Medicine for preventing and curing liver injury
A technology for liver damage and liver damage, applied in the direction of drug combinations, pharmaceutical formulas, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Preparation of icariin (see Ye Haiyong et al., Zhejiang University Journal (Medical Science), 2005, 34(2): 131-136)
[0049] Take 2 kg of Epimedium sagittarius collected in the mountains of southern China, grind it into powder, and then extract it with 95% ethanol (10 L). 250 g of concentrated material were obtained, which was then further extracted with 2.5 L of chloroform, ethyl acetate and n-butanol. Pack 8 g of ethyl acetate extract and 10 g of n-butanol extract into a silica gel chromatography column, and wash with CHCl 3 -MeOH-HCOOH (15:1:0.5) and CHCl 3 -MeOH (8:2) eluted separately. Through this process, 300 mg of icariin and noricariin precursor icariin can be obtained. The precursor is then hydrolyzed according to the method briefly described below to obtain icariin. Solution A: 80 mg precursor was ultrasonically dissolved in 18 ml methanol solution; Solution B: 500 U (0.433 g) cellulase was dissolved in 180 ml (0.1 mol / L), pH 5.0 acetate buffer. Solution...
Embodiment 2
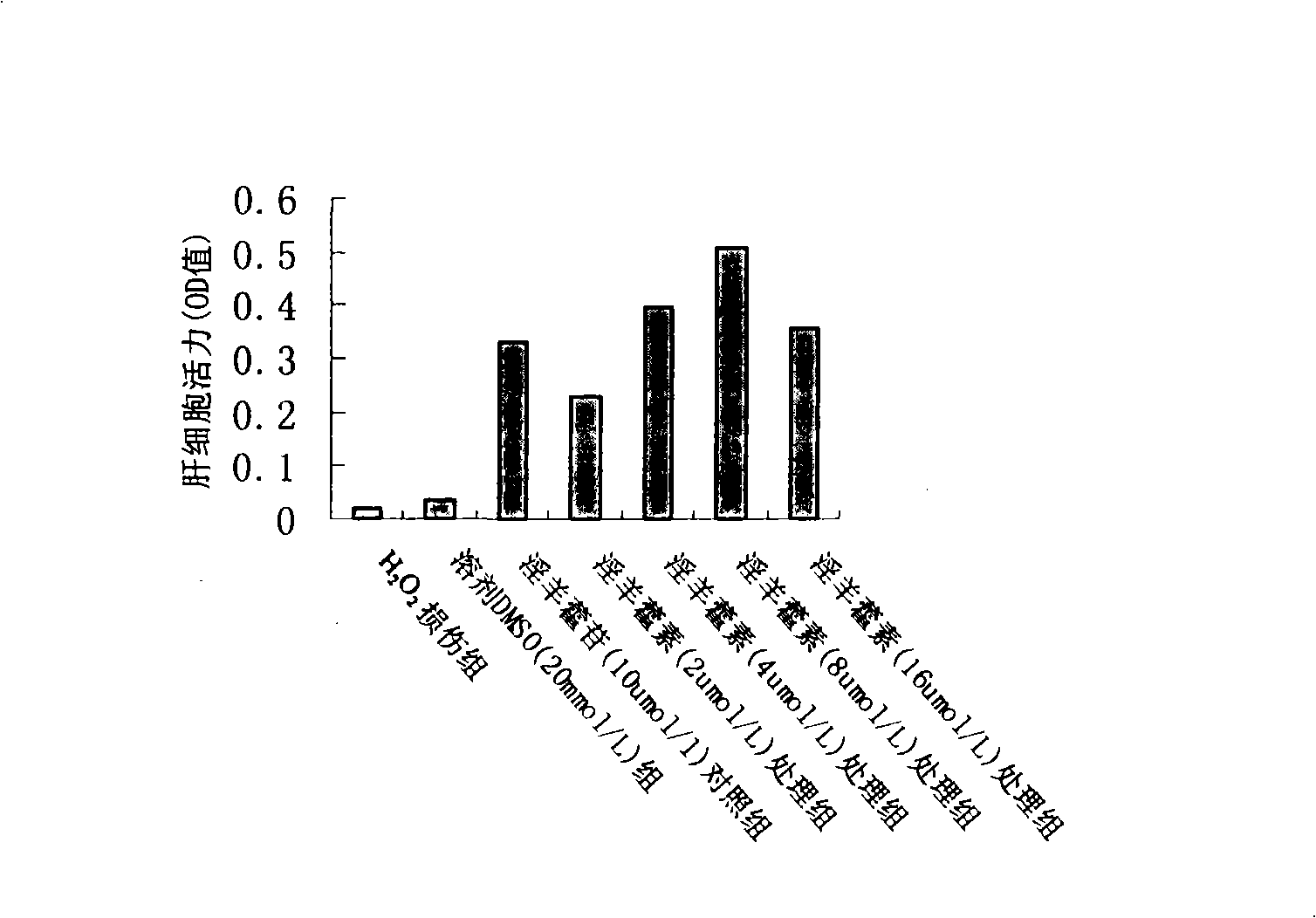
[0051] Icaritin attenuates H in rat primary hepatocytes 2 o 2 damage model test
[0052] 1. Acquisition and culture of primary rat hepatocytes
[0053] Refer to Seglen's two-step collagenase perfusion method and make improvements (Xu Zhe et al., Journal of Postgraduate Medicine, 2003, 16(5): 342-344). SD rats (purchased from the Experimental Animal Center of the Second Military Medical University) were anesthetized and injected with 1000 U of heparin into the tail vein. The abdominal cavity was opened under aseptic conditions, and the distal end of the portal vein and inferior vena cava and the superior vena cava were separated and ligated. Intubate at the end of the heart, cut off the proximal end of the inferior vena cava, and open the perfusion with 4°C D-Hanks solution (containing 0.5mol / L EDTA) at a constant flow rate of 40ml / min. After the liver turns khaki, put the Put the liver in a sterilized plate, use 300ml of 0.03% collagenase at 37°C, and circulate perfusion at...
Embodiment 3
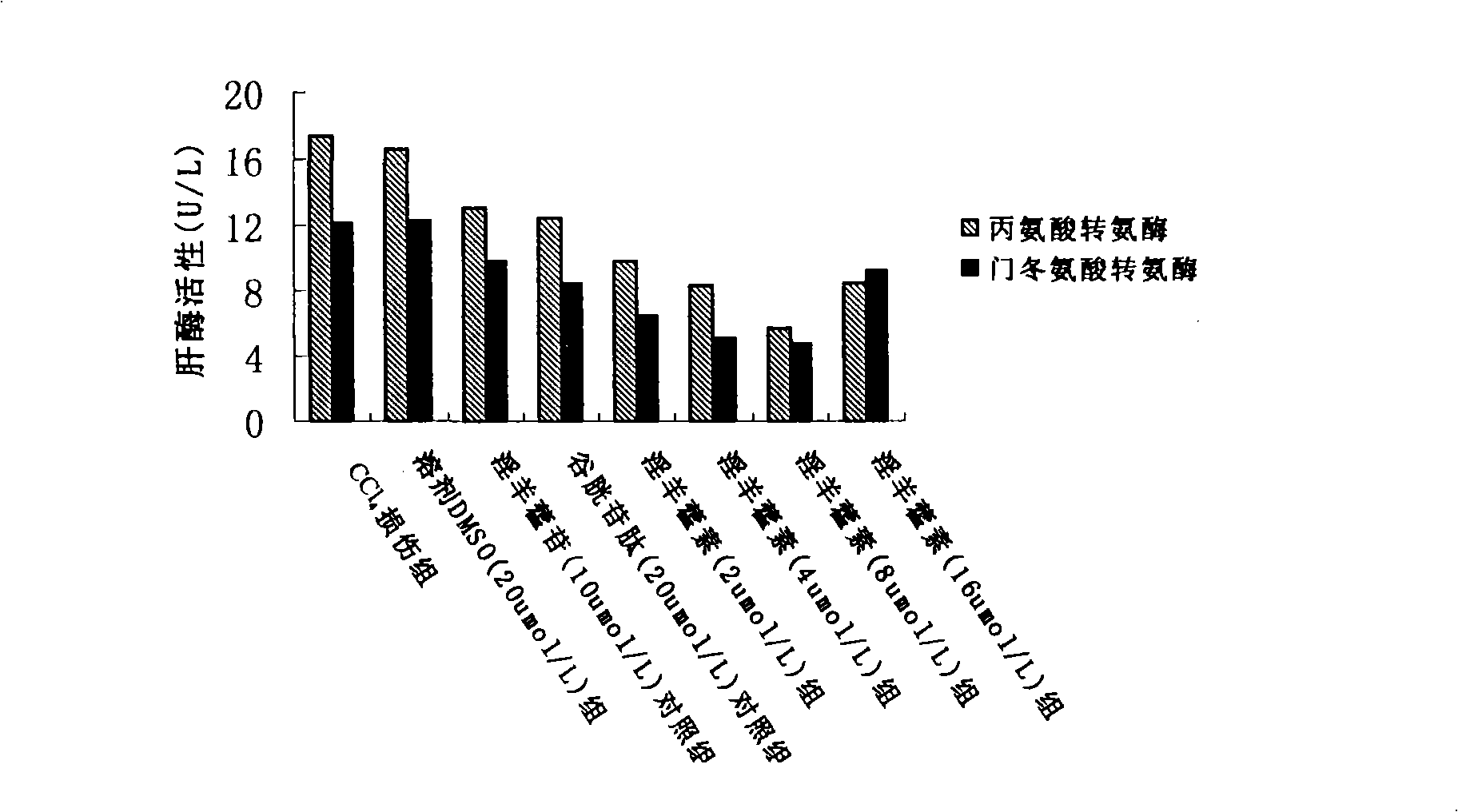
[0056] Icaritin attenuates CCl in rat primary hepatocytes 4 damage model test
[0057] 1. See Example 2 for the acquisition and culture of primary rat hepatocytes.
[0058] 2. Rat primary hepatocytes were cultured in 24-well plates and randomly divided into CCl 4 Injury group, solvent DMSO group, icariin and reduced glutathione control group, icariin 2 μmol / L treatment group, icariin 4 μmol / L treatment group, icariin 8 μmol / L treatment group , Icaritin 16μmol / L treatment group. Each group has 6 replicate wells. Reduced glutathione is a commonly used hepatoprotective drug in clinical practice, and it was set as the positive control group together with icariin in this experiment. Two days after primary rat hepatocytes were cultured, culture medium with different concentrations of drugs were added to each group, and the CCl-containing medium was replaced after 24 hours. 4 The culture medium (concentration is 8mmol / L), after acting for 6 hours, the cell culture supernatant wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com