Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
89 results about "Chronic liver injury" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
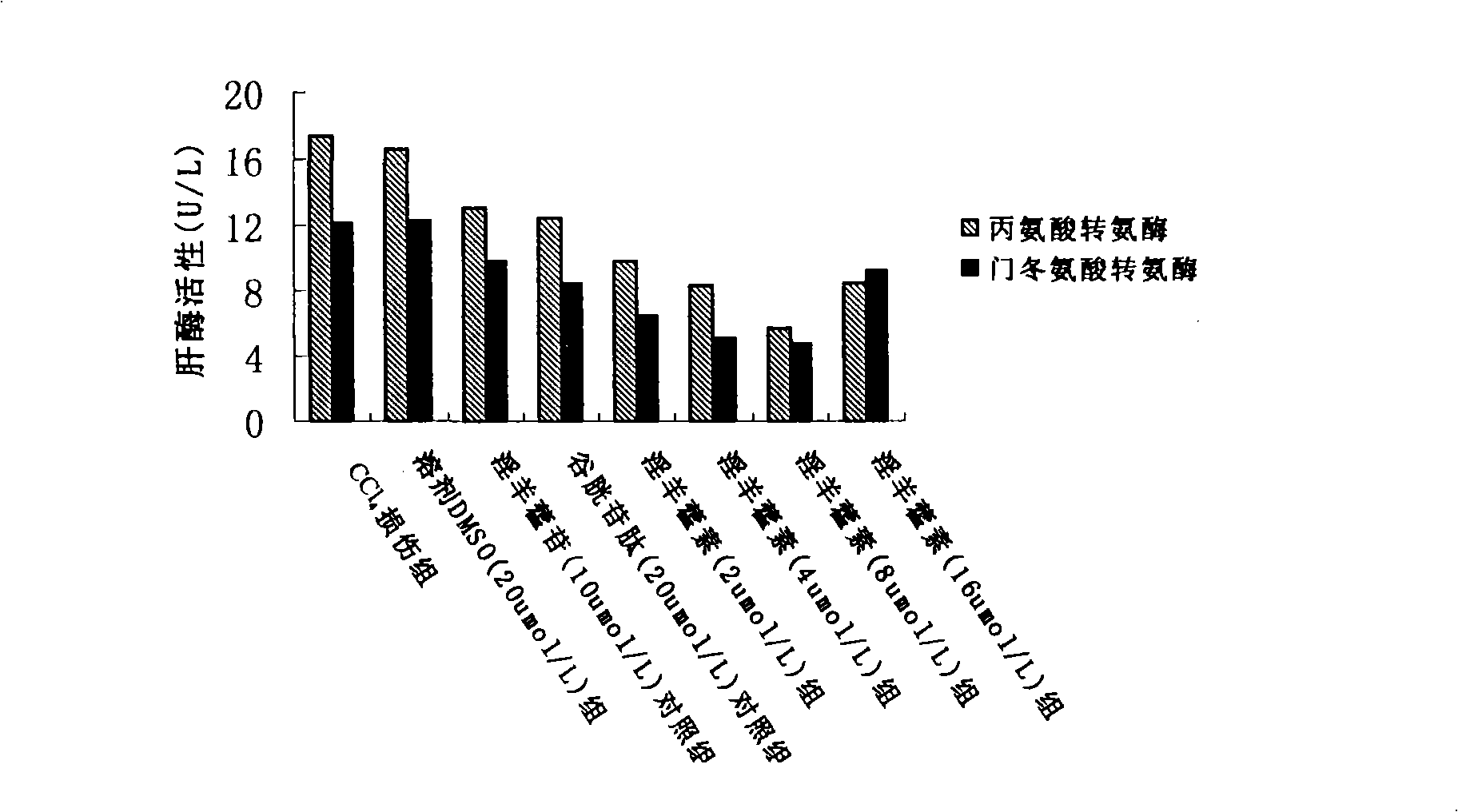
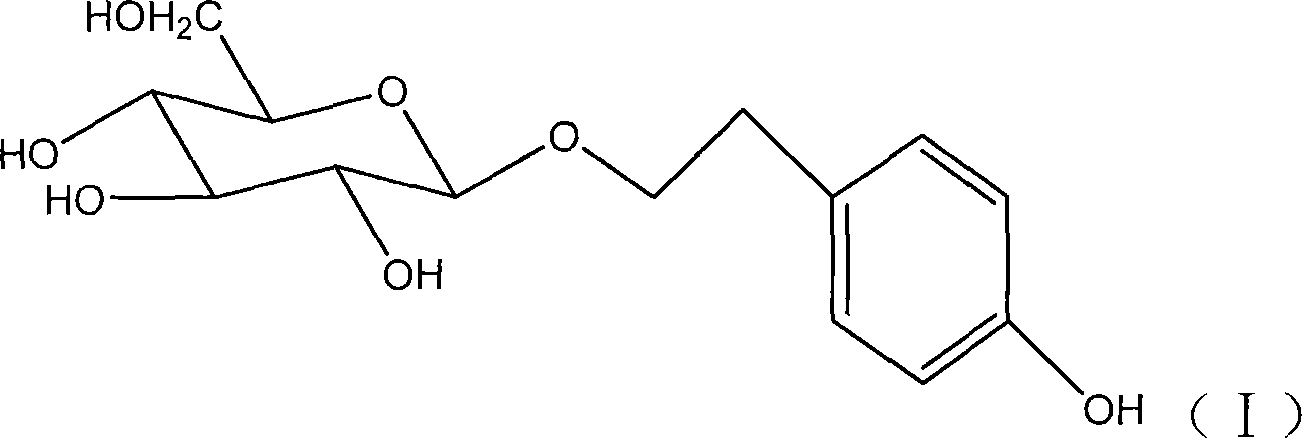
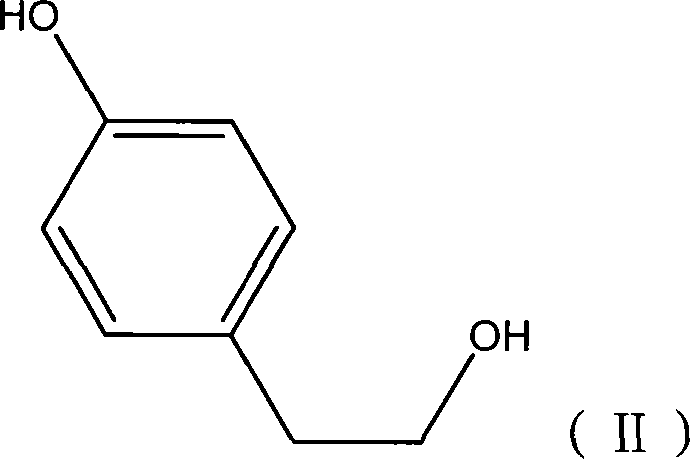
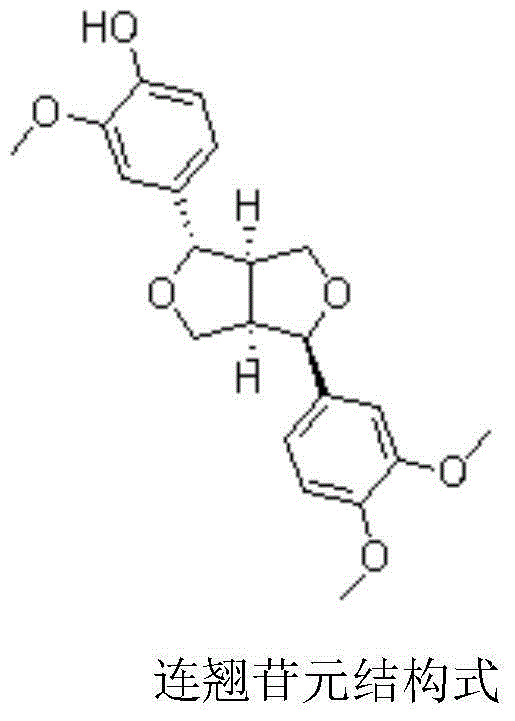
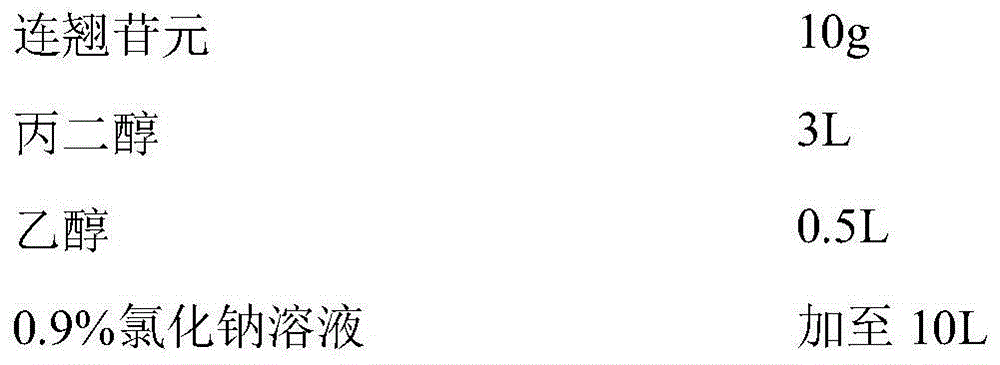
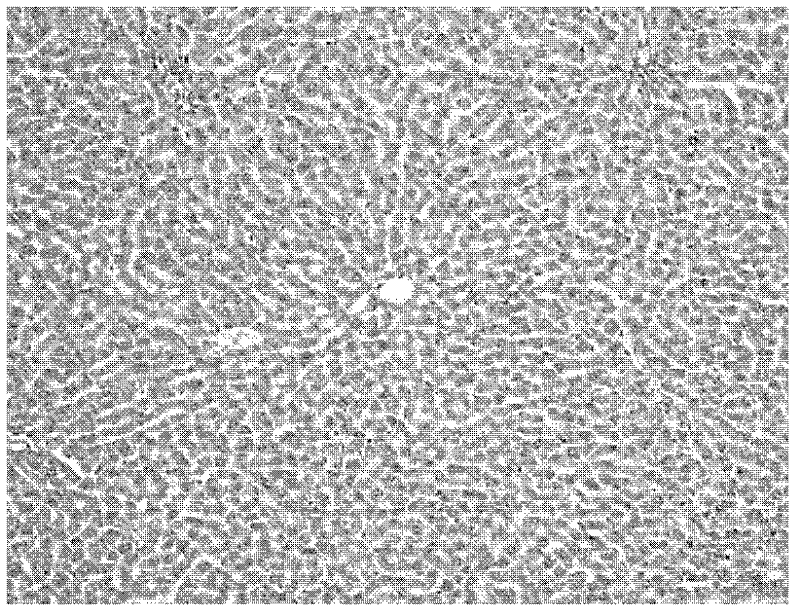
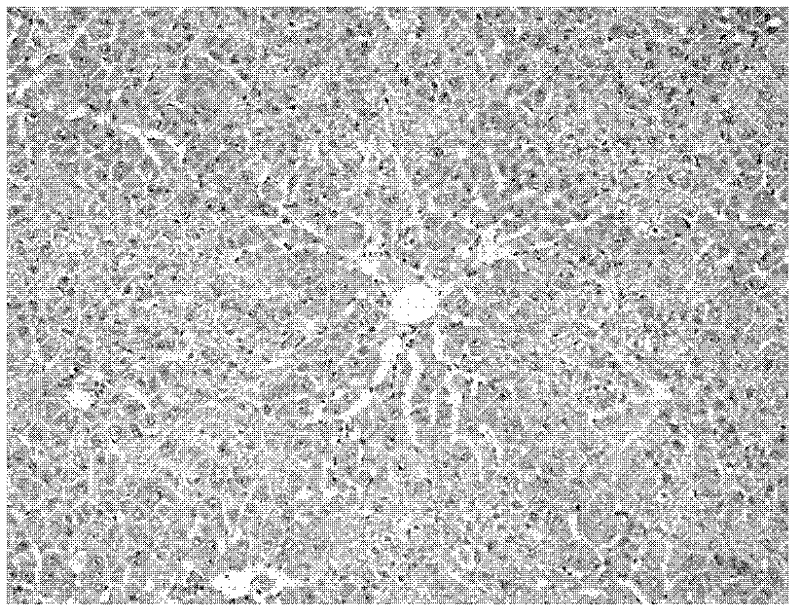
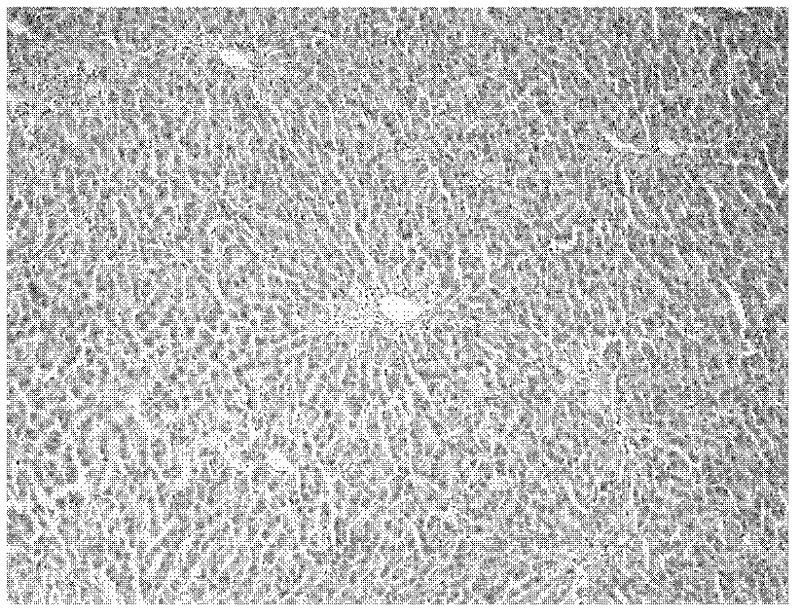
Application of fructus forsythiae aglycone in preparing medicament for preventing or treating liver injury or liver failure
InactiveCN103989668ASignificant preventionGood treatment effectDigestive systemHeterocyclic compound active ingredientsSide effectAglycone
The invention discloses application of fructus forsythiae aglycone in preparing a medicament for preventing or treating liver injury or liver failure, and belongs to the medical field. The fructus forsythiae aglycone is a traditional Chinese medicine monomer obtained by extracting from the conventional fructus forsythiae, and is shown to have better treatment effect for the liver injury caused by acetaminopben, the livery injury caused by anti-tumor drug cis-platinum, acute or chronic livery injury caused by carbon tetrachloride, the livery injury caused by D-galactosamine and the liver failure caused by the D-galactosamine and lipopolysaccharide according to an animal test, has effect, which is better than that of fructus forsythiae and fructus forsythiae aglycone, in treating liver injury or liver failure. The fructus forsythiae aglycone is exact in curative effect and low in side effect for treating the liver injury and the liver failure, and has wide medical application prospect.
Owner:LUNAN PHARMA GROUP CORPORATION
Health preserving composition and application thereof
InactiveCN105595163AImprove constipationImprove loose stool symptomsNatural extract food ingredientsFood ingredient functionsFreeze-dryingBlood sugar
The present invention discloses a health preserving composition and application thereof. The health preserving composition comprises 10-80 parts by weight of whole grain and medicinal and edible traditional Chinese medicinal materials, 0-30 parts by weight of fruit and vegetable powder, 1-10 parts by weight of freeze-dried probiotic powder and 3-30 parts by weight of prebiotics. After taking of the health preserving composition, constipation and loose stool symptoms may be improved significantly. By long-term use of the health preserving composition, chronic gastritis, enteritis and the like can be significantly improved. By improving of gastrointestinal functions, high blood fat, high blood sugar and various types of chronic liver injuries and other chronic diseases can be improved.
Owner:吴志大
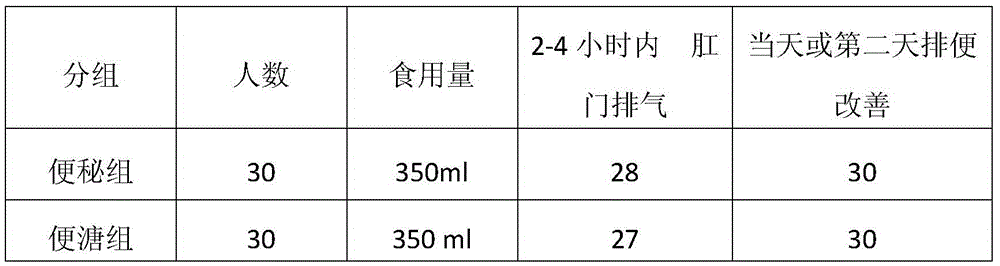
Application of forsythiaside B in preparing medicament for treating or preventing acute and chronic liver damnification and liver fibrosis
The invention relates to application of forsythiaside B in preparing a medicine for treating or preventing hepatopathy, in particular to application of the forsythiaside B in preparing a medicine for treating or preventing acute and chronic hepatic injury and hepatic fibrosis. The medicine can be added with corresponding auxiliary materials according to the preparation requirements, adopts the form of injection, tablets, pills, granules, capsules, syrup and the like, and preferably adopts the form of freeze-dried powder and capsules. Various preparation formulations can be prepared by a conventional pharmaceutical method.
Owner:SHANDONG LUYE PHARMA CO LTD
Application of phillyrin in the preparing of medicine for treating or preventing acute and chronic liver injury and hepar fibrosis
The invention provides the application of phillyrin in preparing medicine that prevents and treats acute or chronic liver injury and liver fibrosis. It can be produced into oral preparation or injection preparation. The dose for injection is 25-800mg, and for oral is 50 -1500mg.
Owner:SHANDONG LUYE PHARMA CO LTD
Compound traditional Chinese medicinal effective component preparation for resisting alcoholic fatty liver disease and application thereof
ActiveCN103800352APromote reversalLowers triglyceride (TG) levelsOrganic active ingredientsDigestive systemChlorogenic acidRat model
The invention belongs to the field of traditional Chinese medicines, relates to a compound traditional Chinese medicinal effective component preparation for resisting alcoholic fatty liver disease and application thereof, and in particular relates to a compound traditional Chinese medicinal component preparation comprising geniposide and chlorogenic acid and application thereof. The compound preparation is prepared from geniposide and chlorogenic acid, and can be processed into clinical common preparations by a conventional method, including granules, tablets, capsules and other oral solid preparations. Animal experiments indicate that the compound preparation can remarkably reduce the content of triglyceride of liver tissues of a rat model, and reduce the degeneration degree of liver fat and liver damage degree. The effects of the compound preparation are superior to those of single component. The compound preparation can be used for improving the effect of resisting alcoholic fatty liver, effectively preventing development of non-alcoholic fatty liver, and can be used for treating and preventing non-alcoholic fatty liver, chronic liver damage and other symptoms.
Owner:SHUGUANG HOSPITAL AFFILIATED WITH SHANGHAI UNIV OF T C M
Hepatoprotective composition as well as preparation method and application thereof
ActiveCN103479833AGood liver protectionPrevent effectOrganic active ingredientsDigestive systemAlpha-Lipoic AcidGreen tea extract
The invention discloses hepatoprotective composition. The hepatoprotective composition is prepared from the following raw materials: a milk thistle extract, a green tea extract, an Acai berry extract, alpha-lipoic acid and N-acetyl-L-cysteine. The invention further discloses a preparation method and an application of the hepatoprotective composition. The hepatoprotective composition combines the milk thistle extract, the green tea extract, the Acai berry extract, the alpha-lipoic acid and the N-acetyl-L-cysteine for use, so that the hepatoprotective composition has a significant hepatoprotective function and can be used for treating and / or preserving acute liver injuries and chronic liver injuries.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
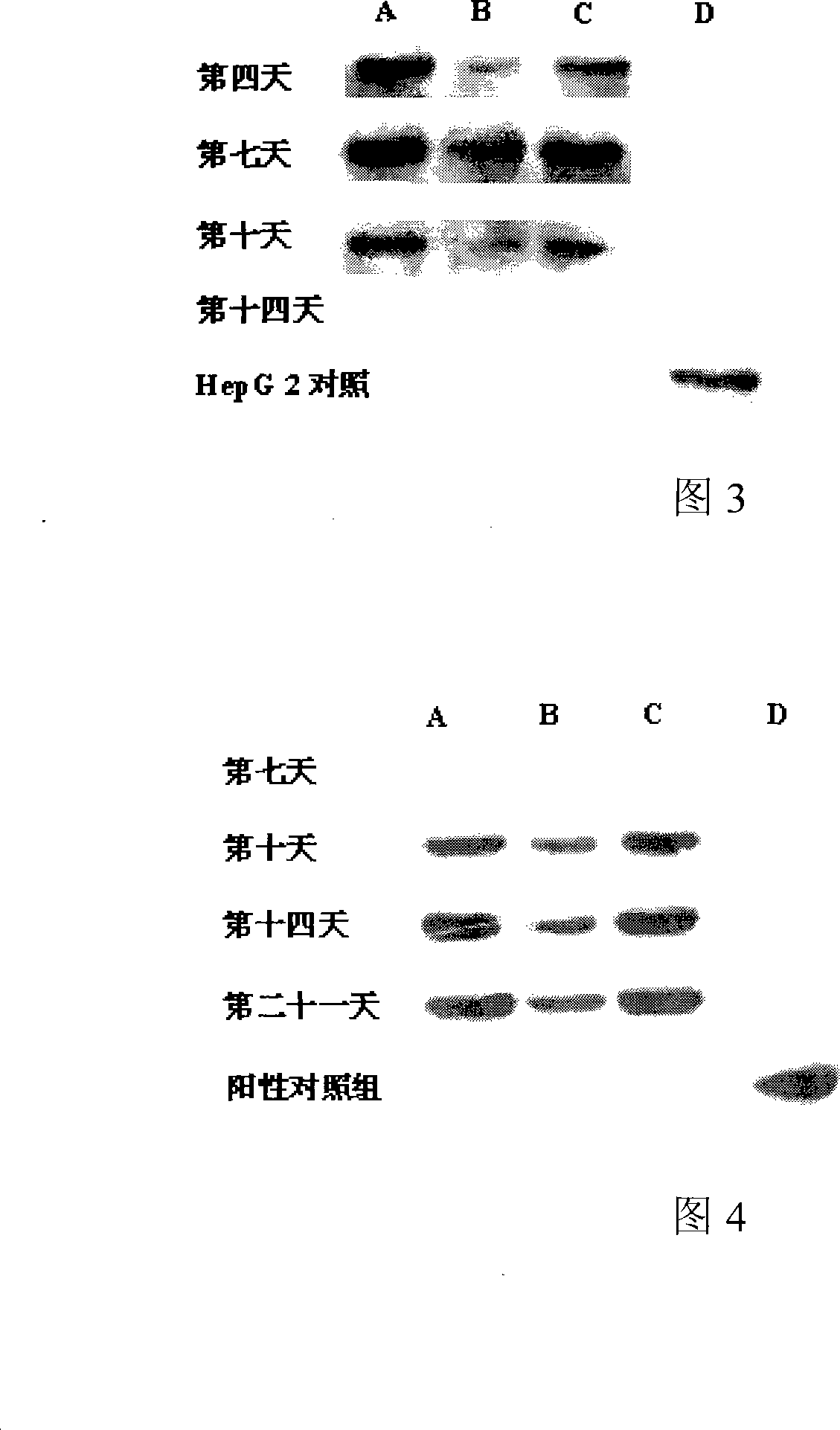
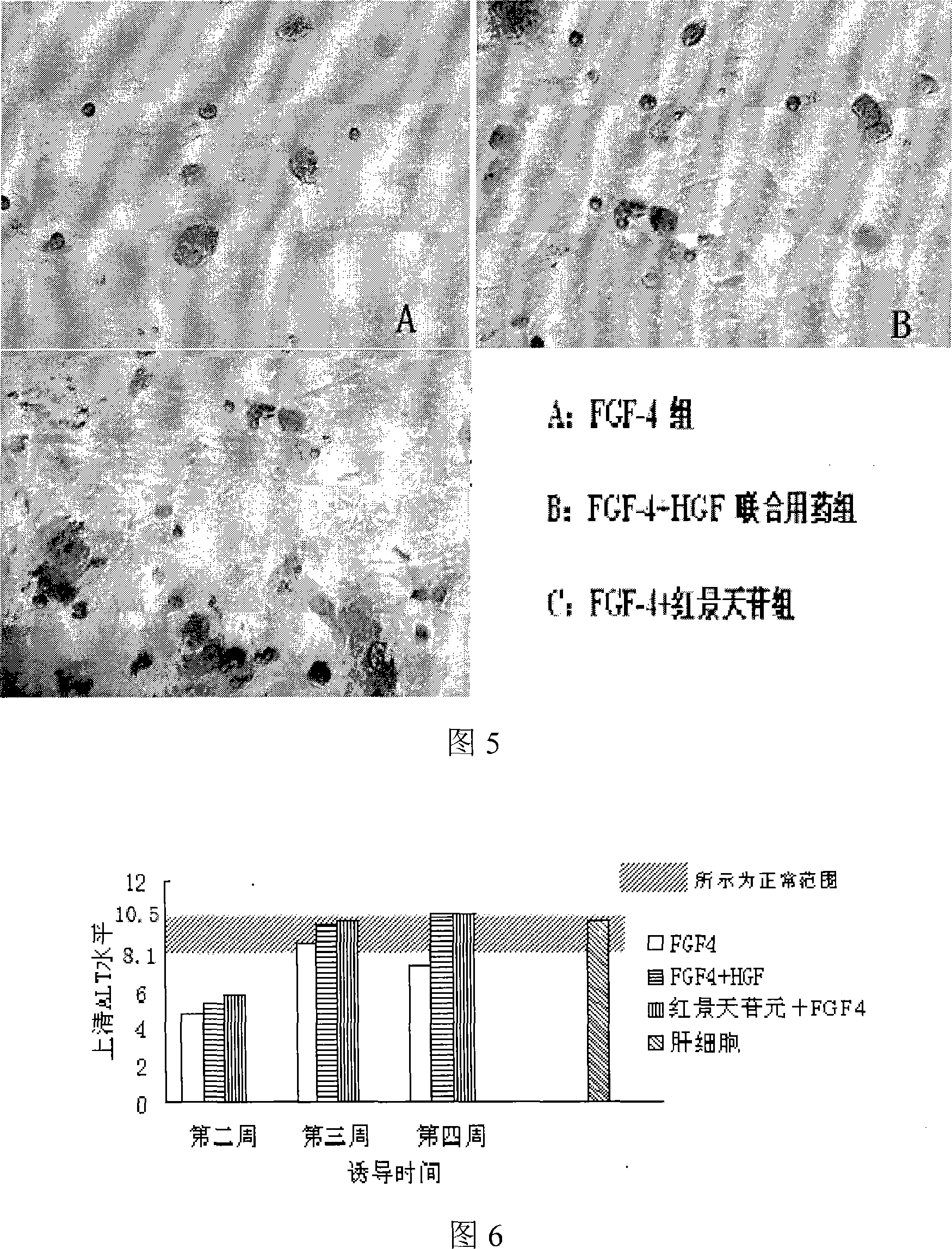
Common stonecrop herb and use of salidroside in stem cell committed differentiation to hepatocyte lineage
ActiveCN101225374AConducive to in-depth researchDigestive systemArtificial cell constructsSalidrosideRHODIOLA ROSEA ROOT
The invention relates to a rhodiola rosea and a rhodioside application for inducing stem cells to directionally differentiate into hepatocytes, in particular to a compound inducer of the rhodioside and cell growth factor FGF-4 used for inducing mesenchymal stem cells to directionally differentiate into hepatocytes. The rhodiola rosea develops the new application of rhodiola, which induces mesenchymal stem cells and other stem cells to directionally differentiate into hepatocytes in vitro, and provides the effect of the rhodiola rosea and the rhodioside on inducing the adult stem cells to directionally differentiate into hepatocytes, which is beneficial to further research of the in vivo stem cells transplantation for treating acute or chronic hepatic injury and middle-advanced liver disease field, and provides the basic for new drug development.
Owner:北京瑞草堂三圣(汤阴)药业有限公司
Preparation method of morroniside and new use thereof
The invention relates to a preparation method of morroniside and a new use thereof, in particular to the application of the morroniside in the preparation of drugs for preventing or treating acute and chronic liver injury and liver fibrosis and the preparation method of the morroniside.
Owner:SHANDONG LUYE PHARMA CO LTD
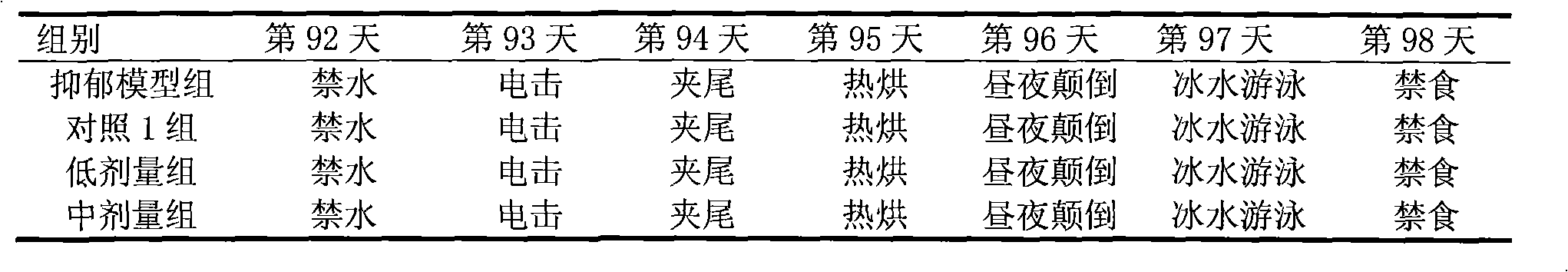
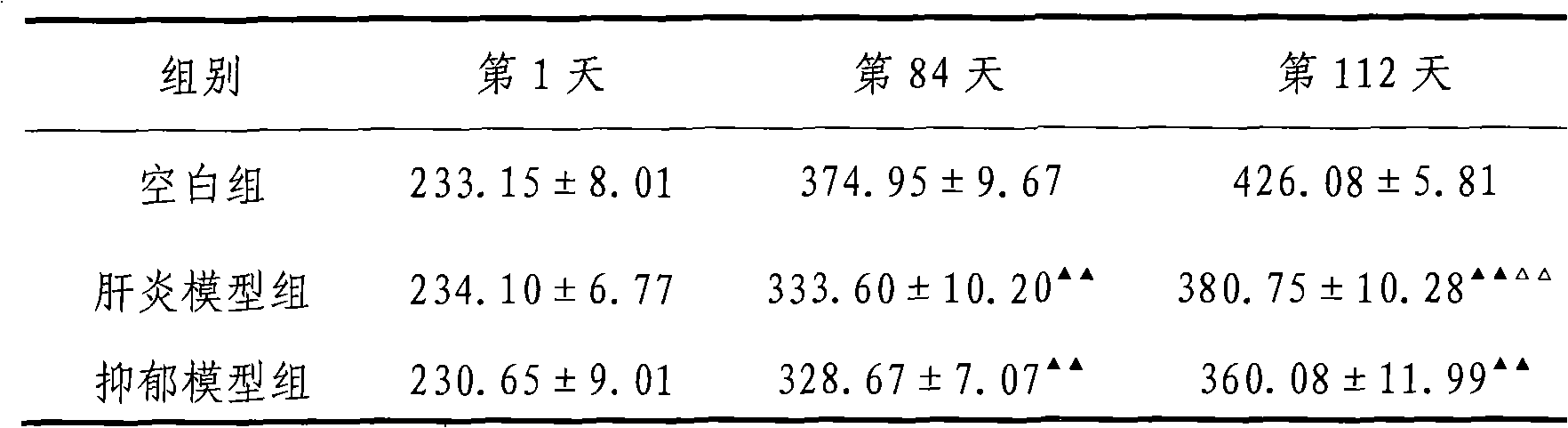
Preparation method of chronic liver injury unit stress depression rat model
A preparation method of chronic hepatic injury complicated with stress depression rat model comprises selecting male Wistar rat (body weight of 180-220 g), acclimating for one week, performing open field test ethology screening, and selecting rat having close integration; normally drinking and eating for the first week to the fourth week, and performing hypodermic injection of 40% CCL4 1ml / kg (twice per week); normally drinking and eating from the fifth to the twelfth week, performing hypodermic injection of 40% CCL4 1ml / kg (twice per week), and performing gastric perfusion of normal saline (one time per day); normally drinking and eating in the thirteenth week, and performing gastric perfusion of normal saline (one time per day); performing open field test in the fourteenth to sixteenth week (one rat per cage), normally drinking and eating, performing gastric perfusion of normal saline (one time per day), and offering chronic stress stimulation; and weighing in 112th day, and performing sweet water consumption and open field test. The preparation method provides reference for modeling research of chronic hepatic injury complicated with stress depression, provides effective experiment base for clinical diagnosis of hepatopathy complicated with depression, and provides experimental verification means for the research of drug for treating the hepatopathy complicated with depression.
Owner:刘铁军
Dehydrogenated silibinin substituted by meta-chlorobenzene formoxyl, preparation method and pharmaceutical applications thereof
InactiveCN101575335ASimple methodShort stepsOrganic active ingredientsNervous disorderDiseaseChlorobenzene
The invention relates to a dehydrogenated silibinin substituted by meta-chlorobenzene formoxyl, a preparation method and pharmaceutical applications thereof, in particular to a preparation method and applications of 2,3-dehydrogenated silibinin substituted by 23-site meta-chlorobenzene formoxyl. The compound has applications of inhibiting activity of production of lipid superoxide induced by free radical and can be expectedly used for preparing medicine for prevention and treatment of acute and chronic liver damage, hepatitis and cerebral atherosclerosis which are caused by the lipid superoxide. The compound further shows very strong resistance for PC12 cell damage caused by free radical and can be expectedly developed into medicine for protecting cranial nerve from oxidation and preventing and treating senile dementia. Besides, the compound has strong xanthine oxidase activity inhibition and can be expectedly developed into medicine for preventing and treating diseases caused by xanthine oxidase.
Owner:WENZHOU MEDICAL UNIV +1
A pharmaceutical composition for preventing or treating acute and chronic liver disease
InactiveCN1879845AReduce fat infiltrationAvoid necrosisOrganic active ingredientsPowder deliveryMedicinal herbsLiver disease
The invention discloses a pharmaceutical composition for treating or preventing acute and chronic hepatic disease, which is prepared from Chinese medicinal herbs including Bupleurum root, licorice root, root of red rooted saliva, notoginseng, schisandra fruit, curcuma longa, picrorhiza rhizome, cow-bezoar, Chinese angelica root, herba patriniae, astragalus root and alisol. The invention also provides the method for preparation and use of the pharmaceutical composition.
Owner:CHENGDU ZIHAO PHARMA
Chinese medicine composition for treating cold fever and liver damage and its preparing method
The present invention discloses a Chinese medicine composition for curing high fever due to exopathy and acute and chronic hepatic injury. It is formed from the following components: (by weight portion) 1-50 portions of taurine, 1-50 portions of baicalin and 1-50 portions of puerarin.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
Application of American ginseng in preparing medicament for preventing and treating alcoholic liver injury
The invention provides a Chinese medicinal composition for preventing and treating alcoholic liver injury, which consists of active ingredients and pharmaceutically acceptable auxiliary materials, wherein the active ingredients are prepared from American ginseng, centella, red-rooted salvia root, hovenia dulcis thunb and other raw materials. Pharmacodynamic experiments prove that the composition has good preventing and treating effect to acute and chronic liver injury caused by alcohol, can be used for improving body immunity, and can be prepared into medicaments or health-care foods with functions of preventing alcoholism and protecting liver.
Owner:JILIN AGRICULTURAL UNIV
Application of Dendrobium nobile total alkaloids
ActiveCN106389879AHas a preventive effectReduce AST activityDigestive systemPlant ingredientsDiseaseTherapeutic effect
The invention discloses an application of Dendrobium nobile total alkaloids in the preparation of medicines for preventing or treating acute and chronic liver injury diseases. The Dendrobium nobile total alkaloids have prevention and treatment effects on CCL4, alcohol or D-galactose induced acute liver injuries and CCL4 induced chronic liver injuries. The Dendrobium nobile total alkaloids are used to obviously reduce the activity of ALT and AST in serum, substantially reduce the content of MDA in liver homogenate and improve the expression of Nrf2, Nqo1 and Ho-1mRNA. A result of liver tissue slice observation shows that after the Dendrobium nobile total alkaloids are used, the liver tissue lesion necrosis disappears, the liver lobule structure is basically complete, and the inflammatory cell infiltration is obviously mitigated.
Owner:ZUNYI MEDICAL UNIVERSITY
Medicine for preventing and curing liver injury
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Application of penthorum chinense pursh monomer in preparation of liver protection medicine
ActiveCN105193833AHas hepatoprotective effectPrevent liver damageOrganic active ingredientsDigestive systemCurative effectPinocembrin
The invention provides an application of pinocembrin dihydrogen chalcone-7-O-[3'-O-galloyl-4',6'-hexahydroxydiphenodicarboxy]-beta-D-glucoside in preparation of a liver protection medicine. The penthorum chinense pursh macrocyclic polyphenol monomer namely pinocembrin dihydrogen chalcone-7-O-[3'-O-galloyl-4',6'-hexahydroxydiphenodicarboxy]-beta-D-glucoside has a liver protection effect, can be used for preventing and treating liver injuries, and has a definite curative effect on hepatic ischemia-reperfusion injuries and chronic liver injuries, and the effect is superior to that of positive medicine namely Gansu Granules, so that the penthorum chinense pursh macrocyclic polyphenol monomer can be used for replacing the Gansu Granules and has a good clinical application prospect.
Owner:四川古蔺肝苏药业有限公司
Use of common stonecrop herb and salidroside in stem cell oriented differentiation to hepatocyte lineage
ActiveCN101225374BConducive to in-depth researchMaterial basis is clearDigestive systemArtificial cell constructsSalidrosideRHODIOLA ROSEA ROOT
The invention relates to a rhodiola rosea and a rhodioside application for inducing stem cells to directionally differentiate into hepatocytes, in particular to a compound inducer of the rhodioside and cell growth factor FGF-4 used for inducing mesenchymal stem cells to directionally differentiate into hepatocytes. The rhodiola rosea develops the new application of rhodiola, which induces mesenchymal stem cells and other stem cells to directionally differentiate into hepatocytes in vitro, and provides the effect of the rhodiola rosea and the rhodioside on inducing the adult stem cells to directionally differentiate into hepatocytes, which is beneficial to further research of the in vivo stem cells transplantation for treating acute or chronic hepatic injury and middle-advanced liver disease field, and provides the basic for new drug development.
Owner:北京瑞草堂三圣(汤阴)药业有限公司
YouguiWan application
InactiveCN103070979ALower ALT levelsReduce collagen areaDigestive systemUnknown materialsRat liverLiver tissue
The invention discloses an application of YouguiWan, and specifically discloses an application of YouguiWan in preparing medicines used for ameliorating chronic liver injury. With YouguiWan, CCl4 mouse serum ALT level can be reduced, liver tissue collagen area can be reduced, and liver tissue Hyp content can be reduced. Also, with YouguiWan, DMN rat liver tissue collagen area can be reduced, liver tissue Hyp content can be reduced, and rat weight can be increased.
Owner:SHANGHAI UNIV OF T C M
Momordica grosvenori liver-protecting preparation and production method thereof
The invention relates to a process for preparation of a hepatoprotective medicament which is prepared from an extract of cucurbitaceous momordica grosvenori and characterized in that momordica grosvenori is firstly disintegrated, heated and extracted through water, and the extracting liquid is separated, concentrated and dried through macroreticular resin column. Purification extracted from momordica grosvenori is prepared into granules, tablets, capsules, oral liquid, dropping pills or syrup, and the medicaments have protective effect to CCI4 chronic liver injury of rats and can decrease activity of ALT and AST in serum and increase TP and ALB, and activity of SOD and GSH-px of liver tissue, reduce MDA content of liver tissue and improve pathologic change of liver tissue.
Owner:防城港市现代科技有限公司
Forsythoside A and preparation method thereof
The invention provides a forsythoside A and a preparation method thereof, and belongs to the field of medicines. In the preparation method, fruits of a traditional Chinese medicine of fructus forsythia are taken as raw materials, and are extracted, separated, crystallized, recrystallized and the like, and the high-content forsythoside A with good effect of clearing heat and releasing toxin and capacity of preventing acute or chronic hepatic injury is prepared; and a high performance liquid chromatography is adopted for measuring, and the content range is 60-99.8 percent. The modern separation and purification technology for traditional Chinese medicines is adopted, so the preparation method is novel in process, simple and convenient to operate and suitable for industrial production, and has high practicability.
Owner:JIANGSU TIANSHENG PHARMA
Pharmaceutical composition composed of saikoside A and taurine and use thereof
InactiveCN104688760ATo promote metabolismEasily damagedAntipyreticAnalgesicsTaurinePharmaceutical Adjuvants
The invention belongs to the technical field of medicine and relates to a pharmaceutical composition with liver protective function. The pharmaceutical composition is composed of saikoside A and taurine; the composition is prepared by supplementing the routine pharmaceutical adjuvants in a routine process. The invention further discloses application of the pharmaceutical composition in preparation of a medicine for treating and / or preventing the acute liver injury or relieving the chronic liver injury. The pharmaceutical composition has an obvious effect for preventing the acute liver injury or reducing the chronic liver injury.
Owner:SHANDONG ACAD OF CHINESE MEDICINE
Compound Chinese medicine for treating chronic liver disease and preparation
InactiveCN1814049AGood effectGreat development and application valueDigestive systemGranular deliveryMedicinal herbsVerbena
The invention is a compound traditional Chinese medicine and preparation for curing chronic hepatopathy, comprising the 12 Chinese traditional medicines (medicinal materials, prepared products and extractives): Phyllanthusurinaria L, verbena, kuh-seng, Spreading Hedyotis Herb, pseudo-ginseng, danshen, peach kernel, radix bupleuri, prepared rhizome of rehmannia, ginseng, fuling, and Liquoricee in a certain proportion in the range of 1%-99%, and each accounts for 2%-15% of total weight. It has efficacies of clearing heat and detoxifying, comforting liver and removing gloomy and promoting blood circulation and tonifying, and has a very good curative effect on liver fibrosis and chronic liver damage.
Owner:广州泽力医药科技有限公司
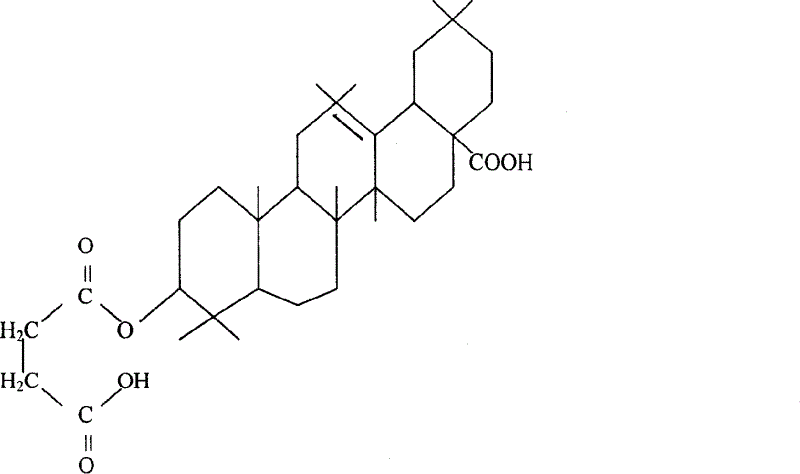
Synthesis of oleanolic acid saccinic acid monoester and its medicinal preparation for treating chronic and acute hepatitis
The present invention belongs to the field of Chinese medicine technology and organic synthesis technology. Oleanolic acid as one natural Chinese medicine component and succinic anhydride in the amount of 7 times produce esterification reaction under the catalysis of anhydrous pyridine in 4 times at 100 deg.c for 8 hr to produce succinyl oleanolic acid monoester with new chemical structure. After reaction, the product is water washed to eliminate residual succinic anhydride and pyridine and further purified through re-crystallization in anhydrous ethanol. The obtained new compound has molecular expression of C34H52O6, molecular weight of 556.77 and melting point of 256-258 deg c. By means of the structural modification, the present invention has raised water solubility and dispersivity and raised bioavailability. The product of the present invention is orally taken to treating acute and chronic liver damage, lower transaminase, reduce liver fibrosis and inhibit immunological liver pathologic changes.
Owner:田振坤 +2
Method for preparing extractive of liuyueqing and its application
InactiveCN101045073APromote modernizationDigestive systemSolution deliverySerum lysozymeEthanol extracts
An extract composed of the aquatic extract, alcohol extract, petroether extract and ethanol extract is prepared from the Chinese-medicinal material 'Liuyueqing' through breaking, extracting and separating. It can be used to prepare the medicines for protecting liver and treating hepatitis B, hepatic injury, and cancer.
Owner:黄仁彬
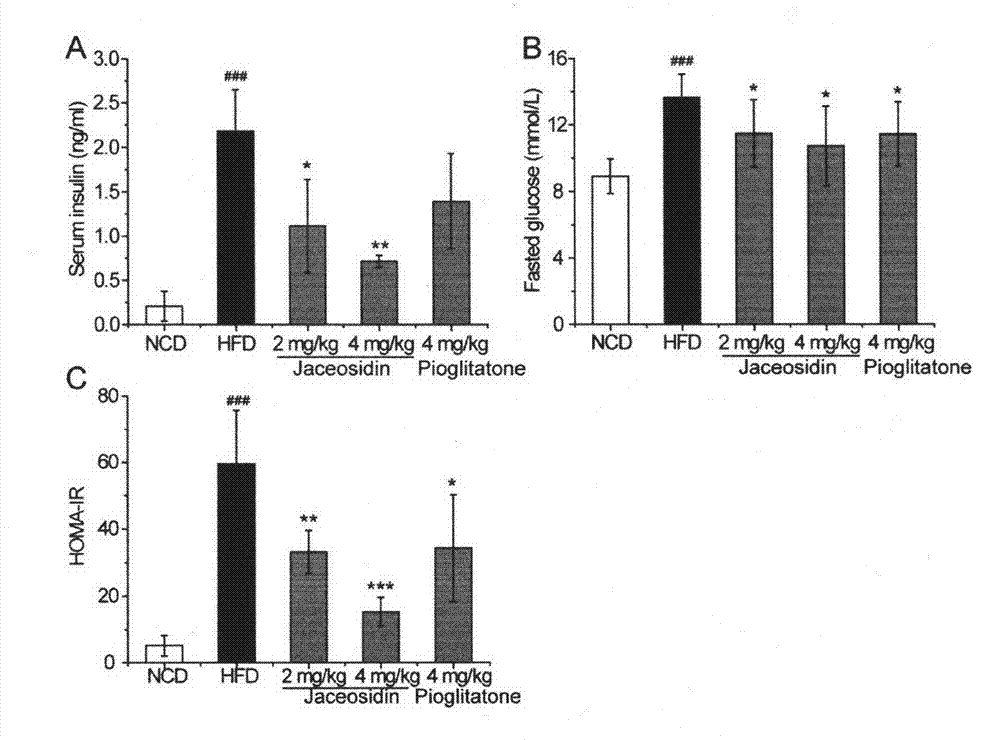
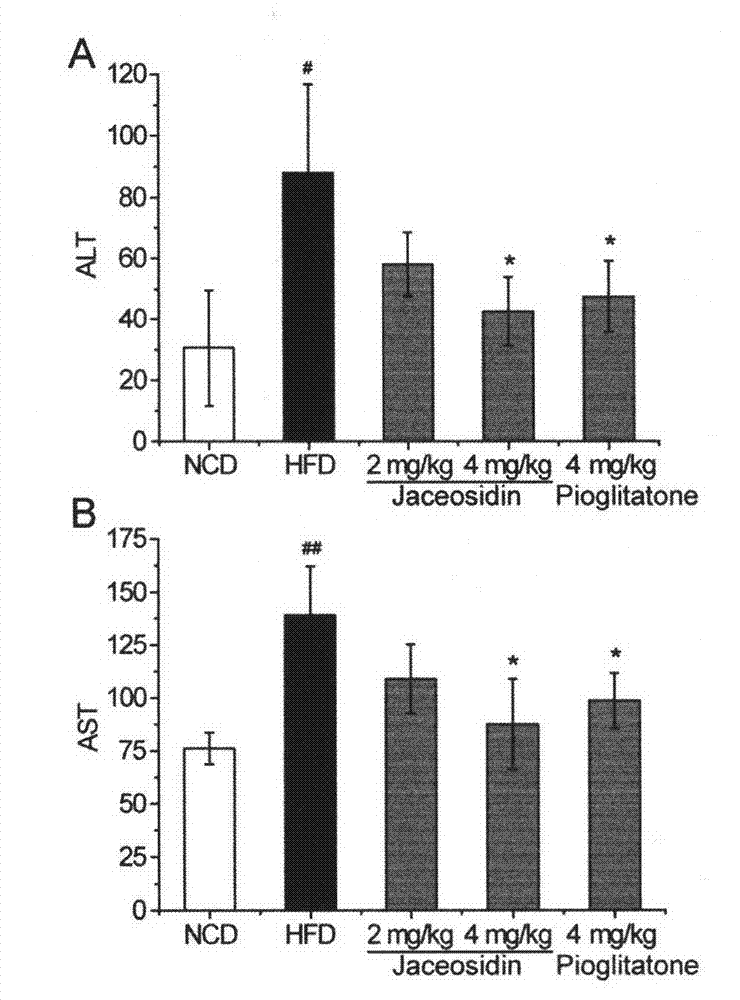
Application of Jaceosidin in preparation of medicine for preventing and treating insulin resistance, nonalcoholic fatty liver diseases and other diseases related to II-type diabetes mellitus
InactiveCN102697767AInhibit the rise of basal blood sugarCurb riseOrganic active ingredientsMetabolism disorderDiabetes modelGlycemic
The invention belongs to the technical field of pharmaceutics. According to the invention, Jaceosidin can be used for remarkably improving mouse insulin resistance induced by high-fat diets, further improving mouse symptoms of the II-type diabetes mellitus models and remarkably improving the nonalcoholic fatty liver diseases and the chronic liver injury caused by the high-fat diets. The Jaceosidin serves as a novel medicine for treating insulin resistance, has activities of controlling blood glucose and improving insulin sensitivity and the like well, meanwhile, can be used for treating the nonalcoholic fatty liver diseases and is capable of effectively reducing liver fat pathological changes and chronic liver injury. Therefore, the Jaceosidin can be used for treating the insulin resistance, the nonalcoholic fatty liver diseases and the diseases related to II-type diabetes mellitus and preparing corresponding therapeutic medicines.
Owner:NANJING UNIV
Application of forsythiaside in the preparation of medicament for treating or preventing acute and chronic liver damnification and liver fibrosis
The present invention is the application of forsythia ester glycoside in preparing medicine for preventing and treating acute and chronic liver injury and liver fibrosis. The dosage of the medicine is 25-800 mg for injection and 50-1500 mg for orally taken preparations. The medicine with forsythia ester glycoside as effective component may be prepared into injection, tablet, capsule, pill or other forms, preferably freeze dried powder for injection and capsule.
Owner:SHANDONG LUYE PHARMA CO LTD
Medicine composition for preparing hepatic diseases and its use thereof
A Chinese medicine for treating hepatofibrosis and heptocirrhosis is prepared from red sage root and notogenseng.
Owner:TIANJIN TASLY PHARMA CO LTD
Application of forsythin aglycone in preparing medicine for preventing or treating liver injury or liver failure
InactiveCN105078956AGood treatment effectSmall toxicityAntipyreticAnalgesicsSide effectTherapeutic effect
The invention discloses application of fructus forsythiae aglycone in preparing a medicament for preventing or treating liver injury or liver failure, and belongs to the medical field. The fructus forsythiae aglycone is a traditional Chinese medicine monomer obtained by extracting from the conventional fructus forsythiae, and is shown to have better treatment effect for the liver injury caused by acetaminopben, the livery injury caused by anti-tumor drug cis-platinum, acute or chronic livery injury caused by carbon tetrachloride, the livery injury caused by D-galactosamine and the liver failure caused by the D-galactosamine and lipopolysaccharide according to an animal test, has effect, which is better than that of fructus forsythiae and fructus forsythiae aglycone, in treating liver injury or liver failure. The fructus forsythiae aglycone is exact in curative effect and low in side effect for treating the liver injury and the liver failure, and has wide medical application prospect.
Owner:LUNAN PHARMA GROUP CORPORATION
Application of Bacillus licheniformis live bacteria in preparation of medicine for preventing and treating intestinal endotoxemia
InactiveCN102552332AImprove pathological changesReduce lipid peroxidation damageAntibacterial agentsBacteria material medical ingredientsBacillus licheniformisAcyl CoA dehydrogenase
The invention relates to an application of Bacillus licheniformis live bacteria which is applied in the field of biotechnology in preparation of medicine for preventing acute and chronic intestinal endotoxemia and medicine for treating chronic intestinal endotoxemia; an application of the Bacillus licheniformis live bacteria in preparation of medicine for preventing acute and chronic intestinal endoxemia; and an application of the Bacillus licheniformis live bacteria in preparation of medicine for treating chronic intestinal endotoxemia. The intestinal endotoxemia is caused by factor causing damage to liver; a dosage of the Bacillus licheniformis live bacteria is 0.45-1.35 billion live bacteria per kilogram every day. The Bacillus licheniformis live bacteria provided by the invention ameliorates the intestinal endotoxemia caused by acute and chronic liver injury in rat induced by thioacetamide, improves histopathology change of the liver injury of rat, relieves lipid peroxidatic injury of hepatic tissue when chronic liver injury occurs, and partially decreases levels of serum glutamic pyruvic transaminase, glutamic-oxalacetic transaminease and lactic dehydrogenase.
Owner:NORTHEAST PHARMA SHENYANG SCI & TECH DEV +1
Application of demethylenetetrahydroberberine hydrochloride in preparation of medicine for preventing or treating liver injury
InactiveCN111012781AHigh damage success rateNot easy to reverseOrganic active ingredientsDigestive systemDiseaseHepatoprotective Drugs
The invention relates to the field of biological medicines, and concretely relates to application of demethylenetetrahydroberberine hydrochloride in the preparation of a medicine for preventing and / ortreating liver injury induced by thioacetamide. The demethylenetetrahydroberberine hydrochloride medicine has the advantages of good solubility, good medicine absorptivity and good anti-inflammatoryand antioxidant activity, and has a remarkable treatment effect on thioacetamide-induced acute mild and chronic liver injury; and compared with the traditional liver protection medicine, the demethylenetetrahydroberberine hydrochloride can achieve the treatment purpose under a low oral administration dosage, can improve the patient compliance, and achieves the treatment effect on diseases.
Owner:CHINA PHARM UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com