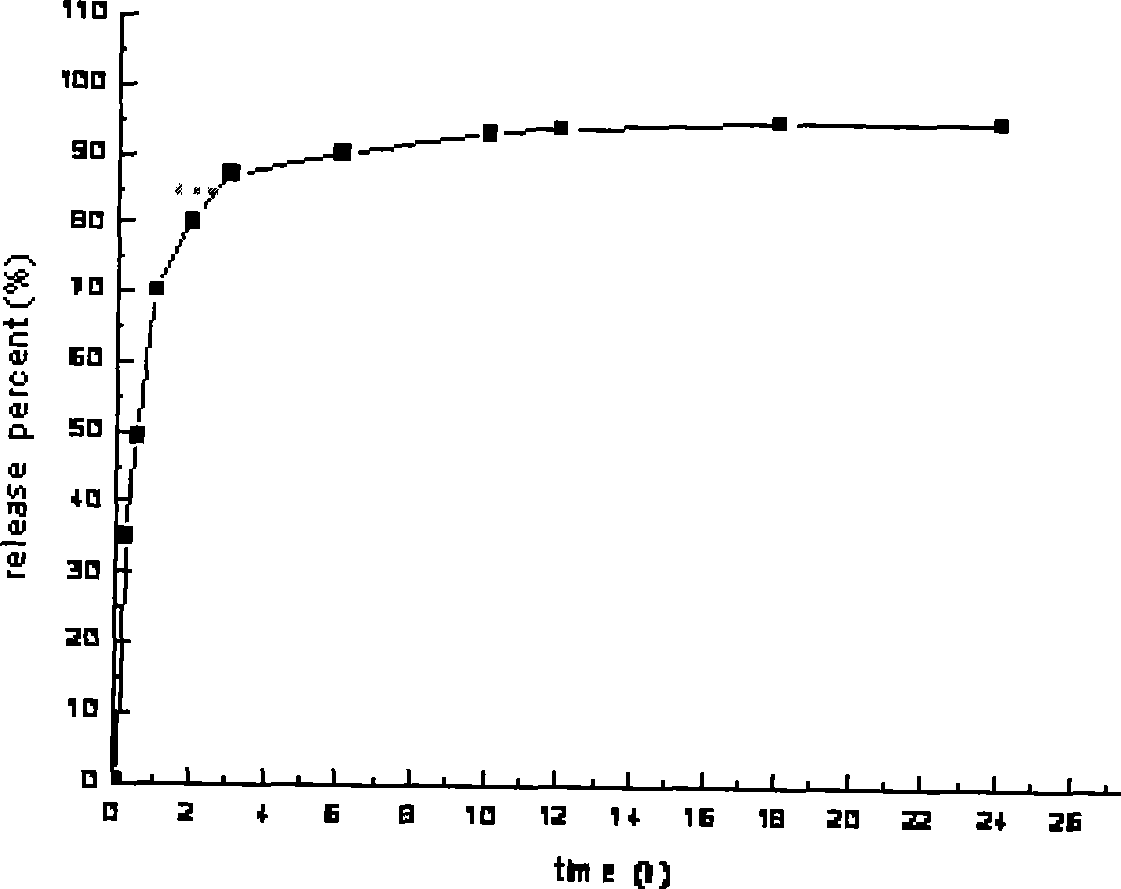
Embodiment 3
[0059] Preparation of Cordyceps capsules (specification: 10mg)
[0060] 1. Prescription (1000 tablets for 10 mg)
[0061] (1) Prepare drug-loaded pellets according to the following prescription:
[0062] 80 parts of Cordyceps active extract, 15 parts of crospovidone, 15 parts of dextrin, 30 parts of PEG-1000, 5 parts of 50% ethanol, 0.5 parts of talcum powder, 5 parts of dilute alkali;
[0063] Immediate-release pellets were prepared according to the following formulation:
[0064] 100 parts of drug-loaded pellets, 37 parts of microcrystalline cellulose, 30 parts of PEG-1000;
[0065] Sustained-release pellets were prepared according to the following prescription:
[0066] 100 parts of drug-loaded pellets, 15 parts of croscarmellose sodium, 30 parts of PEG-4000;
[0067] 2 Preparation method:
[0068] (1) Take the active extract of Cordyceps sinensis of prescription quantity and place in the mixer with crospovidone, PEG-1000, dextrin and mix evenly, add appropriate amount...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com