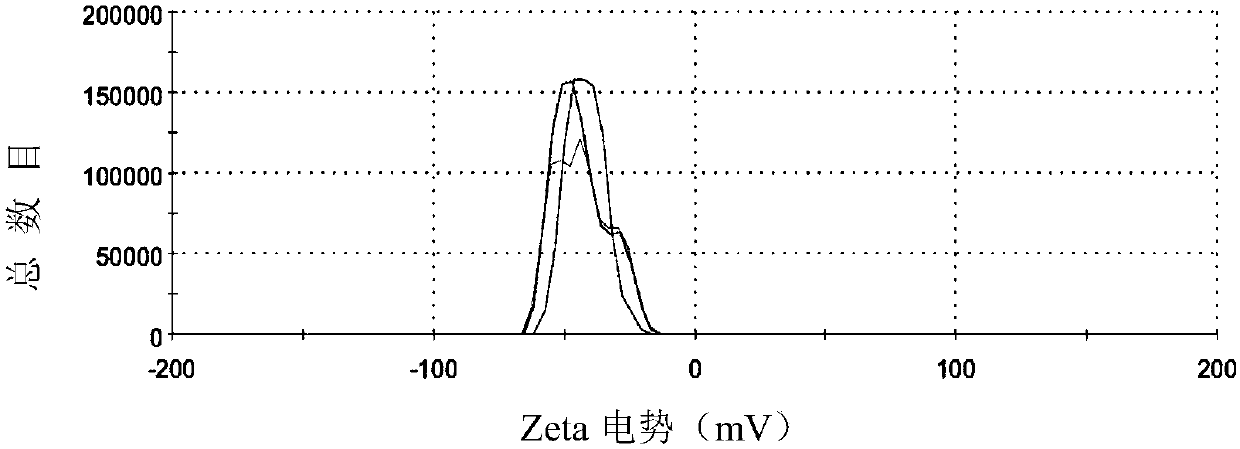
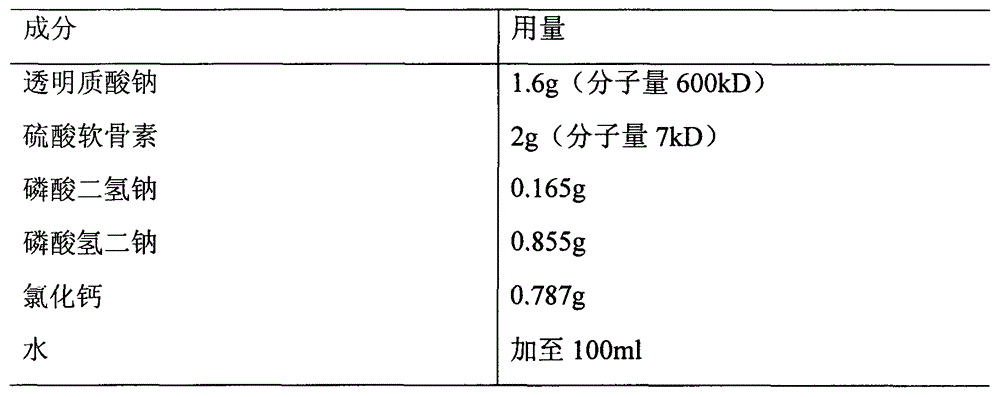
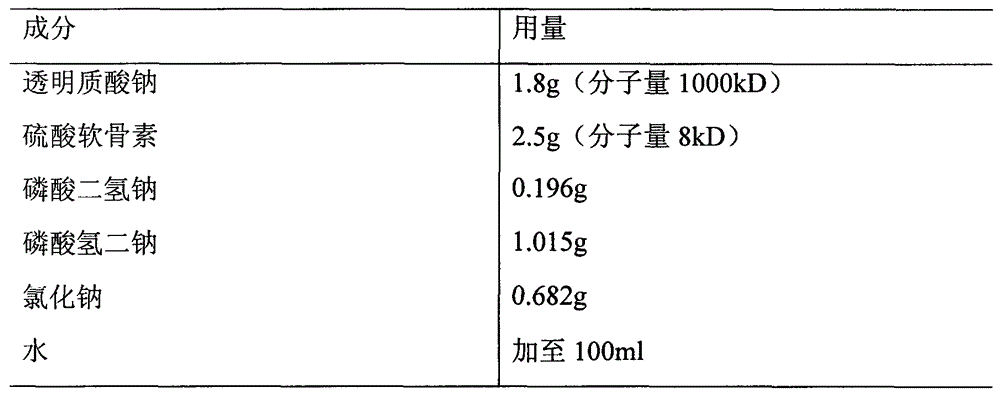
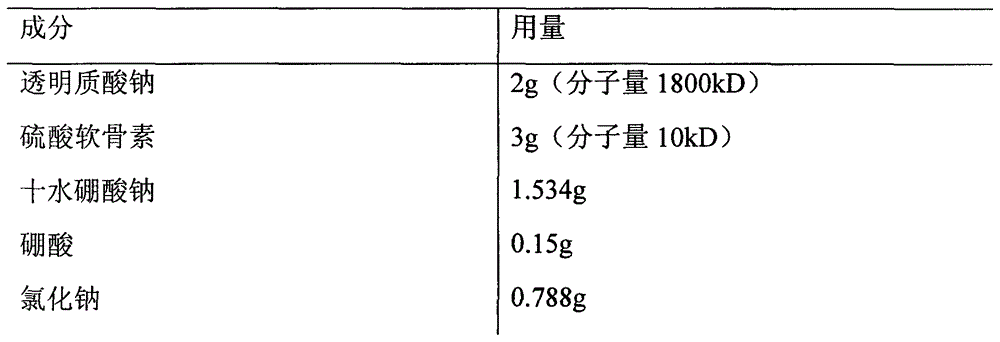
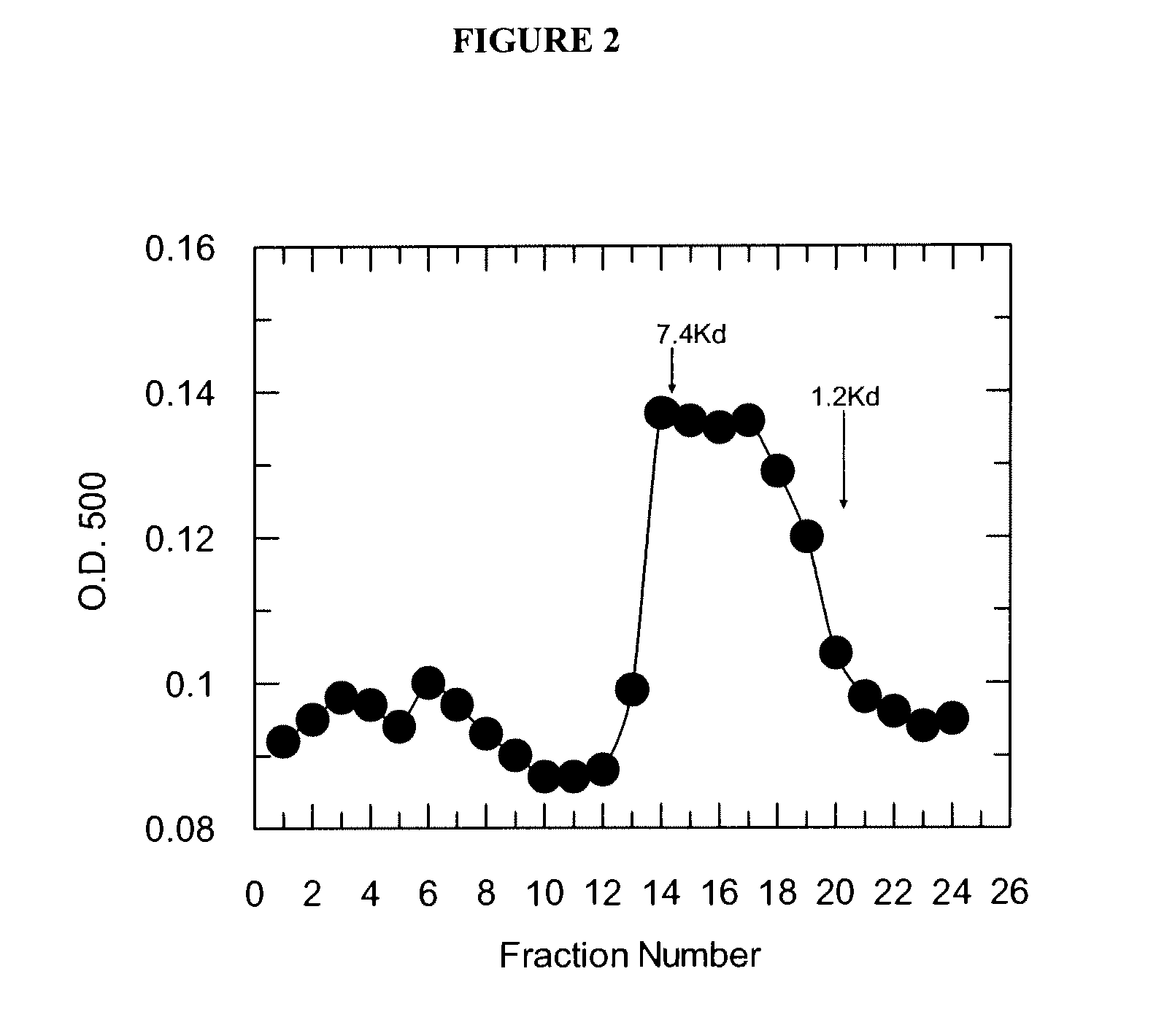
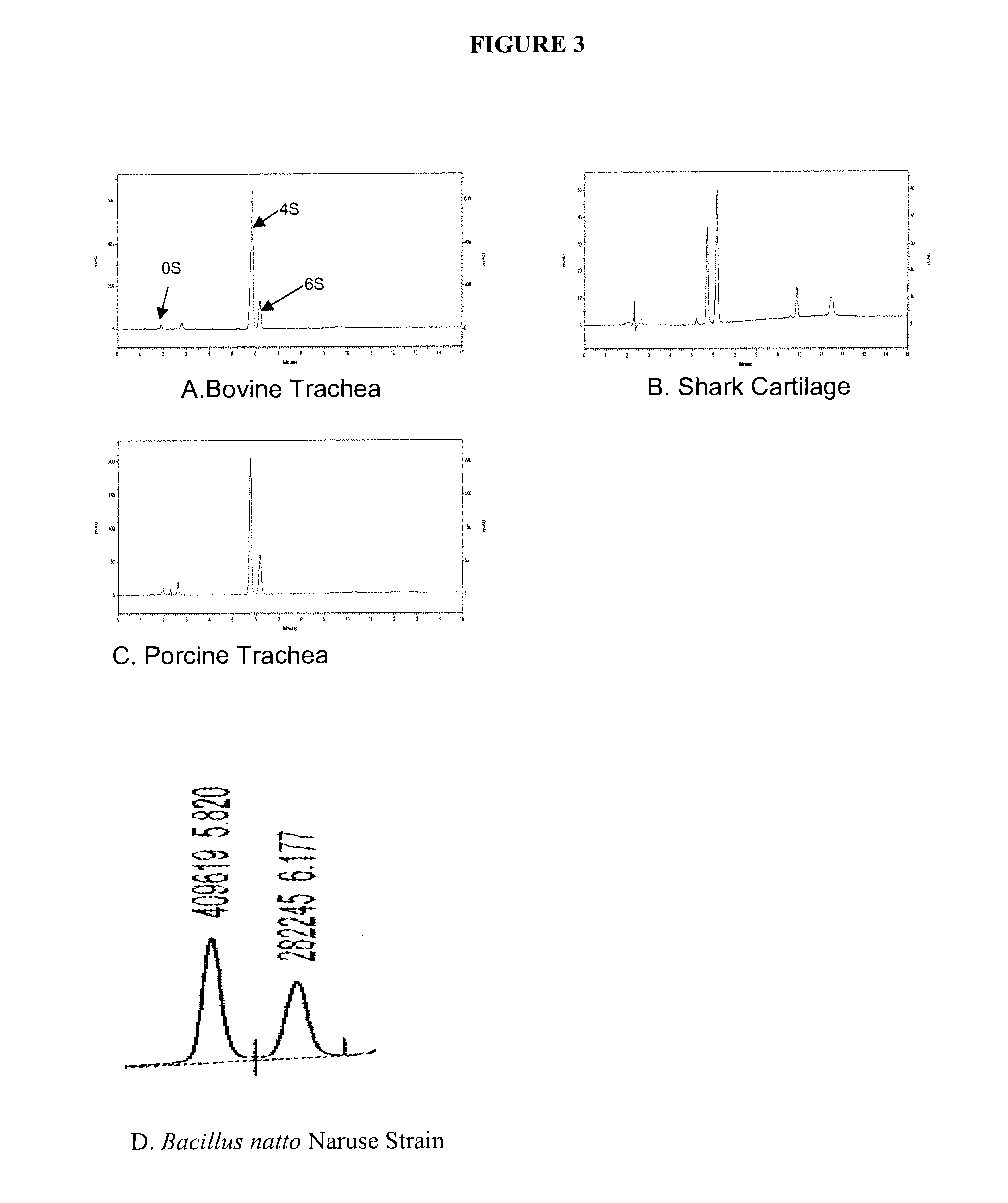
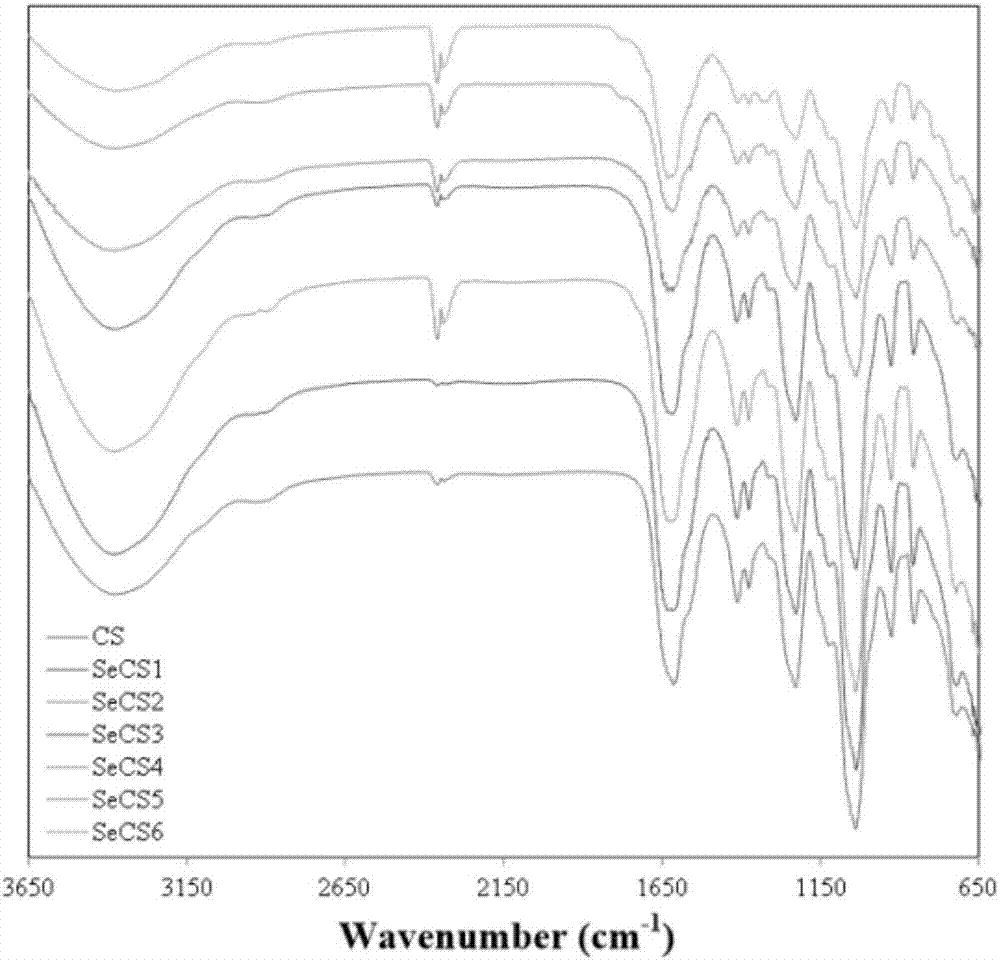
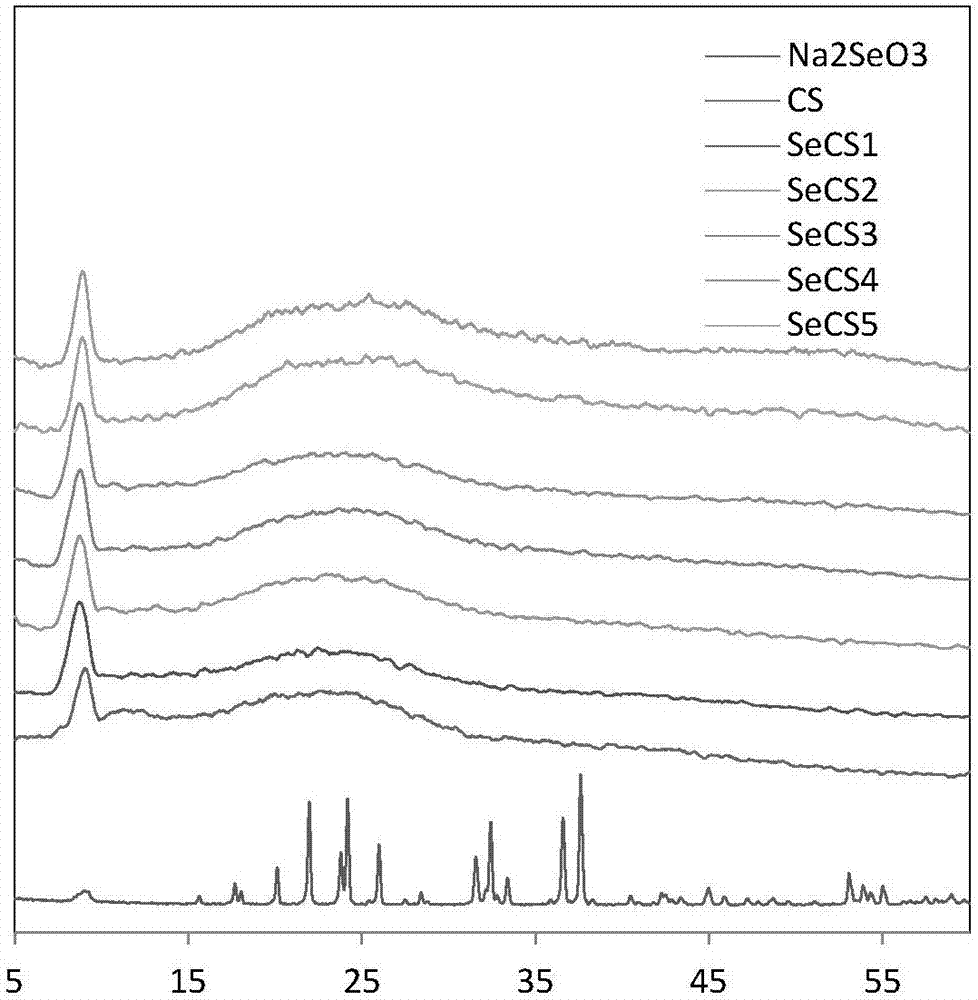
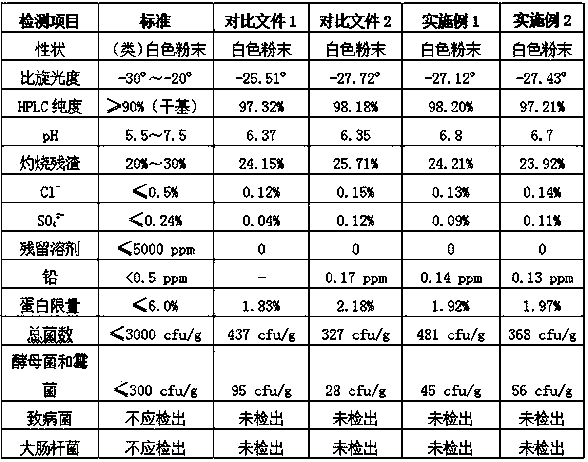
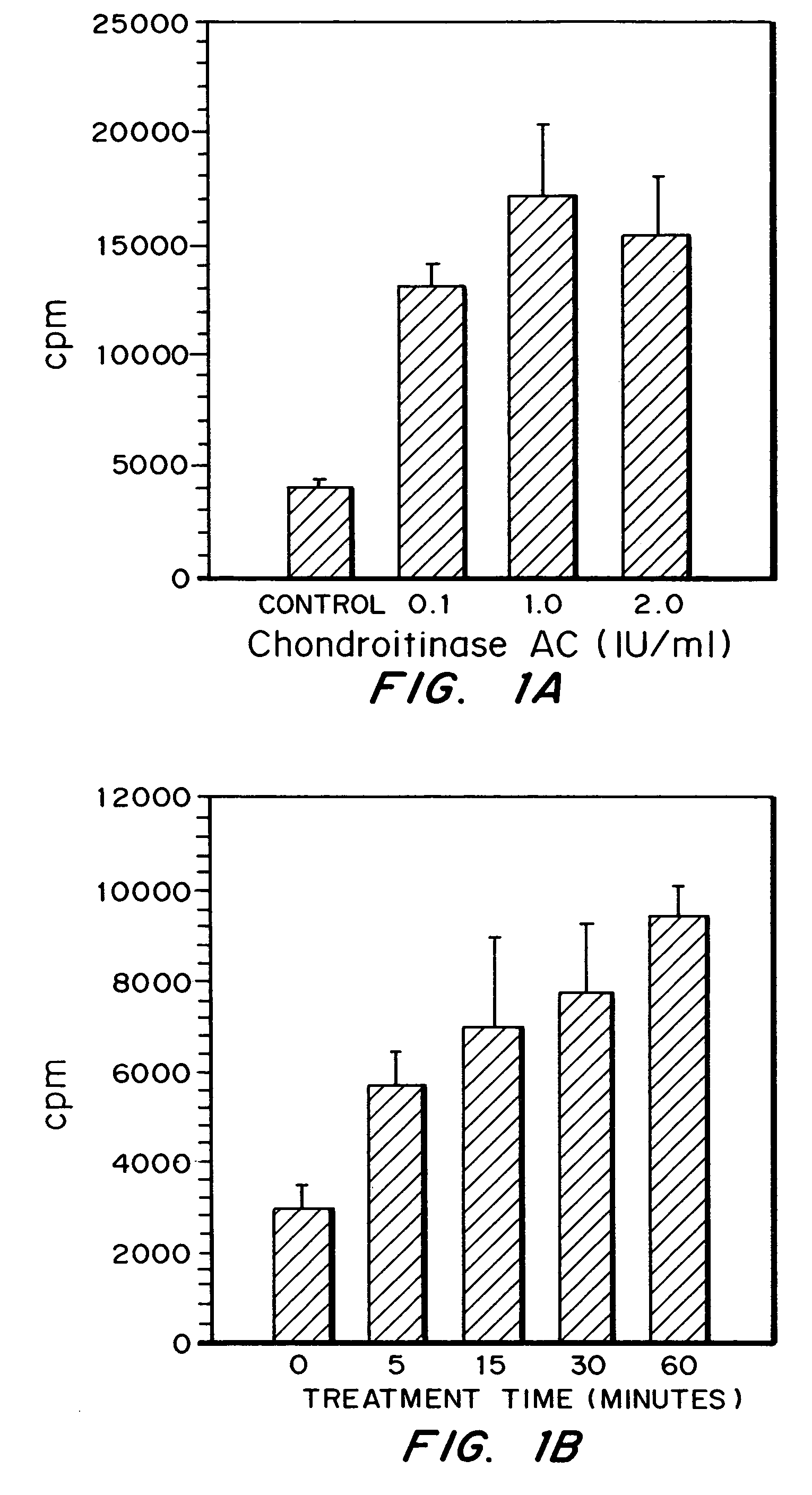
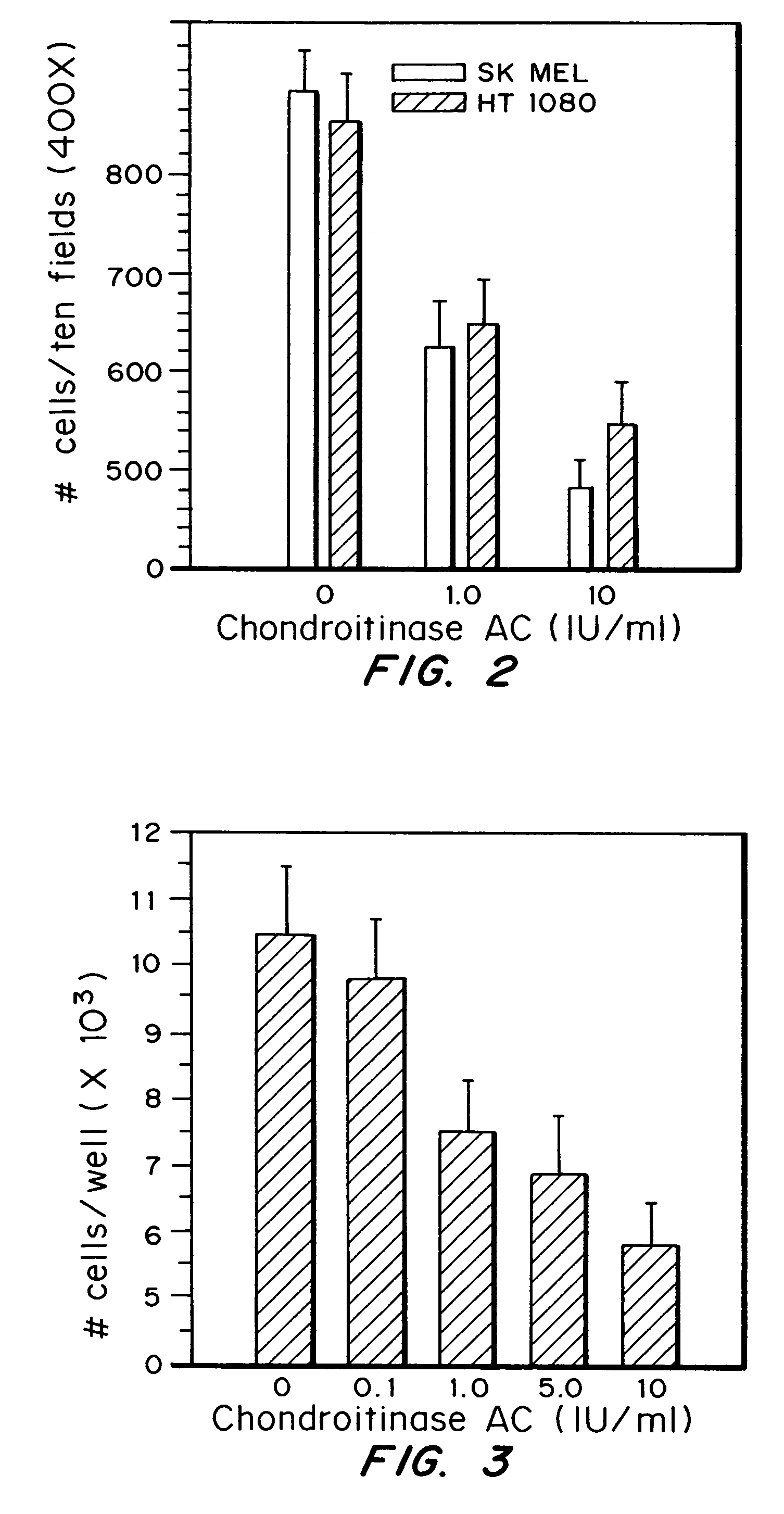
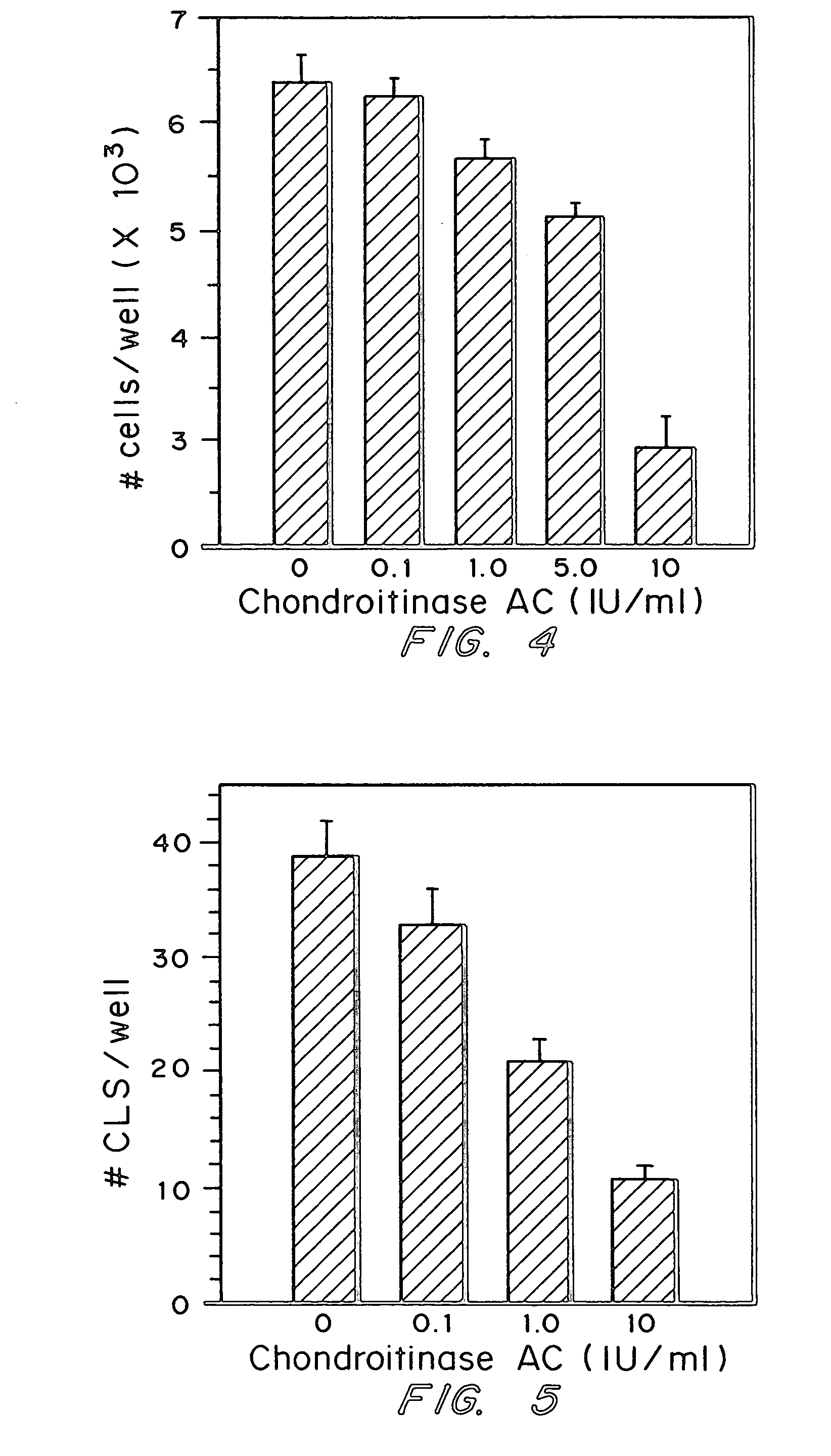
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
62 results about "Chondroitin sulfate A" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Production method of fish cartilage extract for preventing and treating osteoarthritis
InactiveCN103623006ARealize simultaneous hydrolysisRealize comprehensive utilizationSkeletal disorderFish material medical ingredientsTherapeutic effectHydrolysis
The invention discloses a production method of a fish cartilage extract for preventing and treating osteoarthritis. According to the method, enzymolysis is carried out on fish cartilages by using mixed enzymes; trapping with an ultrafiltration membrane to obtain an extract containing low-molecular-weight chondroitin sulfate of which the molecular weight is less than 1000 Dalton and collagen peptide. In the method, a compound enzyme hydrolysis technology is adopted, so that synchronous hydrolysis of the chondroitin sulfate and collagen is realized, and comprehensive utilization of the fish cartilages is realized. The obtained product is low-molecular-weight chondroitin sulfate, so that the problem of low bioavailability of the chondroitin sulfate on the aspect of clinical application is solved. The extract can remarkably improve the symptoms of osteoarthritis and has good health care and treatment effects.
Owner:QINGDAO BETTER BIO TECH
Placenta targeted delivery system and preparation method and application thereof
PendingCN109568268AStrong targetingIncrease contrastEchographic/ultrasound-imaging preparationsMacromolecular non-active ingredientsDiseaseLipid formation
The invention provides a placenta targeted delivery system. The placenta targeted delivery system comprises a hydrophobic core, a monolayer lipid molecular layer wrapping the hydrophobic core and a hydrophilic shell. The hydrophobic core comprises a hydrophobic polymer and a targeted delivery object loaded by the hydrophobic polymer, and the targeted delivery object includes at least one of a pregnancy drug and an ultrasound contrast agent. The hydrophilic shell is an amphiphilic macromolecular compound grafted with a polypeptide of targeted placenta-like chondroitin sulfate A, the hydrophobicend of the amphiphilic macromolecular compound is interspersed in the monolayer lipid molecular layer, and the hydrophilic end of the amphiphilic macromolecular compound is linked to the polypeptidethrough an amido bond. The polypeptide is exposed outside the monolayer lipid molecular layer, wherein the amino acid sequence of the polypeptide is selected from one or more of amino acid sequences shown in SEQ ID NO: 1-SEQ ID NO: 3. The invention further provides a preparation method of the placenta targeted delivery system and application of the placenta targeted delivery system to diagnosis and treatment of a pregnancy disorder caused by the placenta.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
Pharmaceutical composition containing sodium hyaluronate and chondroitin sulfate
The invention provides a pharmaceutical composition containing sodium hyaluronate with the relatively medium molecular weight and chondroitin sulfate with the relatively low molecular weight. The molecular weight of sodium hyaluronate is 200 kD-1,000 kD, the concentration of sodium hyaluronate is 1%-2%, the molecular weight of chondroitin sulfate is 5 kD-10 kD, and the concentration of chondroitin sulfate is 1.5%-3%. The pharmaceutical composition contains chondroitin sulfate with the relatively low molecular weight, has the better treatment effect than single sodium hyaluronate and can be used for treating refractory nonbacterial cystitis, Chronic recurrent bacterial cystitis, radiation-induced cystitis, interstitial cystitis (the chronic bladder pain syndrome) and neurogenic bladder urinary system infection.
Owner:成都金思唯生物技术有限公司
Preparation method of sea cucumber fucosan sulfate and chondroitin sulfate oligosaccharide
The invention provides a preparation, separation and purification method of sea cucumber fucosan sulfate and chondroitin sulfate oligosaccharide. The method comprises the following steps: taking sea cucumber sulfated polysaccharide extracted from sea cucumbers as a raw material, adjusting the pH value of sea cucumber sulfated polysaccharide solution to 5.0-6.0, heating and degrading the solution with 110-115 DEG C high pressure steam for 60-90 min to enable fucosan sulfate to be degraded into oligosaccharide and performing ultrafiltration separation to obtain fucosan sulfate oligosaccharide; adjusting the pH value of ultrafiltration trapped fluid with the molecular weight of more than 5 kDa to 3.0-4.0, performing thermal degradation with 120-130 DEG C high pressure steam for 90-120 min andperforming concentration and freeze drying, so as to obtain sea cucumber fucosyl chondroitin sulfate oligosaccharide. According to the method, the steps of ion chromatographic separation, gel chromatographic purification, desalination and the like of fucosan sulfate and chondroitin sulfate in the sea cucumbers are saved, and the degradation, separation and purification of sea cucumber fucosan sulfate and chondroitin sulfate can be further completed, so that the preparation process is greatly simplified.
Owner:OCEAN UNIV OF CHINA
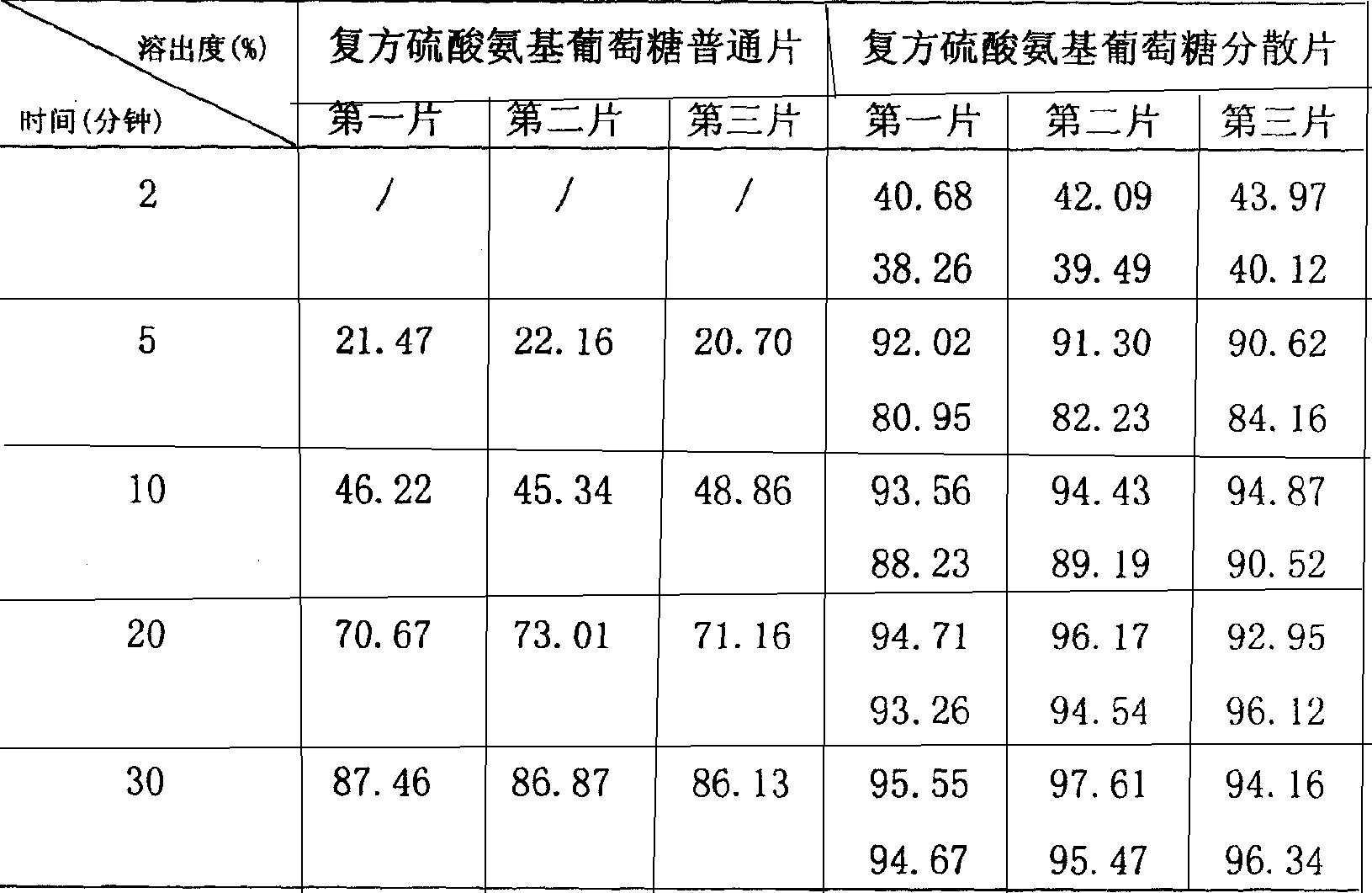
Compound glucosamine sulfate dispersible tablet formulation and its preparation method
ActiveCN1762379AMolding reductionGuaranteed moldingOrganic active ingredientsSkeletal disorderCross-linkAlcohol
The invention relates to the field of pharmacy, particularly a compound glucosamine sulfate dispersible tablet preparation, wherein the main compositions include glucosamine sulfate and chondroitin sulfate, the auxiliary materials include excipient (e.g. starch and microcrystalline cellulose), disintegrating agent (e.g. cross-linked polyplasdone), lubricant (e.g. magnesium stearate) and humectant (e.g. anhydrous alcohol).
Owner:GUANGZHOU YIPINHONG PHARMA +4
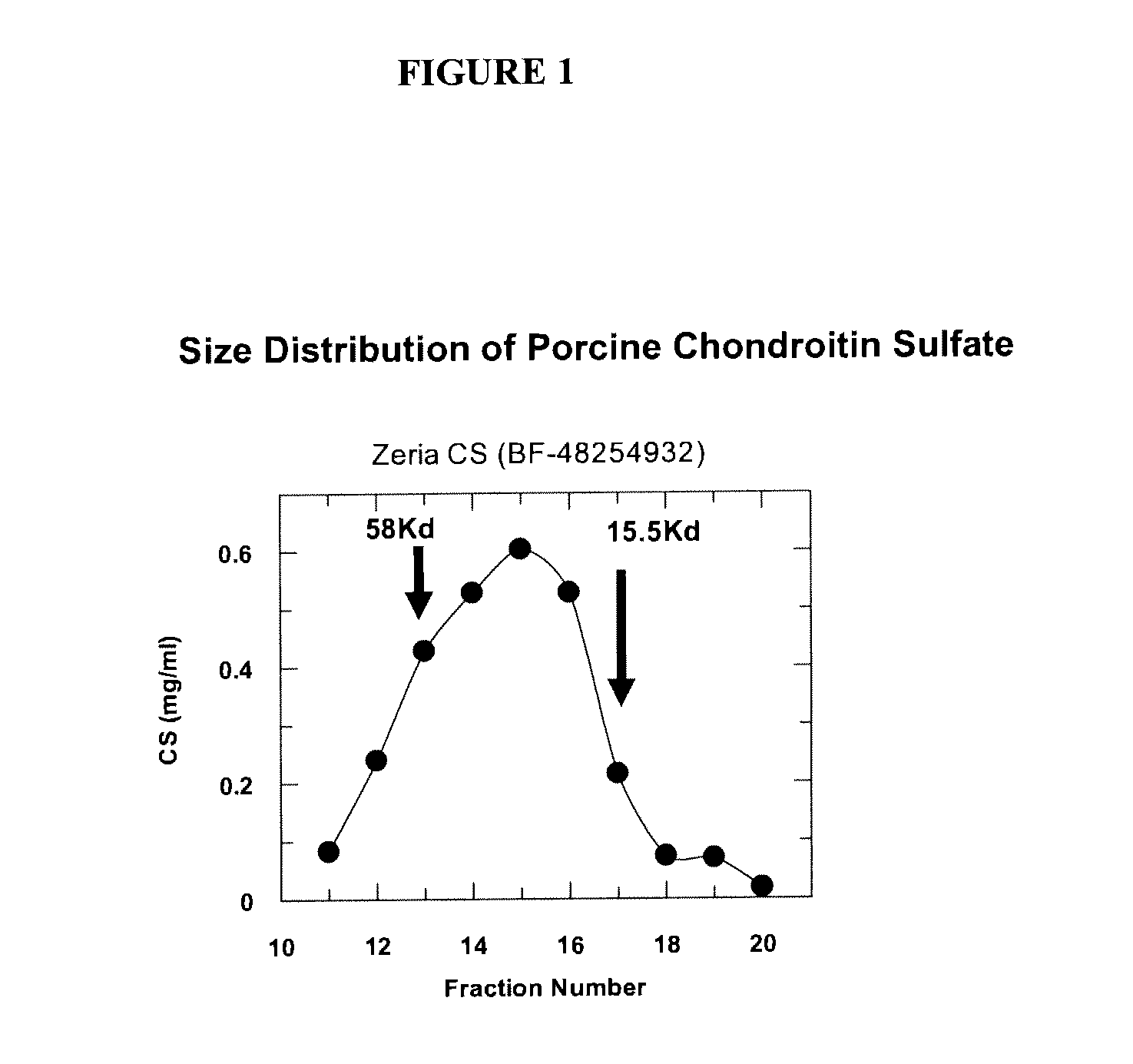
Method for preparing high-purity chondroitin sulfate A from rabbit ear cartilage
The invention relates to a technology for preparing high-purity chondroitin sulfate from animal organ, and particularly relates to a method for preparing high-purity chondroitin sulfate A from rabbit ear cartilage. The method comprises the following steps: skinning and fleshing the rabbit ear to obtain fresh rabbit ear cartilage; performing degreasing, trypsin enzymolysis, ethanol precipitation and anion exchange resin purification to obtain high-purity chondroitin sulfate A with molecular weight of 16-59kDa. The method provided by the invention is used for preparing chondroitin sulfate A from rabbit ear cartilage, has the characteristics of abundant sources of raw material, high product yield and high purity, and can meet the need for high-purity chondroitin sulfate A in the medical market.
Owner:OCEAN UNIV OF CHINA
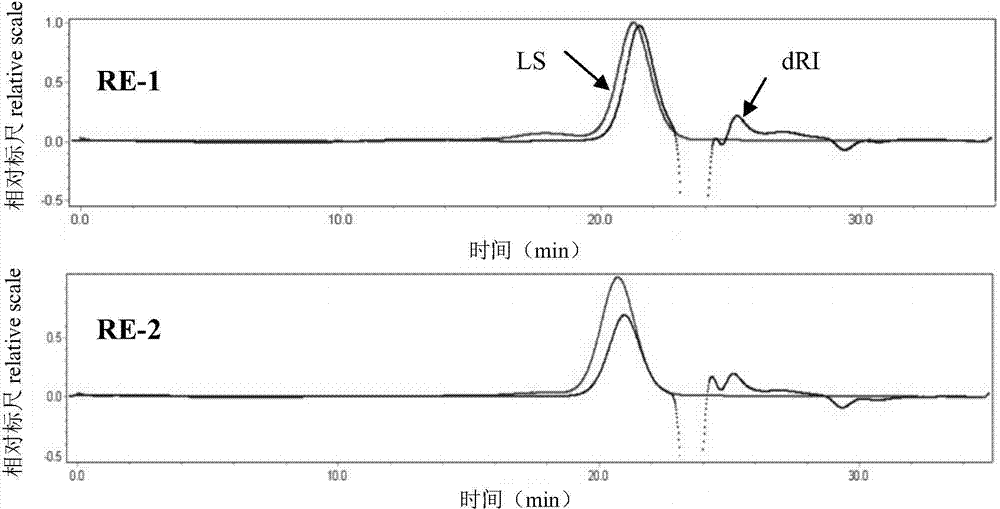
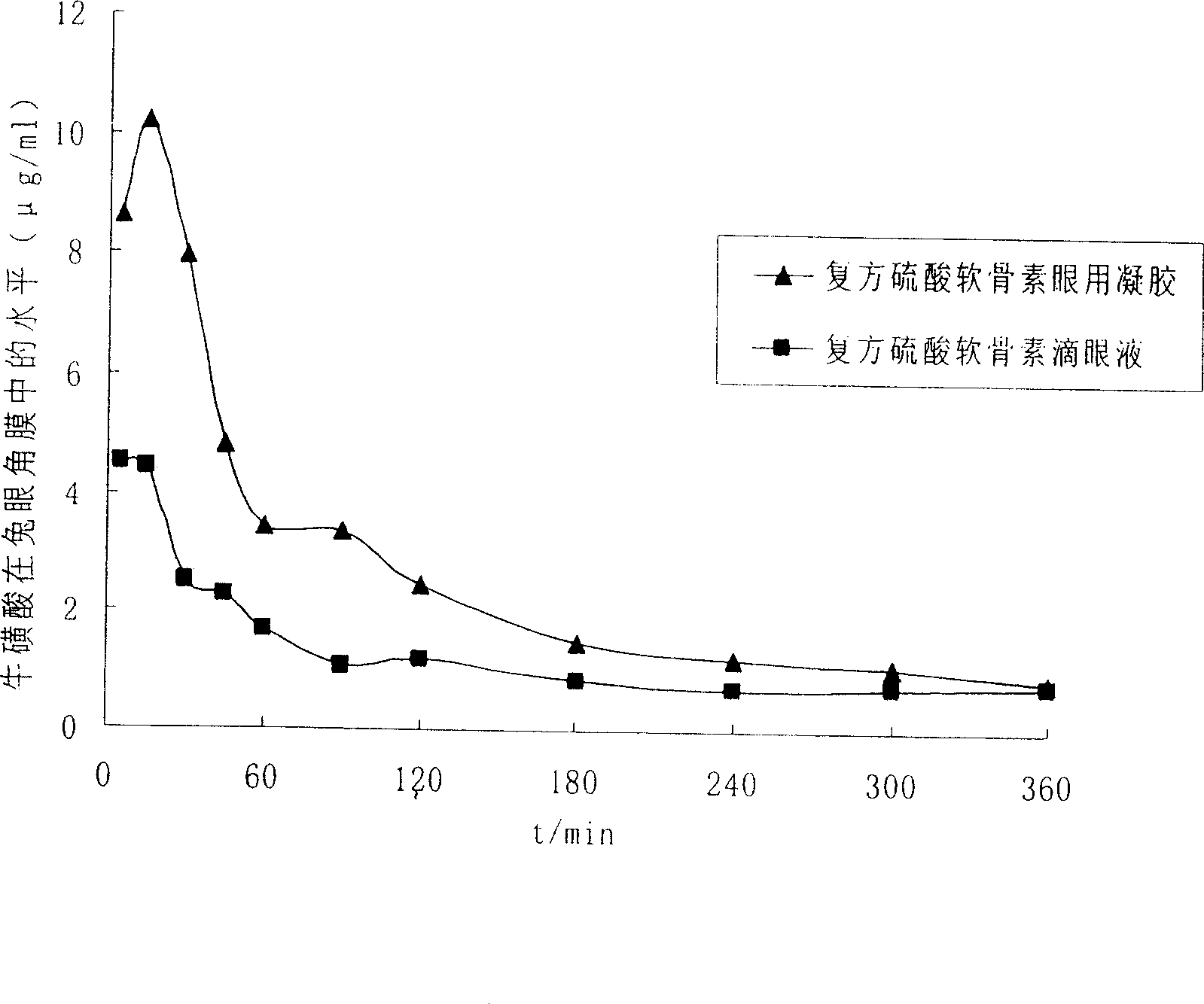
Ophthalmic gel containing chondroitin sulfate and method for preparing same
ActiveCN101543509AGood lookingSmooth appearanceSenses disorderPharmaceutical delivery mechanismIrritationBiocompatibility Testing
The invention relates to an ophthalmic gel containing chondroitin sulfate and a method for preparing the same. The ophthalmic gel comprises chondroitin sulfate and taurine in effective dose and an aqueous gel substrate in proper amount. Compared with eyedrop, the ophthalmic gel has the advantages of good biocompatibility, small stimulation, simple preparation process and the like.
Owner:SHENYANG XINGQI PHARM CO LTD
Microbial-derived chondroitin sulfate
Described is chondroitin sulfate obtained from microbial sources, and related compositions and methods.
Owner:AMANO ENZYME USA CO LTD +1
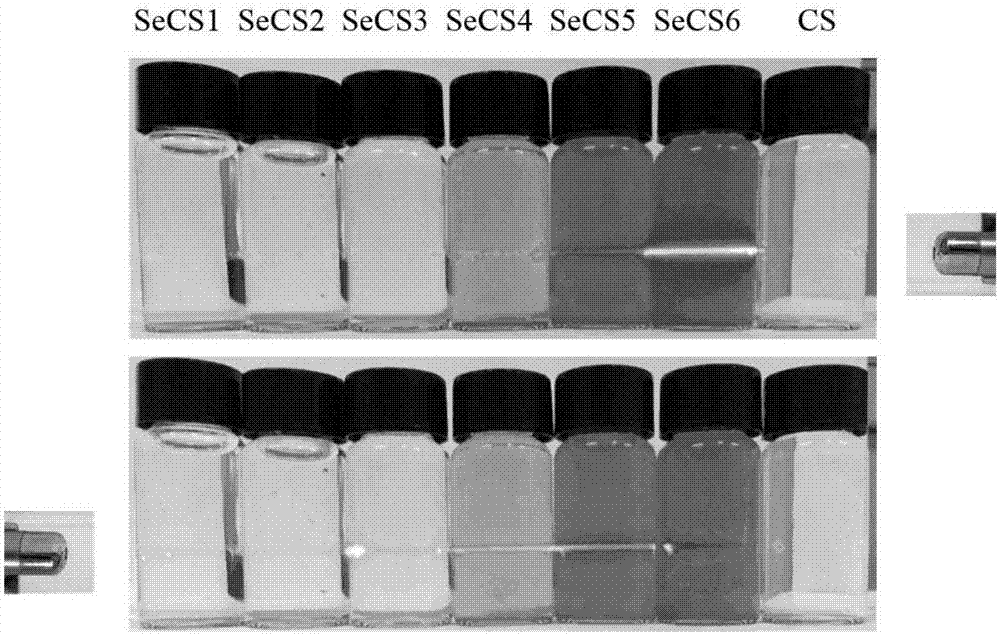
Chondroitin sulfate A nano-selenium micelle particle, and preparation method and application thereof
ActiveCN107260668AImprove solubilityReduce biological activityPowder deliveryOrganic active ingredientsBioavailabilityInorganic selenium
The invention discloses a chondroitin sulfate A nano-selenium micelle particle, and a preparation method and application thereof, which belong to the technical field of medical nanometer materials. The preparation method comprises the steps of adopting chondroitin sulfate A as a carrier, combining nano-selenium on the chondroitin sulfate A through physical adsorption so as to form the nano-selenium micelle particle, wherein the mass fraction of selenium is 0.1 percent to 25 percent. The chondroitin sulfate A nano-selenium micelle provided by the invention combines dual pharmacological activity of the chondroitin sulfate A and the nano-selenium, so that the toxicity of inorganic selenium is reduced, and the bioavailability is improved; meanwhile, by combining the biological activity of the chondroitin sulfate A, the chondroitin sulfate A nano-selenium micelle particle can be better developed to be a novel nanomicelle preparation for preventing and improving osteoarthrosis.
Owner:XI AN JIAOTONG UNIV
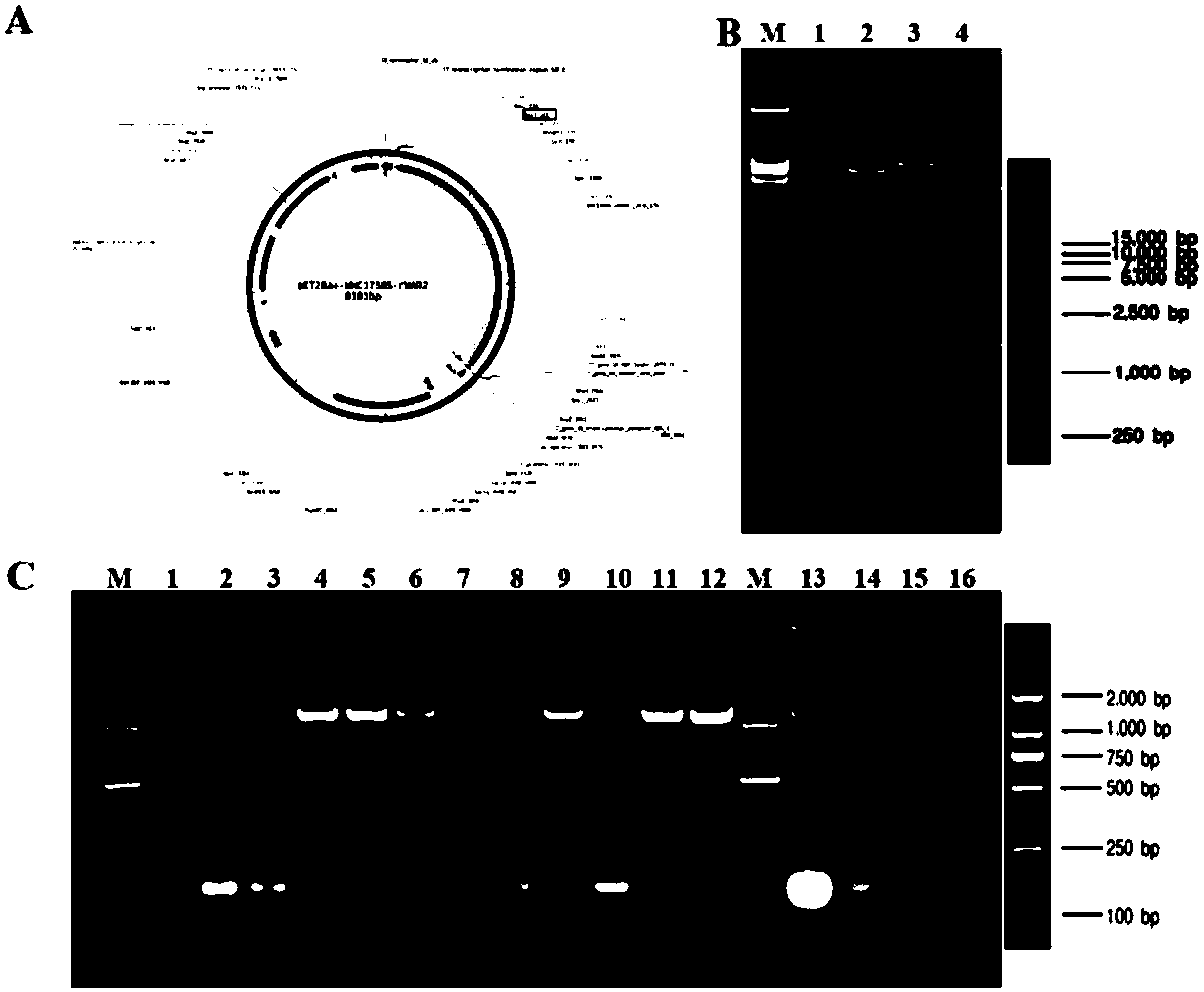
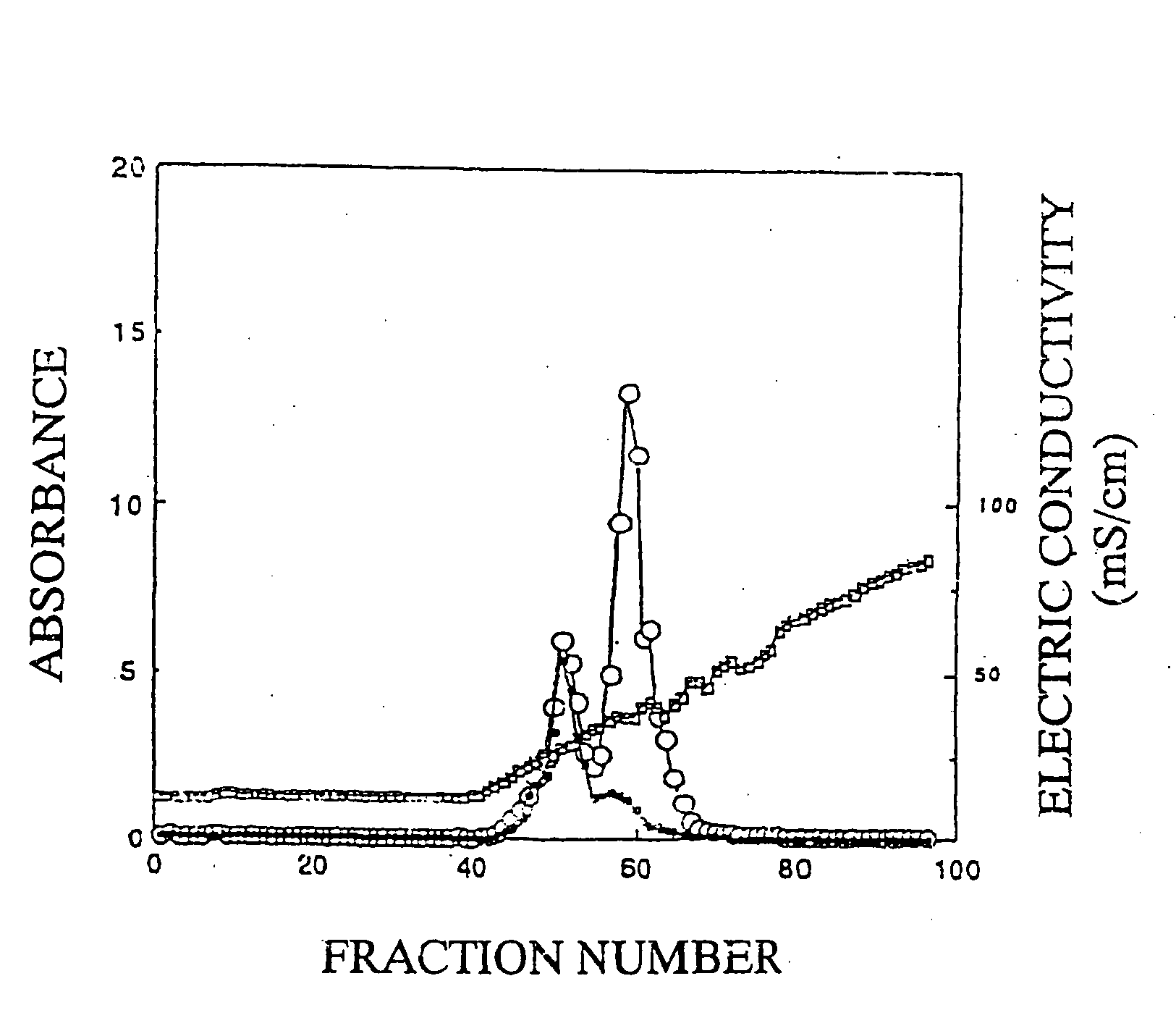
Affinity chromatography purification method of placenta sample chondroitin sulfate A or derivative thereof
ActiveCN108570118AOvercoming Difficulties in PurificationHigh recovery rateAgainst vector-borne diseasesAntigenPurification methods
The invention relates to an affinity chromatography purification method of placenta sample chondroitin sulfate A or a derivative thereof, and particularly discloses a purification method of the placenta sample chondroitin sulfate A or the derivative thereof. The placenta sample chondroitin sulfate A or the derivative thereof are chromatographically purified by the affinity chromatography method, and an affinity chromatography column is a conjugate of a recombinant plasmodium infected red cell surface antigen protein and an affinity chromatography substrate. According to the chromatography purification method, crude products of the placenta sample chondroitin sulfate A or the derivative thereof are loaded onto the affinity chromatography column and washed by washing liquid until no impurities flow out, and then the crude products are eluted by eluent, and pure products of the placenta sample chondroitin sulfate A or the derivative thereof are collected. The purification method can solvethe problem of difficulty in polysaccharide purification, other polysaccharides with similar molecular sizes and structures cannot be adsorbed by rVAR2 and can be easily washed and removed to obtainhigh-purity pl-CSA, and the washing liquid pl-CSA is firmly combined by an rVAR2 ligand and easily released to obtain high recovery rate.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
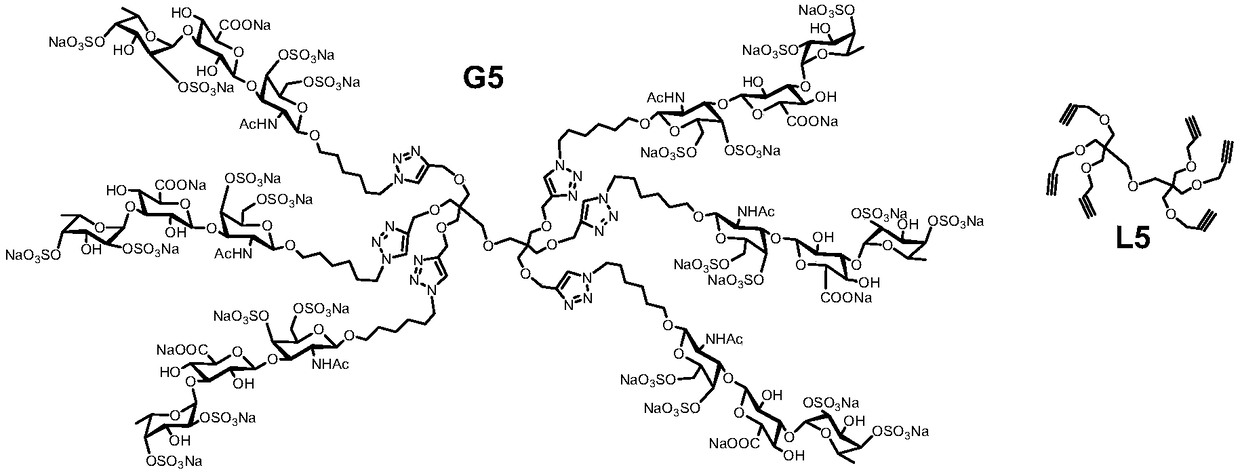
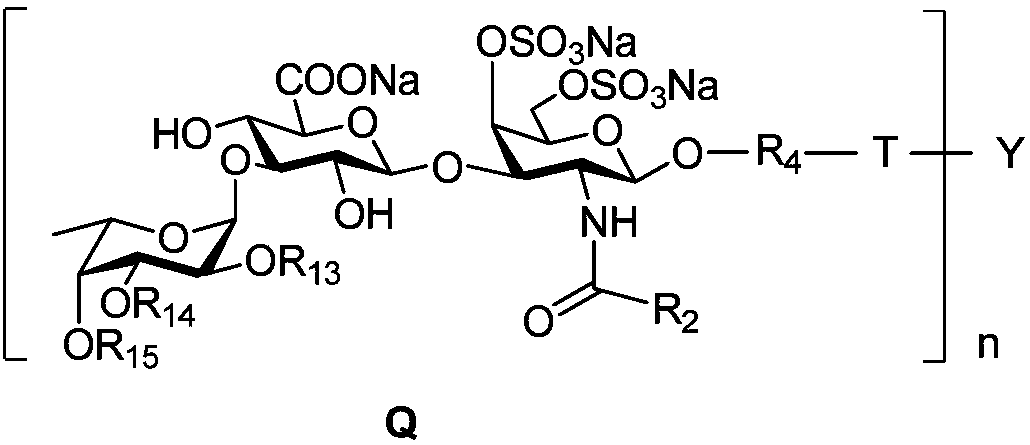
Fucosylated chondroitin sulfate oligosaccharide glycocluster and preparation method thereof
The invention discloses a fucosylated chondroitin sulfate oligosaccharide glycocluster with a structure show in general formula Q in the description. Definitions of substituent groups are detailed inthe description. The glycocluster compound is synthesized from chondroitin sulfate A salt as a raw material by acidolysis, protecting group operation and glycosylation in sequence on the basis of molecules of multifunctional groups as molecular skeletons. The glycocluster can simulate activity of natural glycosaminoglycan based on glycocluster effect, and can be applied to the medical field due tostructural certainty.
Owner:YANTAI DONGCHENG PHARMA GRP
Placental chondroitin sulfate A based cancer screening and early stage diagnosis reagent and method
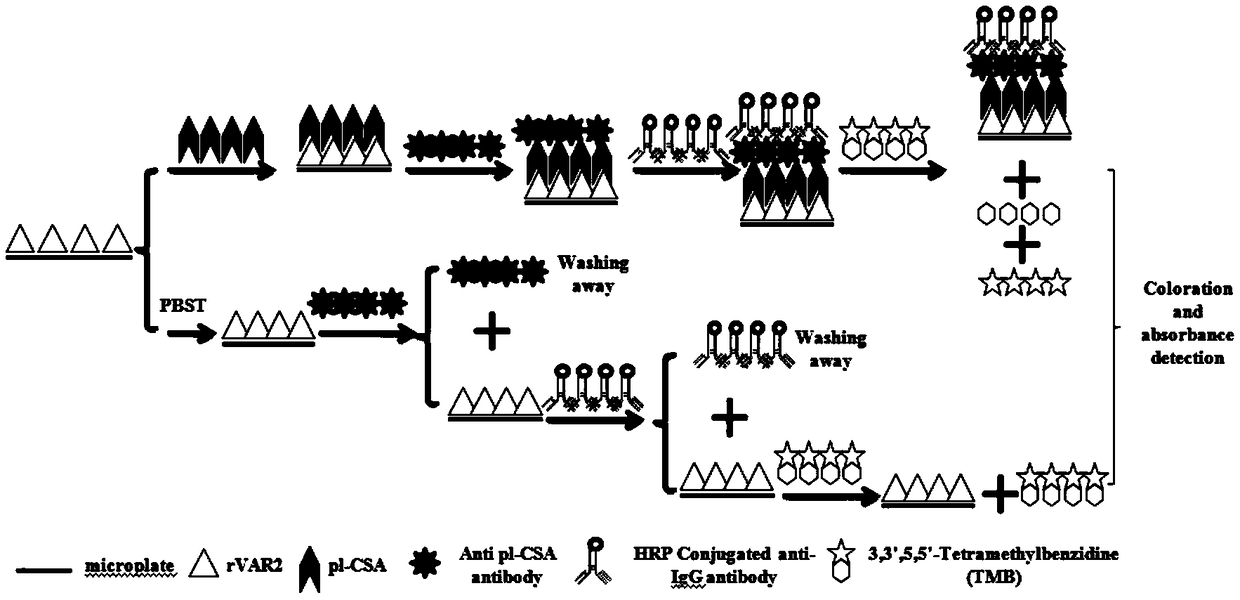
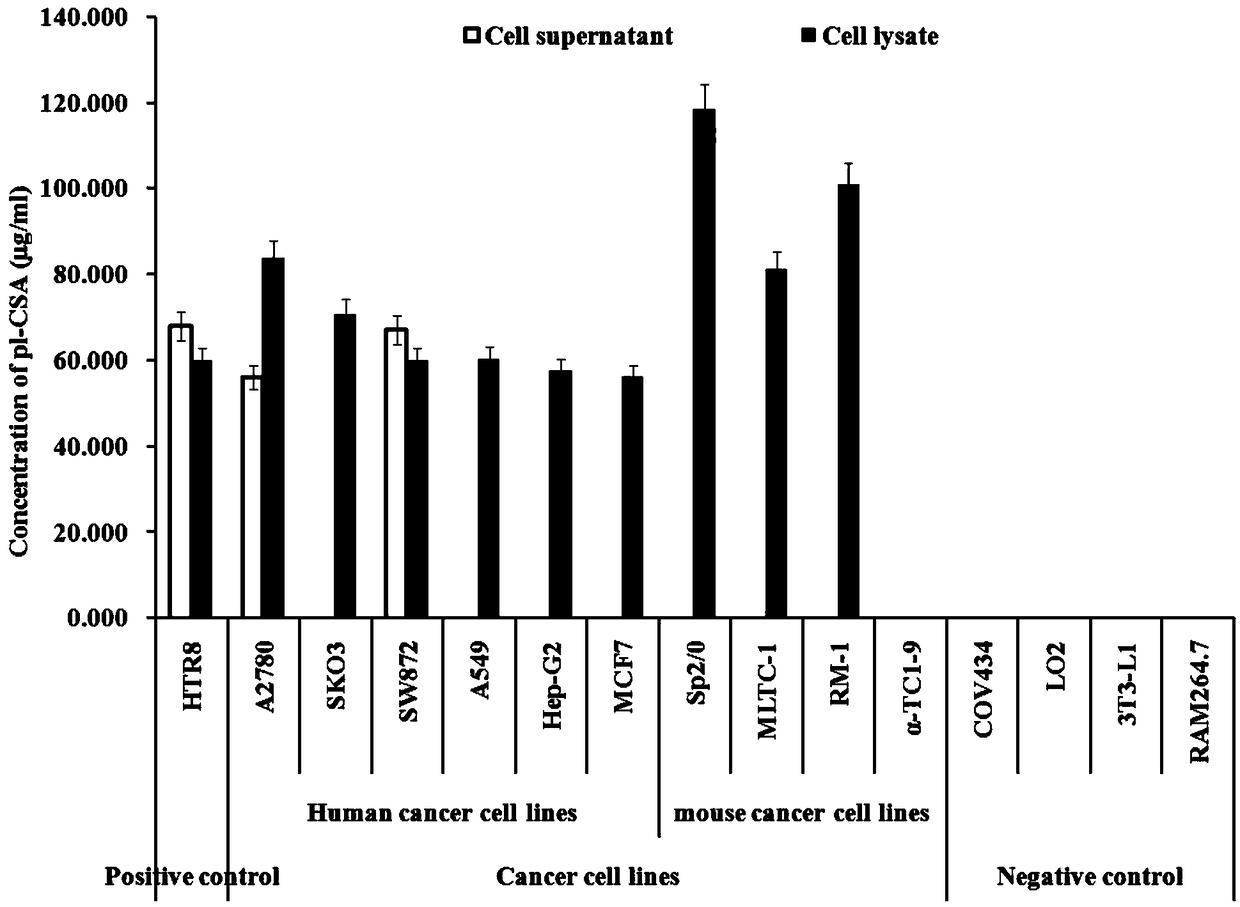
ActiveCN109387627ASimple methodLow detection limitMaterial analysisAgainst vector-borne diseasesAntigenElisa kit
The invention relates to a placental chondroitin sulfate A based cancer screening and early stage diagnosis reagent, and a method, and more specifically discloses an ELISA kit used for tumor detection. The ELISA kit comprises a capturing protein combined with placental chondroitin sulfate A (pl-CSA), and the capturing protein is preferably selected from the minimum bonding peptide segments of plasmodium infected erythrocyte surface antigen (VAR2CSA, rVAR2), and more preferably, the sequence of the minimum bonding peptide segment is represented by SEQ ID No.1. It is found for the first time that pl-CSA can be detected in a plurality of kinds of body fluid, so that basis is provided for in vitro qualitative or quantitative biological detection of pl-CSA. The method is low in detection limit,excellent in repeatability, and high in specificity.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
Pharmaceutical formulations comprising chondroitin sulfate and hyaluronic acid derivatives
ActiveUS20150072954A1Overcomes drawbackOrganic active ingredientsBiocideInterstitial cystitisTendinosis
The present invention relates to pharmaceutical formulations containing a combination of specific high-molecular weight hyaluronic acid derivatives and chondroitin sulfate to be used in the treatment of osteoarthritis, of subchondral damage, osteoporosis, synovitis, tenosynovitis, tendinitis, tendinosis, as an intra-articular washing liquid and as a viscous substitute of synovial fluid following osteochondral surgery. These formulations are also suitable for the treatment of interstitial cystitis.
Owner:FIDIA FARM SPA
Method of extensive culture of antigen-specific cytotoxic T cells
InactiveUS20080166325A1Improve securitySsRNA viruses negative-senseBiocideAdditive ingredientOligosaccharide
The present invention provides methods for inducing, maintaining and expanding CTL (cytotoxic T cell) having an antigen-specific cytotoxic activity at a high level, which is useful in the adoptive immunotherapy, by using as an effective ingredient at least one compound selected from the group consisting of acidic polysaccharides, acidic oligosaccharides, acidic monosaccharides, and salts thereof. The above-mentioned compounds include fucoidans, heparins, alginic acid, chondroitin sulfate A, chondroitin sulfate B, pectic acid, hyaluronic acid, degradation products of fucoidans, sulfated glucose, sulfated fucose and salts thereof.
Owner:SAGAWA HIROAKI +2
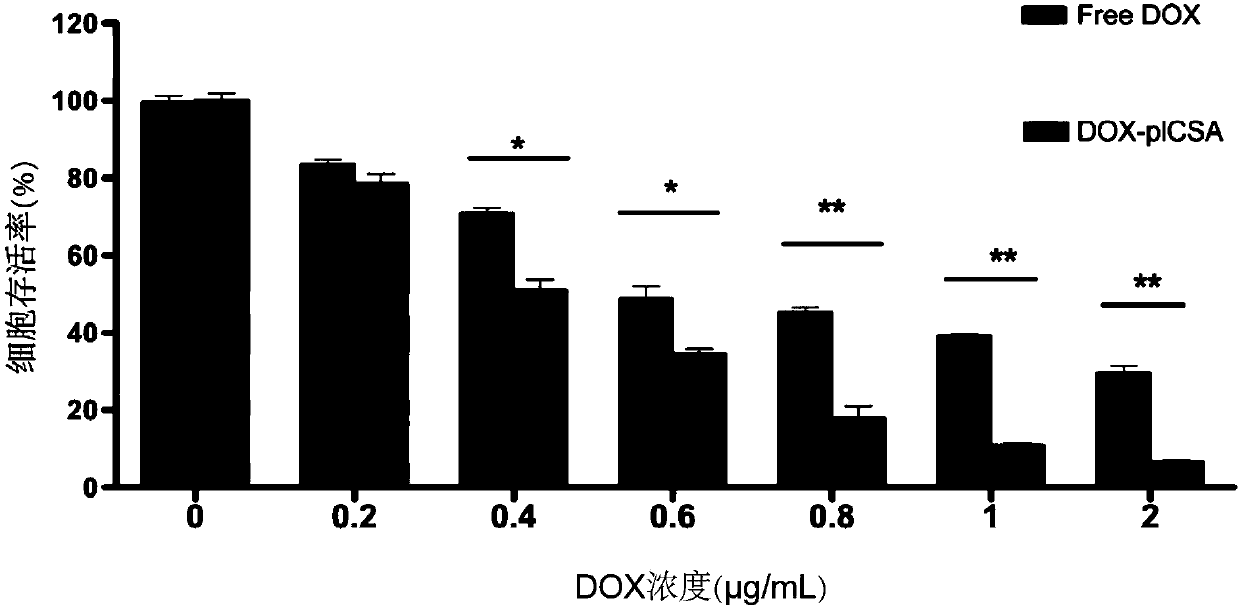
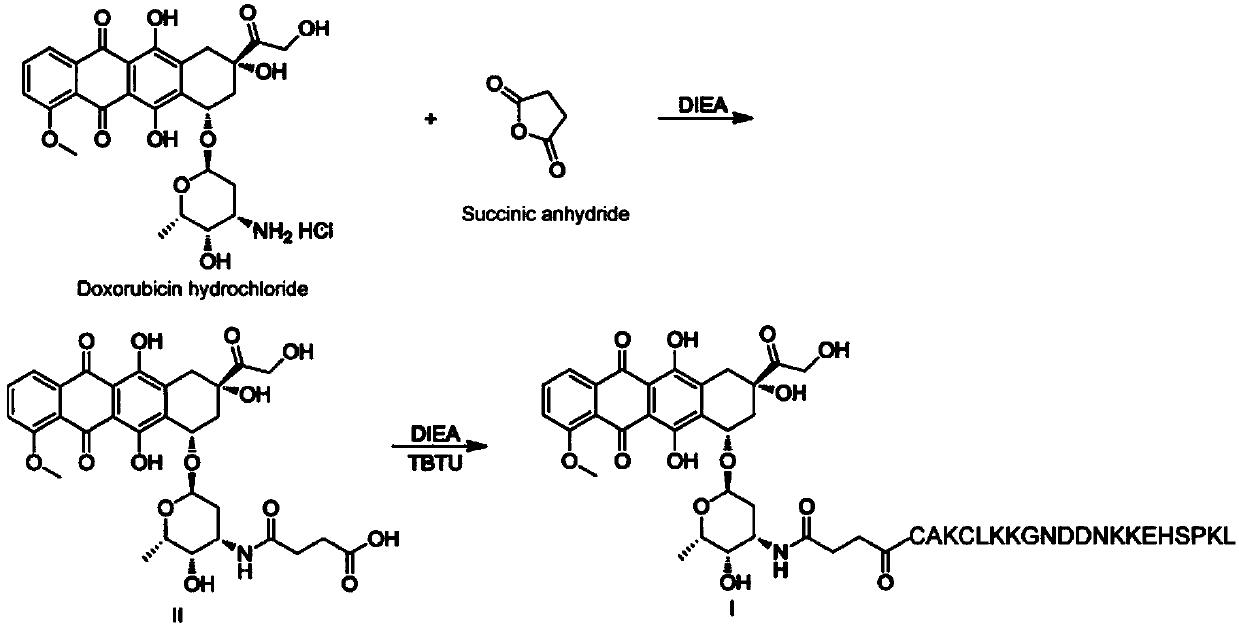
Peptide-drug conjugate of target placenta-like chondroitin sulfate A, and preparation method and application of peptide-drug conjugate
ActiveCN109568597AImprove stabilityImprove utilizationOrganic active ingredientsGeneral/multifunctional contrast agentsSide effectNormal tissue
The invention provides peptide-drug conjugate of target placenta-like chondroitin sulfate A. The peptide-drug conjugate comprises a small molecule drug part, a peptide part and a connecting sub-part connected with the small molecule drug part and the peptide part, wherein peptide corresponding to the peptide part can target the placenta-like chondroitin sulfate A specifically, and the amino acid sequence of the peptide is selected from one or more of amino acid sequences shown as SEQ ID NO:1 to SEQ ID NO:3. The peptide-drug conjugate can target inappropriately-expressed target tissue of the placenta-like chondroitin sulfate A specifically, a drug is controlled to be released near the target tissue, and the toxic and side effects of the drug on normal tissue are reduced. The invention further provides a preparation method and application of the peptide-drug conjugate.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
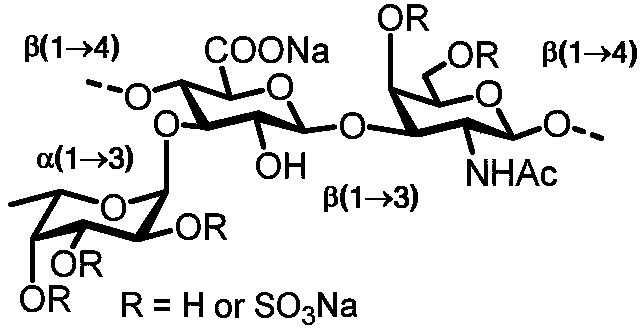
Fucosylation chondroitin sulfate oligosaccharide and preparation method, compound and application thereof
ActiveCN110724209AHigh anticoagulant activityProlonged thrombin timeEsterified saccharide compoundsOrganic active ingredientsFucosylationChemical compound
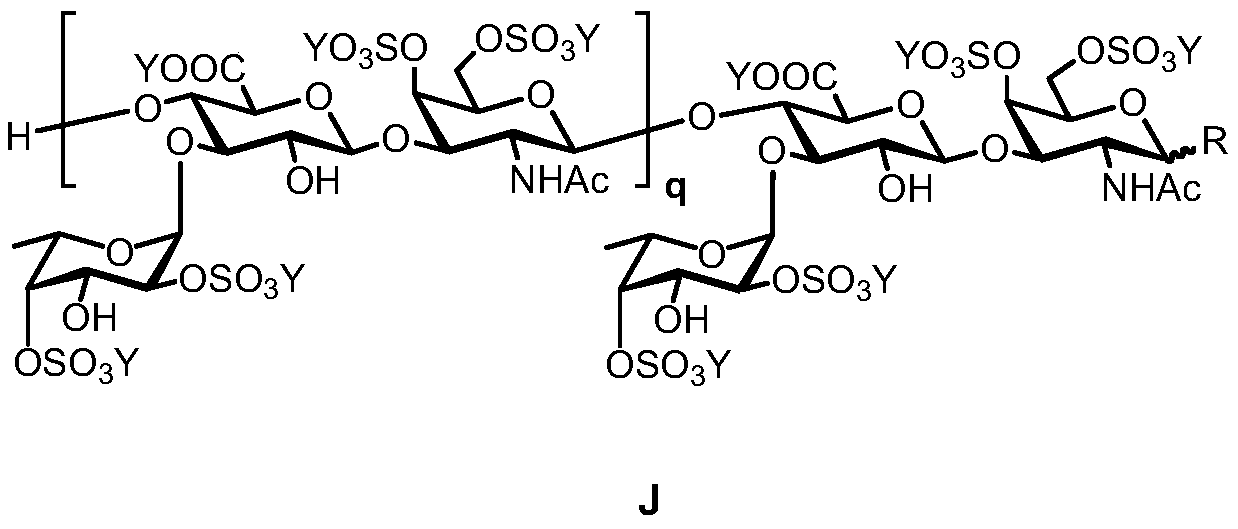
The invention discloses fucosylation chondroitin sulfate oligosaccharide provided with a structure shown in J. The definition of a substituent group is shown in the description in detail. Besides, theinvention further discloses a preparation method of the fucosylation chondroitin sulfate oligosaccharide. According to the preparation method, chondroitin sulfate A salt is used as a raw material andis subjected to enzymolysis, protecting group operation and glycosylation sequentially, the oligosaccharide compound is synthesized, and the certainty of the structure enables the compound to be applied to the medicine field.
Owner:YANTAI DONGCHENG PHARMA GRP
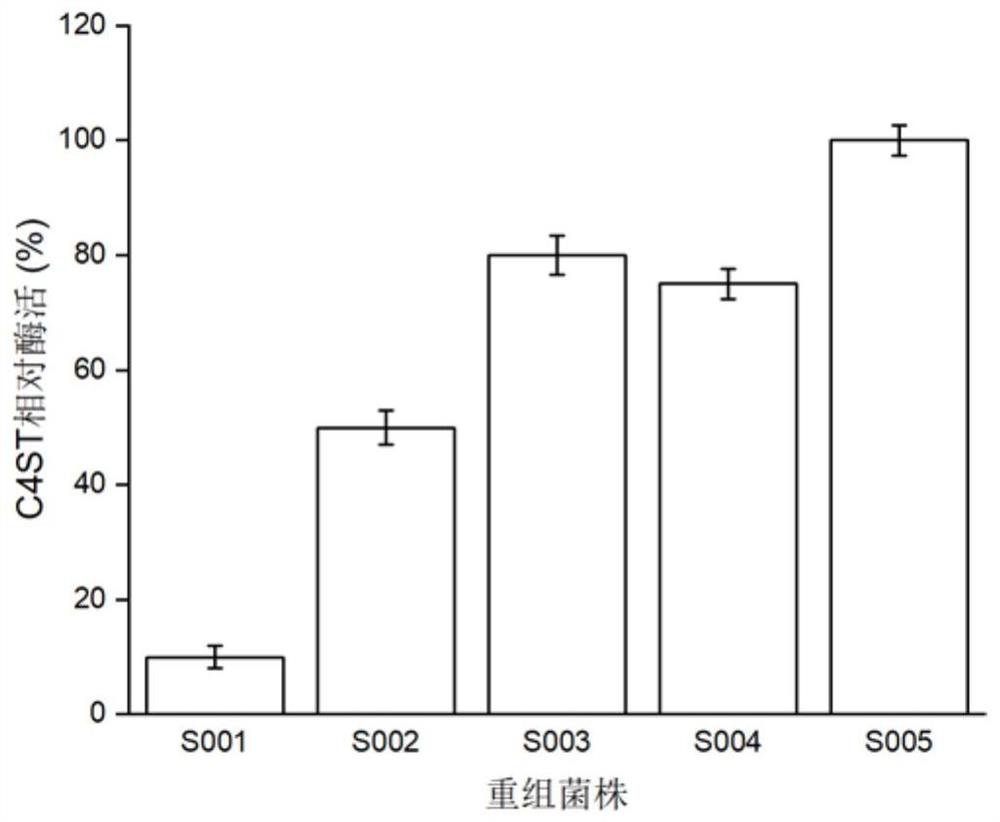
Recombinant strains expressing chondroitin 4-sulfotransferase gene and application of recombinant strain
ActiveCN109897812AIncrease valueBacteriaMicroorganism based processesEscherichia coliBacillus subtilis
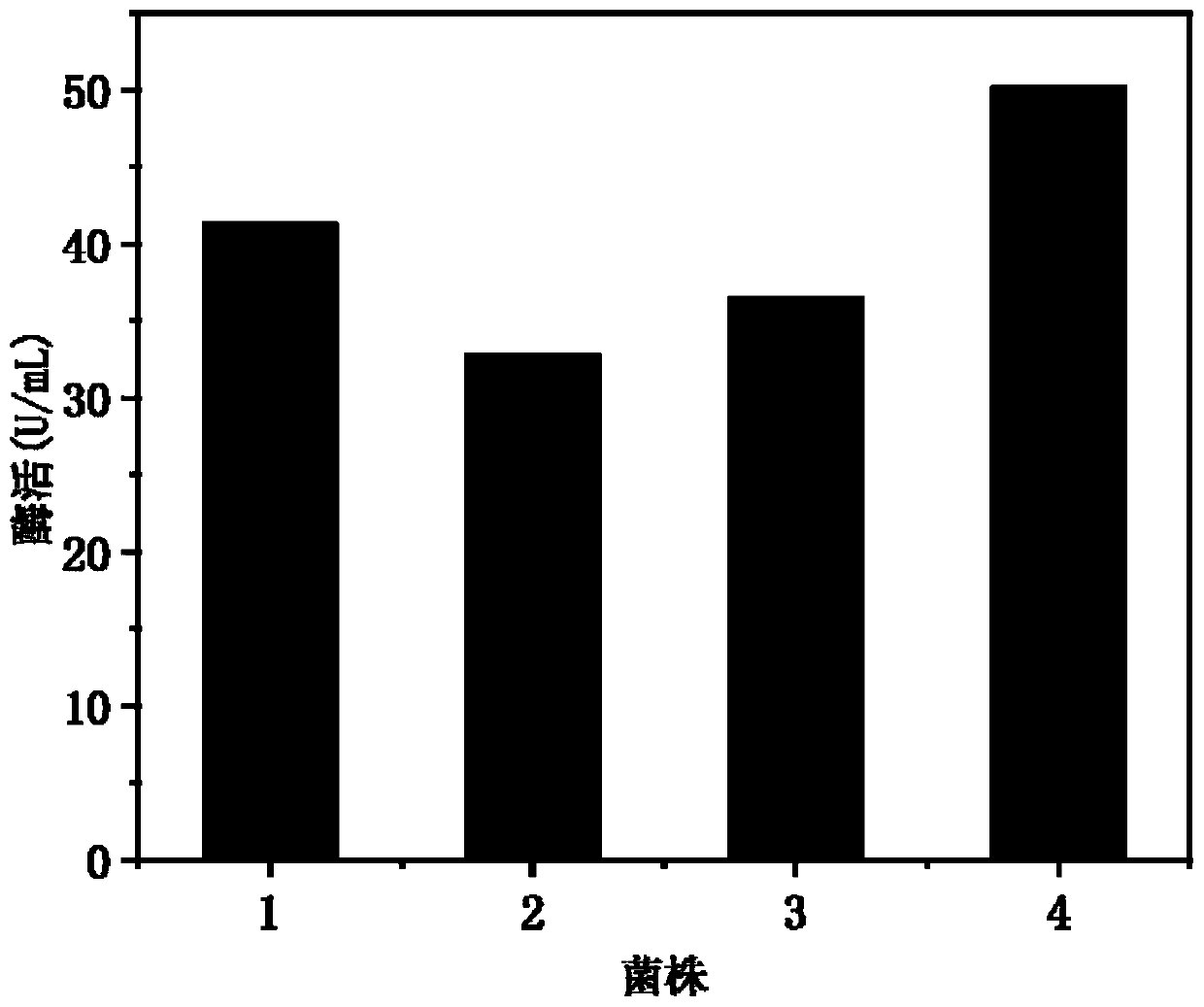
The invention discloses recombinant strains expressing a chondroitin 4-sulfotransferase gene and an applicationof the recombinant strain, and relates to the technical field of bioengineering. Chondroitin 4-sulfotransferase gene with the amino acid sequence shown in SEQ ID NO.1 is expressed with Escherichia coli or bacillus subtilis as a host, four recombinant strains are constituted, the high-efficiency expression of chondroitin 4-sulforansferase gene is realized, and final enzyme activity respectively reaches 41.3 U / mL, 32.8 U / mL, 36.5 U / mL and 50.2 U / mL. By using the enzyme as a catalyst, chondroitin sulfate A is catalyzed and synthesis in one step with chondroitin as a substrate, the final conversion efficiency reaches 80%. The method has potential and quite wide values in industrial synthesis of chondroitin sulfate A.
Owner:JIANGNAN UNIV +1
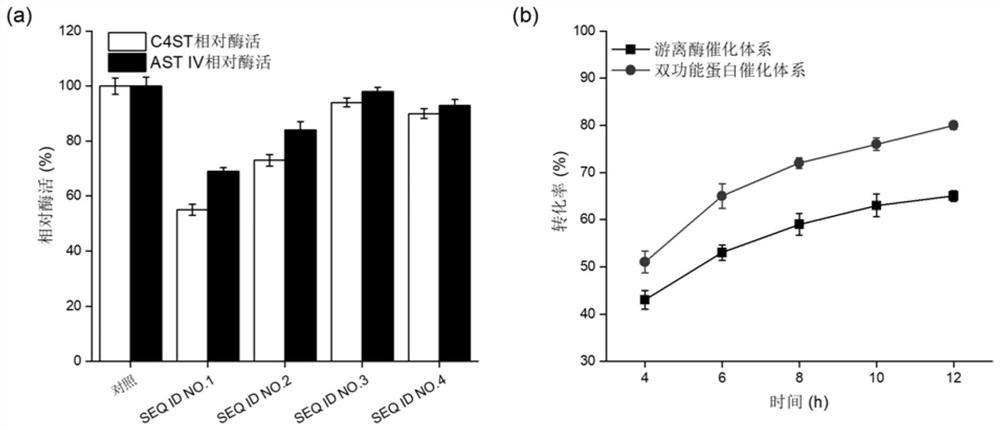
Method for efficiently producing chondroitin sulfate A by artificial enzymatic method
ActiveCN111621533AUniform structureNo potential pathogenic factorFungiAntibody mimetics/scaffoldsHeterologousArylsulfotransferase
The present invention discloses a method for efficiently producing chondroitin sulfate A by an artificial enzymatic method and belongs to the technical field of bioengineering. In the method, a bifunctional protein chondroitin 4-O-sulfotransferase C4ST and an arylsulfotransferase AST IV are heterologously expressed by microorganisms, and are used to catalyze synthesis of the chondroitin sulfate Afrom chondroitin, and site-directed mutation and optimization of reaction system components are combined to accelerate synthesis efficiency of the chondroitin sulfate.
Owner:JIANGNAN UNIV
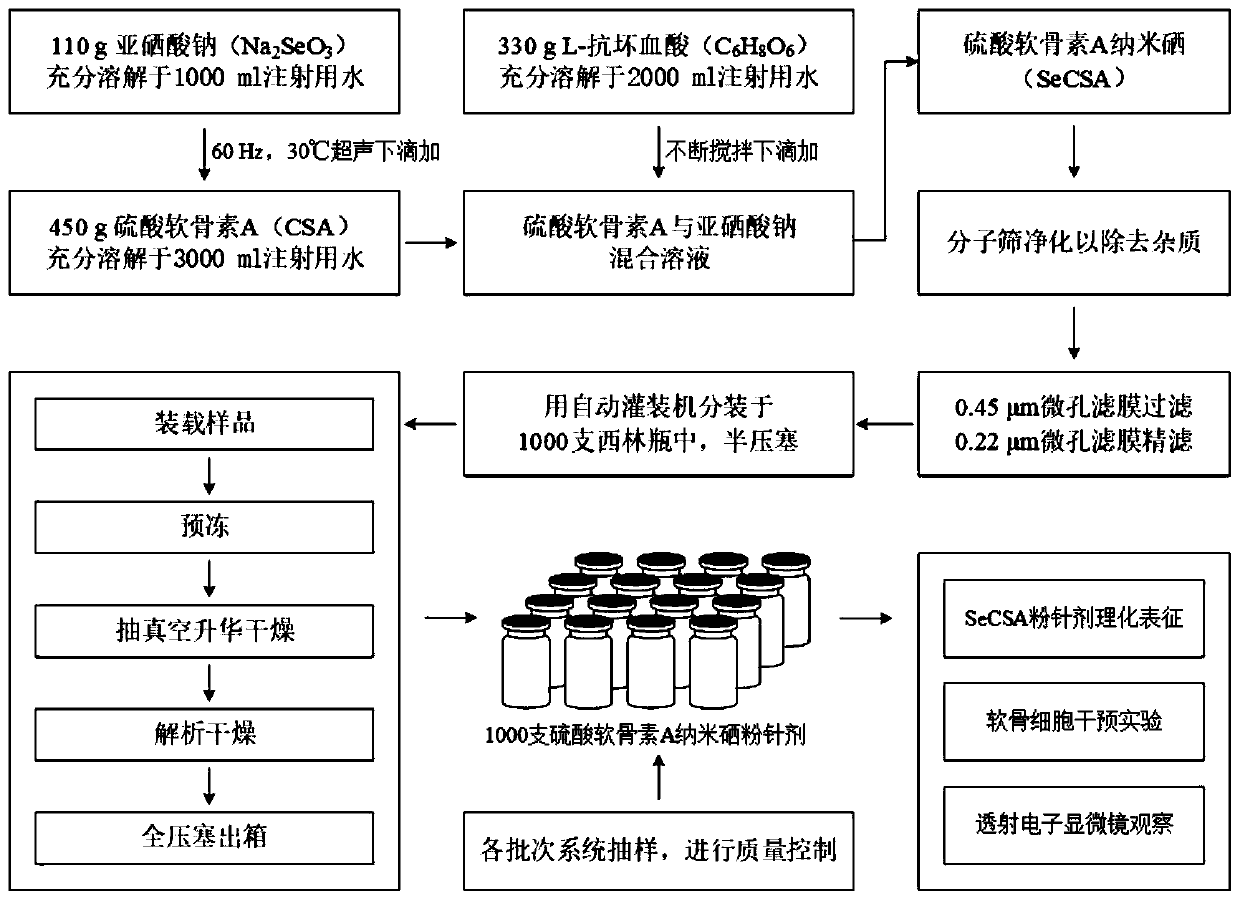
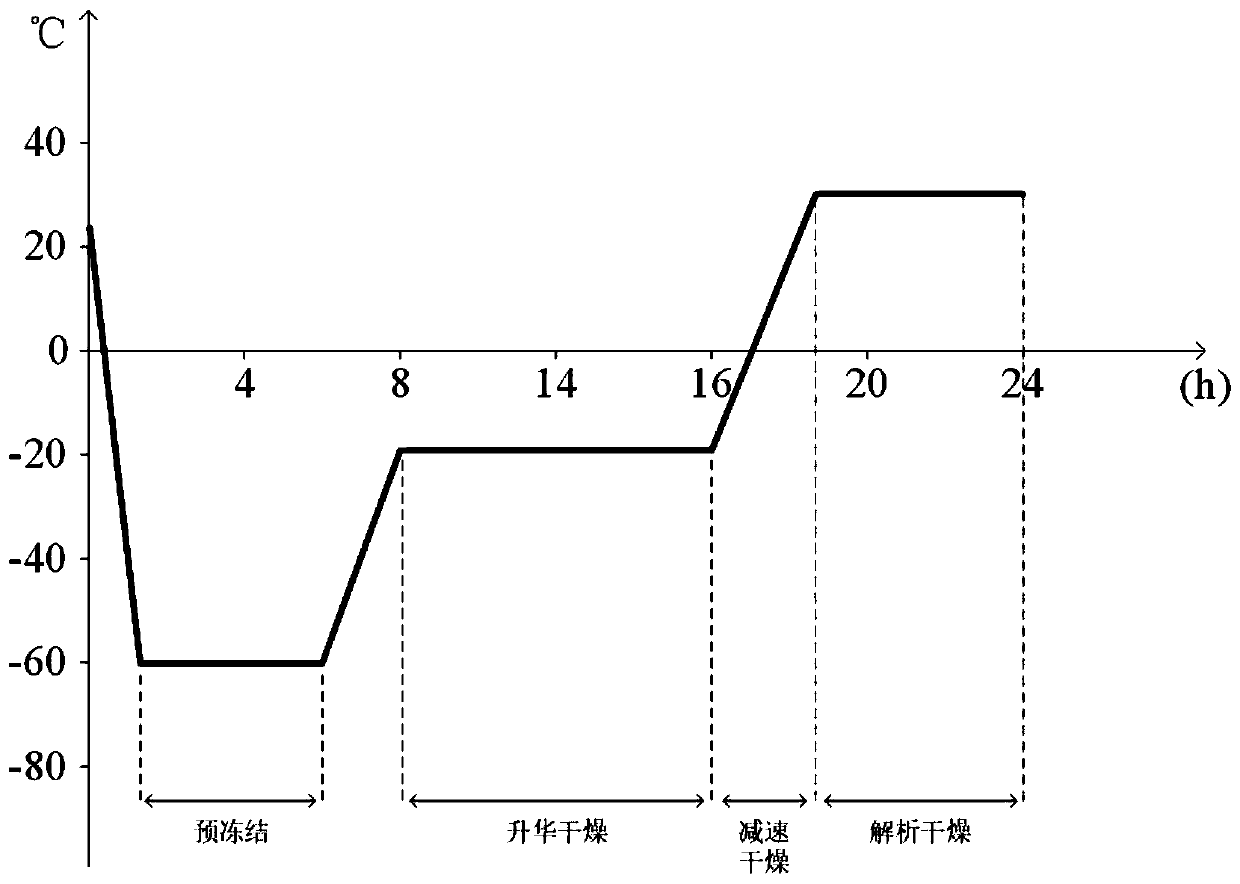
Preparation technology of chondroitin sulfate A nano selenium freeze-dried powder injection
InactiveCN110420185ASimple preparation processStable and controllable qualityPowder deliveryOrganic active ingredientsPenicillinFreeze-drying
The invention discloses a preparation technology of a chondroitin sulfate A nano selenium freeze-dried powder injection, and belongs to the technical field of preparation of biomedical preparations. According to the adopted scheme, chondroitin sulfate A serves as a carrier to carry nano selenium particles obtained by reduction of sodium selenite and L-ascorbic acid for reaction in water so as to generate chondroitin sulfate A nano selenium liquid; unreacted substances in the prepared chondroitin sulfate A nano selenium liquid are removed through a molecular sieve, then filtering is conducted to remove bacteria and impurities, after physical characteristics are checked, the chondroitin sulfate A nano selenium liquid is split-charged into penicillin bottles, half plug-compressing is conducted, then vacuum freeze-drying treatment is conducted, plug-compressing is conducted for compressing aluminum caps, and the chondroitin sulfate A nano selenium freeze-dried powder injection is prepared,and stored in a frozen mode for standby application. According to the preparation technology, the process is simple, the quality is stable and controllable, physicochemical properties of prepared products are stable, and the preparation technology is suitable for industrial scale production.
Owner:XI AN JIAOTONG UNIV +1
Technology for shortening extracting time of chondroitin sulfate
A technology for shortening the extracting time of chondroitin sulfate comprises the following steps: (1) cartilage pretreatment; (2) dual enzymolysis: regulating pH (Potential of Hydrogen) value to be 8.8 to 9.3, adding alkaline protease 2709, stirring at a stirring speed of 20 to 60 rpm, and carrying out enzymolysis; regulating the pH value to be 8.4 to 8.8, adding activated porcine pancreas, stirring at the stirring speed of 20 to 60 rpm, and carrying out enzymolysis; (3) deproteinization: heating to 70 to 80 DEG C, stirring at the stirring speed of 90 to 110 rpm, regulating pH to an isoelectric point, treating for 10 to 30 minutes, stopping stirring, and standing; (4) filter pressing; (5) disk type centrifuging; (6) ion exchange; (7) eluting; (8) refining of the chondroitin sulfate. According to the technology disclosed by the invention, on the technological basis that the chondroitin sulfate is extracted through a dual enzymolysis method in the prior art and by improving a technological path and technological parameters and adopting a proper stirring speed, the enzymolysis speed is increased, and the enzymolysis time is shortened, so that the extracting time is shortened, andthe yield is increased.
Owner:HUNAN WUXING BIOLOGICAL TECH CO LTD
Attenuation of tumor growth, metastasis and angiogenesis
InactiveUS6979563B1Reduce capacityInhibition formationPeptide/protein ingredientsEnzymesDiseaseAbnormal tissue growth
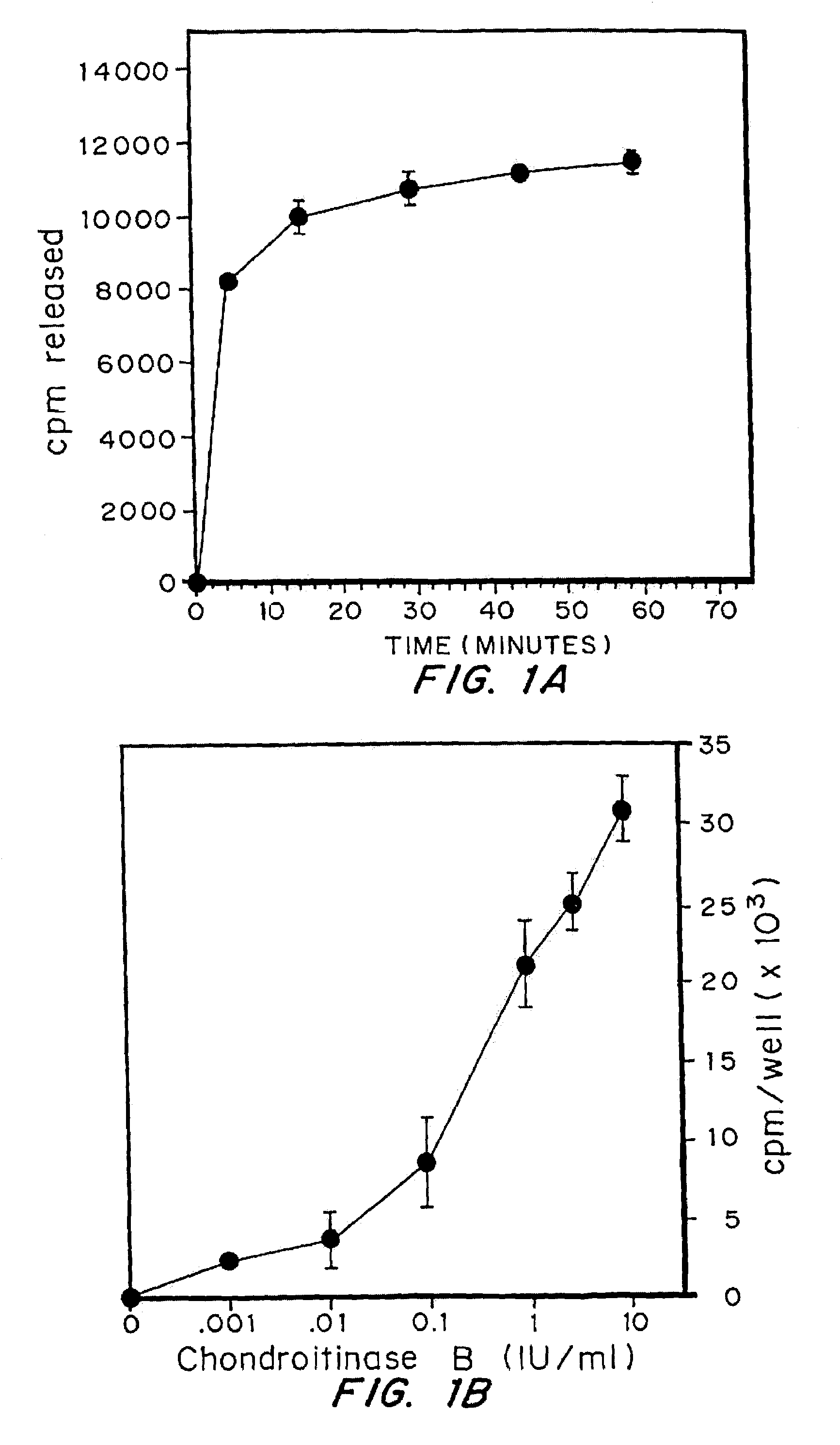
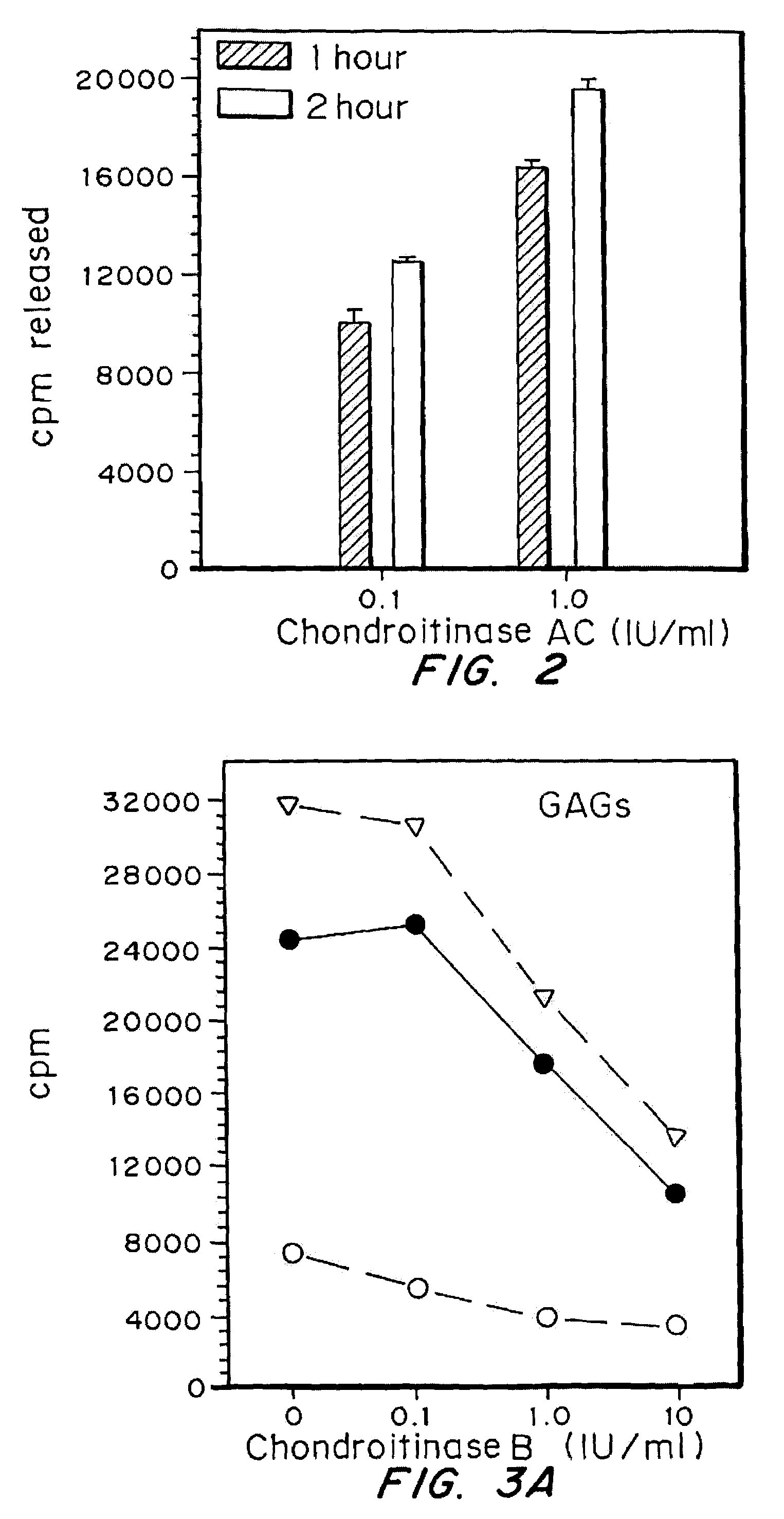
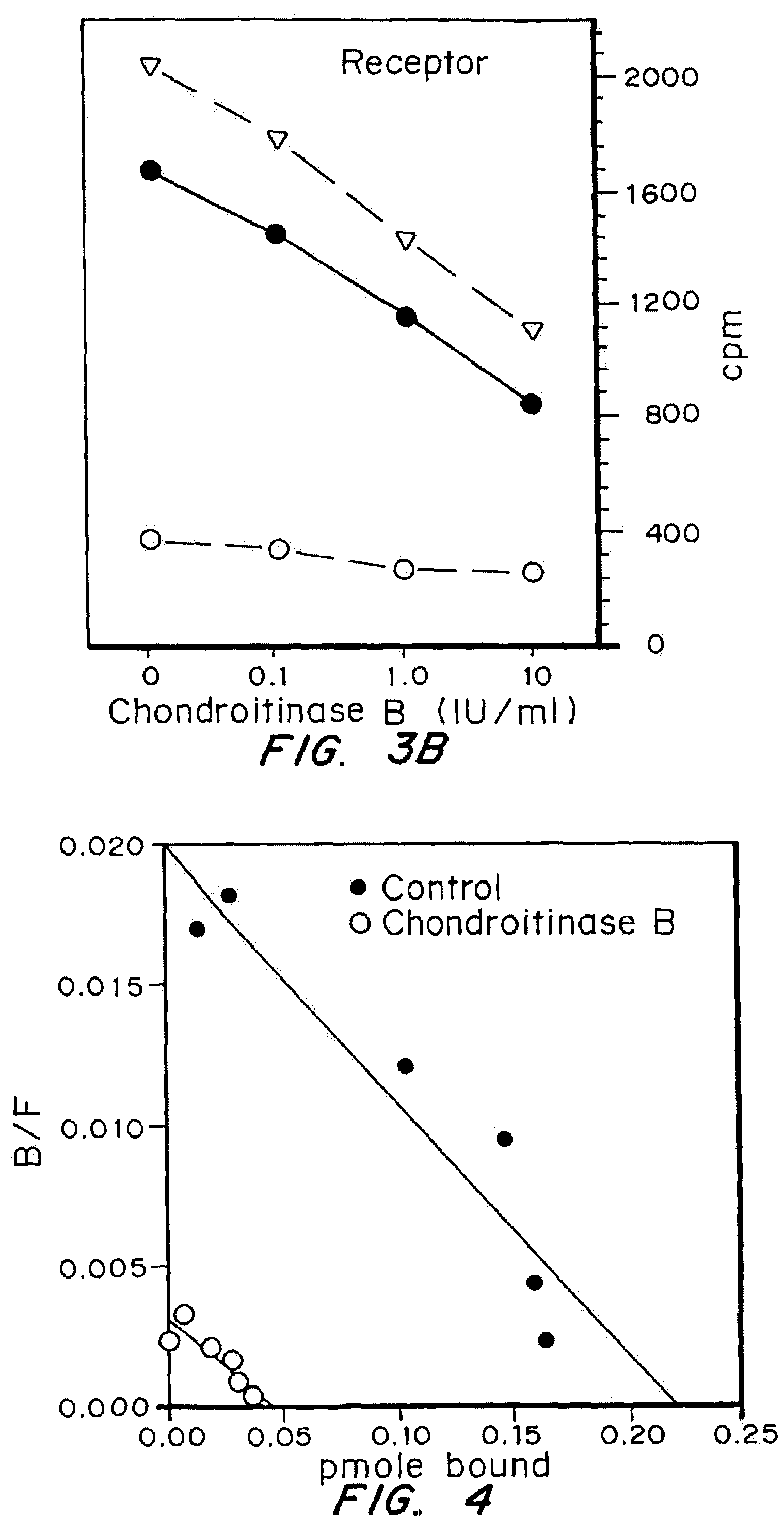
A highly purified and specific glycosaminoglycan degrading enzyme, chondroitinase AC, and to a lesser extent, chondroitinase B, can be used in the treatment of metastatic cancers and in other disorders characterized by angiogenesis. The enzymatic removal of chondroitin sulfates A and C, and to a lesser extent, chondroitin sulfate B, from cell surfaces directly decreases the ability of tumor cells to invade blood vessels and thus prevents the formation of metastatic, or secondary tumors; inhibits tumor cell growth; and decreases angiogenesis by inhibiting both endothelial cell proliferation and capillary formation. Decreasing the formation of new blood vessels into the tumor in turn decreases the potential for tumor growth, and further decreases the ability of tumor cells to invade the bloodstream. These effects are opposite to the prometastatic effects of tumor-secreted heparanase.
Owner:BIOMARIN PHARMA INC
Chondroitinase ABC producing recombinant yeast strain and structuring and applying methods thereof
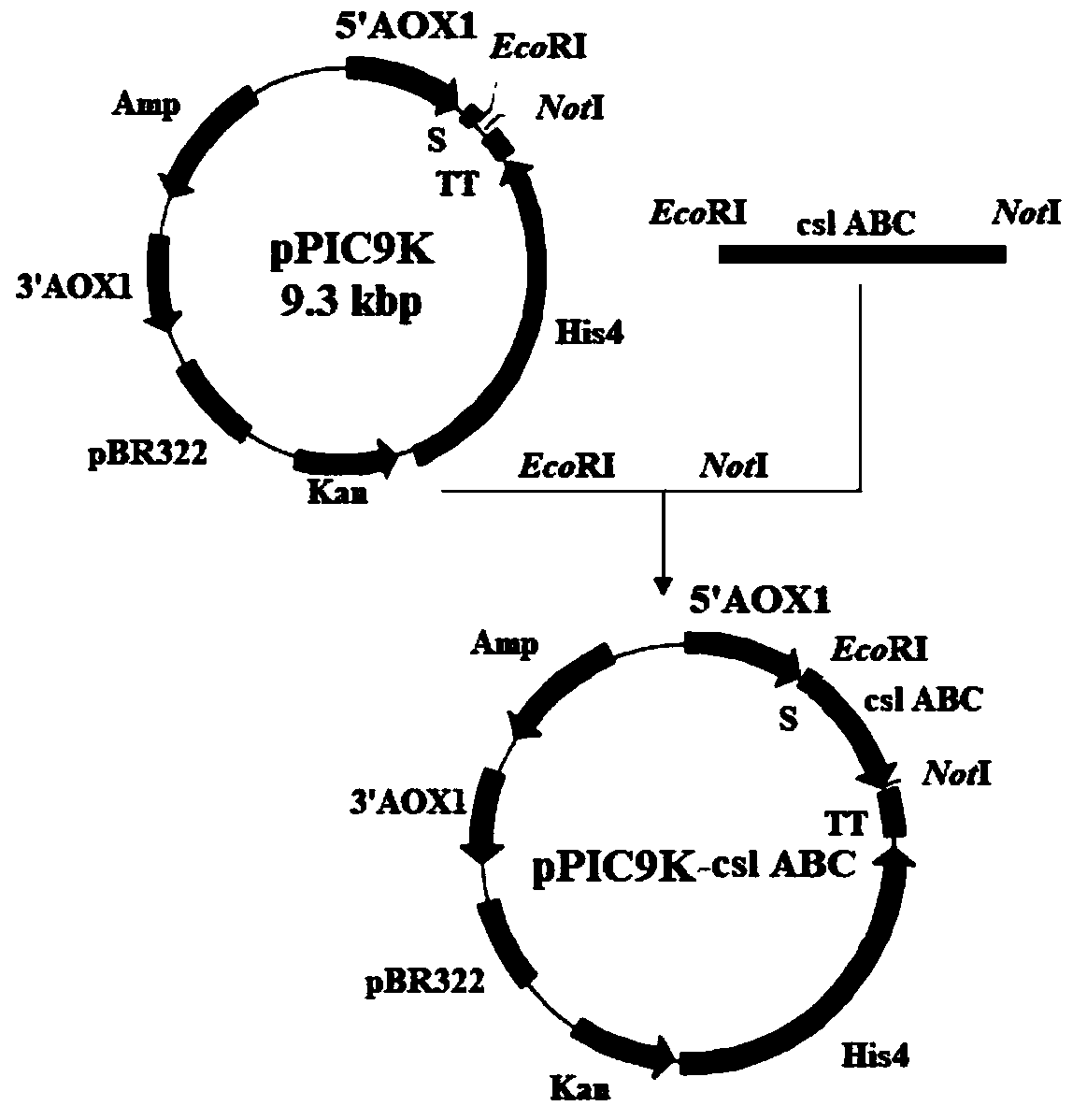
ActiveCN109988723AMeet health careFulfil requirementsBioreactor/fermenter combinationsFungiPichia pastorisBiotechnology
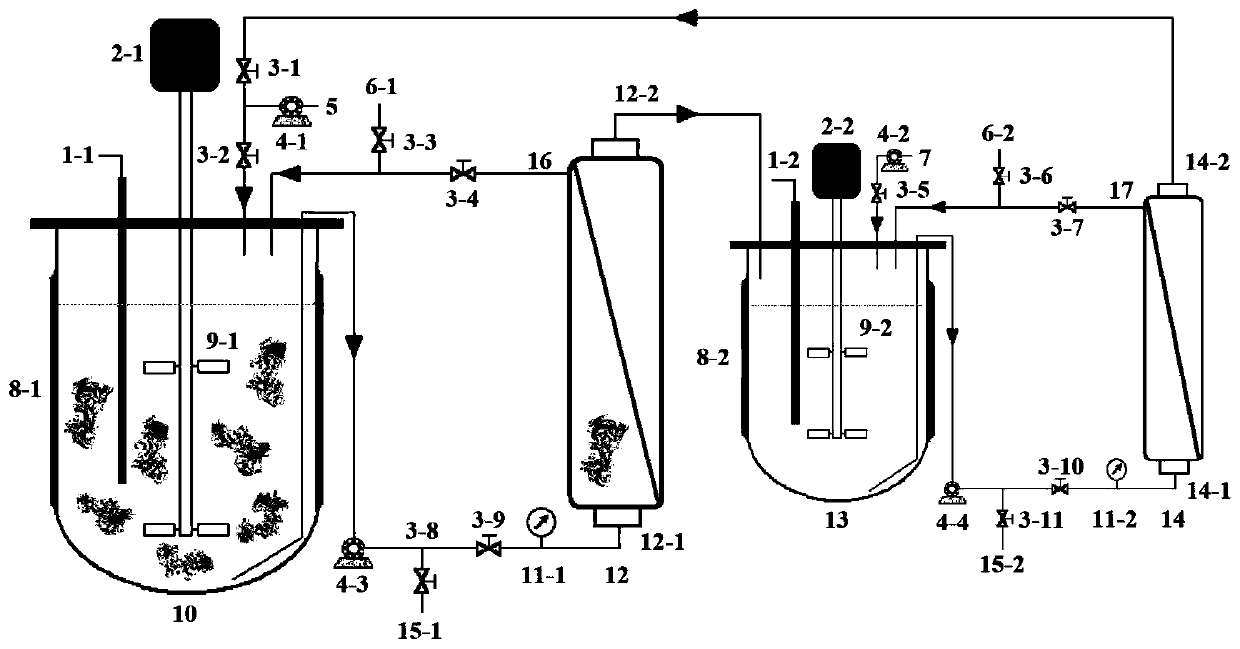
The invention discloses a chondroitinase ABC producing recombinant yeast strain and application thereof and belongs to the technical field of bioengineering. According to the chondroitinase ABC producing recombinant yeast strain, chondroitinase ABC from Proteus vulgaris ATCC33420 is subjected to heterogeneous expression, and with pPIC9K carriers and through induction of methanol, secretory expression of the chondroitinase ABC in pichia pastoris GS115 can be achieve; by means of a designed chondroitinase ABC-enzymatic membrane reactor, efficient and continuous production of small-molecular chondroitin sulfate can be achieved, the chondroitinase ABC can be recycled and reutilized, and the production cost can be greatly reduced. By taking food-grade yeast as host strains, the chondroitinase ABC producing recombinant yeast strain can be safe and reliable, provide effective references for industrialized and green production of small-molecular chondroitin sulfate A, B and C, and meanwhile, save energy, reduce emission and achieve significant economic and social benefits.
Owner:CHANGSHU INSTITUTE OF TECHNOLOGY
Method for reducing ethanol residues in sodium chondroitin sulfate
The invention relates to a method for reducing ethanol residues in sodium chondroitin sulfate. Sodium chondroitin sulfate is a mucopolysaccharide substance extracted from animal cartilage such as pig nasal bone and the like. Ethanol is mainly used for precipitation purification and dehydration. According to the method, the content of ethanol in chondroitin sulfate is decreased through thermal precipitation and dehydration of ethanol, and ethanol residues are reduced. The method adopts a simple process and mild conditions, production is safer, the ethanol residue content is low, the yield of chondroitin sulfate can be increased, and the purity of chondroitin sulfate can be increased, and mass industrial production can be realized.
Owner:QINGDAO JIULONG BIO PHARMA
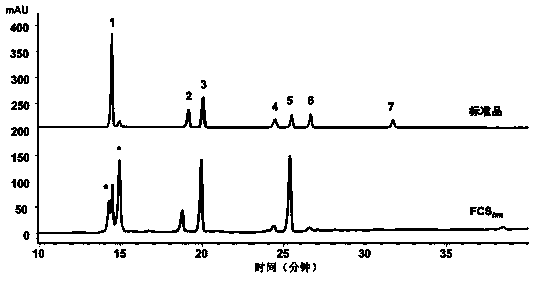
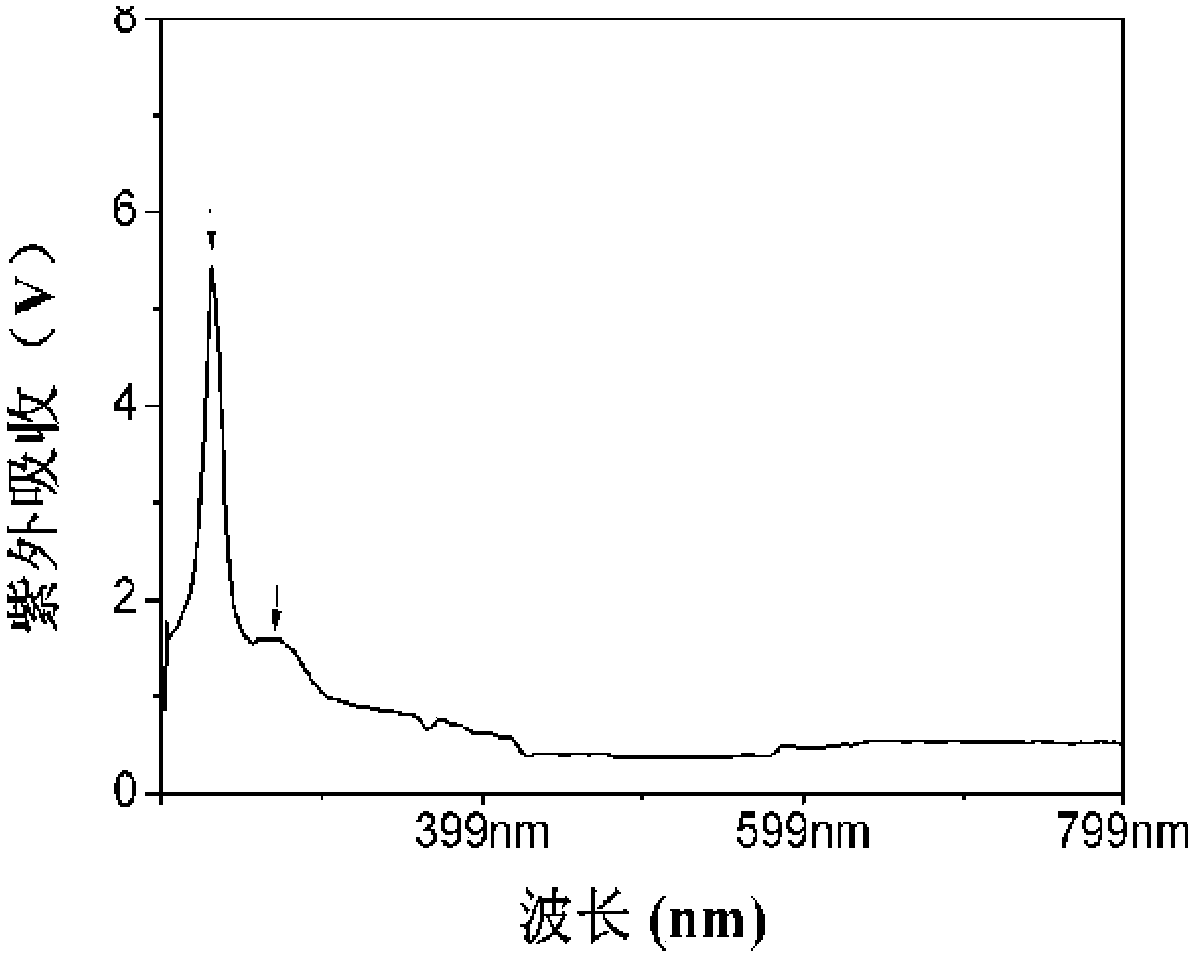
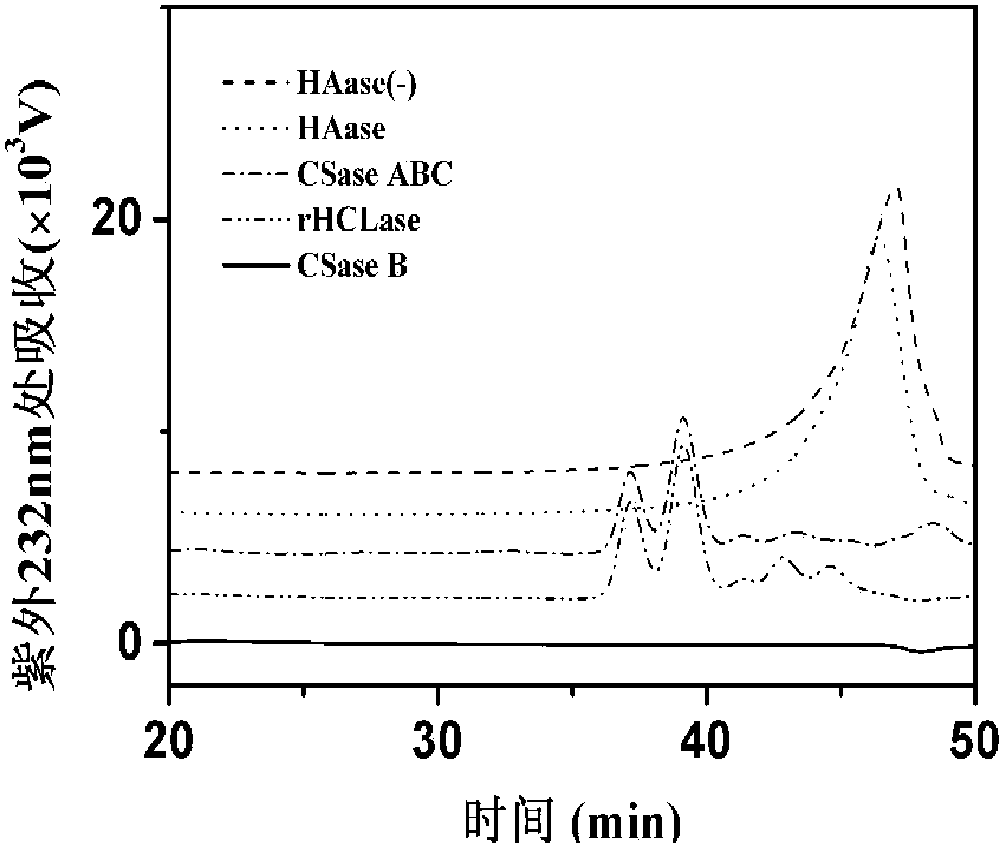
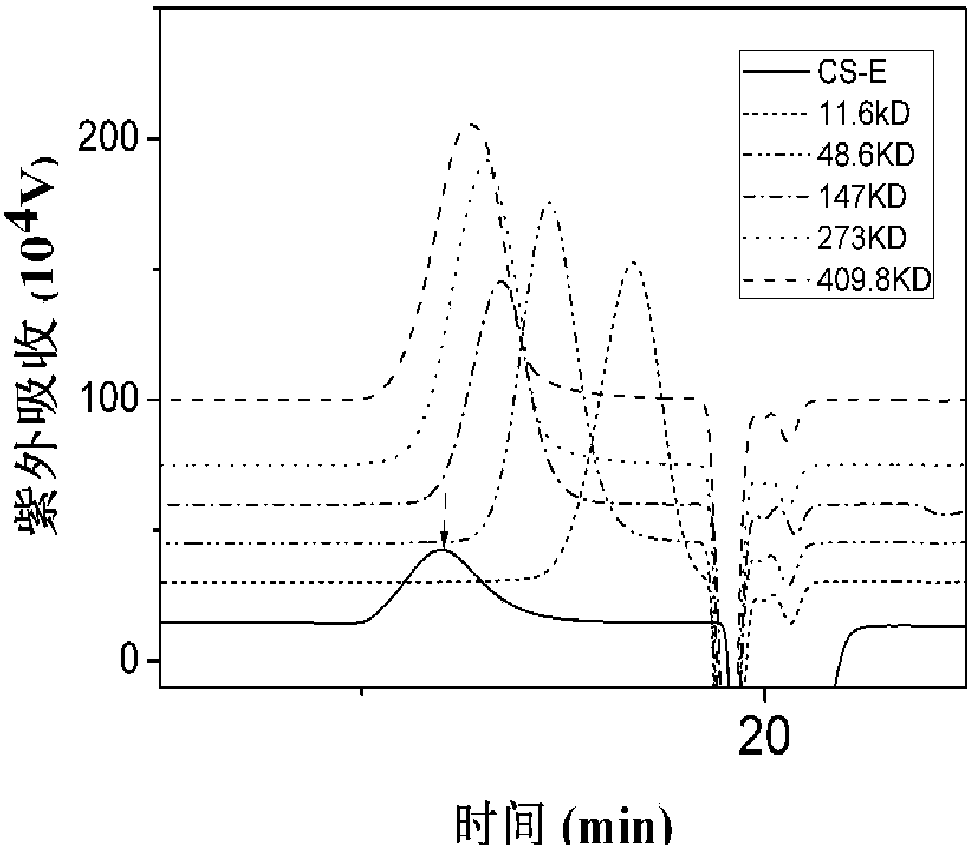
Novel fucosylated chondroitin sulfate FCS hm and preparation method and application thereof
ActiveCN109251255ALow manufacturing process costEasy to operateBlood disorderExtracellular fluid disorderSide chainBlood vessel
Owner:OCEAN UNIV OF CHINA
A kind of highly sulfated chondroitin sulfate and its preparation method and application
ActiveCN105924544BShorten the growth cycleIncrease productionOrganic active ingredientsAntineoplastic agentsHumboldt squidDisaccharide
Owner:SHANDONG UNIV
Preparation method of polysulfated chondroitin sulfate
The invention discloses a preparation method of polysulfated chondroitin sulfate, which comprises the steps of preparation of polysulfated chondroitin sulfate, separation and purification, formamide removal, decolorization, precipitation, lyophilization and the like. According to the invention, a chlorosulfonic acid- formamide mixed solution is adopted to performing sulfonation and heat degradation on chondroitin sulfate, then alcohol precipitation, filtration, decolorization and the like are carried out, and further purificatoion is conducted to obtain polysulfated chondroitin sulfate which can improve bone and joint function. The sulfonation and the degradation reaction are separated, firstly chlorosulfonic acid and formamide are added for sulfonation, and then heating is carried out fordegradation, and then through multi-step alcohol precipitation and filtration, polysulfated chondroitin sulfate meeting key quality indexes such as the molecular weight, the sulfur-carboxyl ratio, glucuronic acid and free sulfate content is obtained, has a high sulfonated chondroitin content of more than 70%. The chondroitin sulfate has a high degree of sulfation and is stable in nature and is not easily degraded by natural chondrosulphatase. The weight average molecular weight Mw is 7300-9300 Da, and the prepared polysulfated chondroitin sulfate has a good joint function improvement effect and can be used to maintain the joint cartilage integrity of humans and animals.
Owner:HEBEI CHANGSHAN BIOCHEM PHARMA
Attenuation of fibroblast proliferation
InactiveUS7056711B2Promote growthReduce secretionPeptide/protein ingredientsSkeletal disorderDiseaseCell-Extracellular Matrix
Highly purified and specific glycosaminoglycan degrading enzymes, chondroitinase B and chondroitinase AC, are used to treat fibroproliferative diseases. The enzymatic removal of chondroitin sulfate B(dermatan sulfate), and to a lesser extent, chondroitin sulfate A or C, from cell surfaces effectively decreases growth factor receptors on the cells and thereby decreases the cell proliferative response to such growth factors. In addition, removal of chondroitin sulfates reduces secretion of collagen, one of the major extracellular matrix components. Through the combined inhibition of fibroblast proliferation and collagen synthesis, treatment with chondroitinase B or chondroitinase AC decreases the size of fibrous tissue found in psoriasis, scleroderma, keloids, pulmonary fibrosis and surgical adhesions.
Owner:BIOMARIN PHARMA INC
Powder injection of medium and/or low molecular weight chondroitin sulfate
The invention discloses a powder injection of medium and / or low molecular weight chondroitin sulfate, in which the average molecular weight of the adopted chondroitin sulfate is in a scope of 2,000-30,000 daltons. The powder injection may be medium and / or low molecular weight chondroitin sulfate A,C or pharmaceutic salt of them, and the powder injection may also contain the its pharmaceutically acceptable auxiliary material. Because the molecular weight of the medium and / or low molecular weight chondroitin sulfate in the inventive powder injection is obviously less than the national standard of the chondroitin sulfate, thus it overcomes shortcoming of big molecular weight fluctuation of the ordinary chondroitin sulfate; it has more notable pharmaceutical role, more helpful for the absorption of the human body; and the powder injection may greatly raise the shelf-life, accordingly thoroughly solving its hidden trouble of going bad or reducing the effect in the storing and transport process.
Owner:汤毅
Placenta-like chondroitin sulfate A targeted delivery system and preparation method and application thereof
ActiveCN109568289AStrong specificityEasy diagnosisOrganic active ingredientsPharmaceutical non-active ingredientsSerum igeSerum albumin
The invention provides a placenta-like chondroitin sulfate A targeted delivery system, and further provides a preparation method and application of the placenta-like chondroitin sulfate A targeted delivery system. The placenta-like chondroitin sulfate A targeted delivery system comprises a serum albumin layer and a saccharide molecule, the saccharide molecule adheres to the serum albumin layer, and the serum albumin layer is further grafted with polypeptides of targeted placenta-like chondroitin sulfate A, wherein the polypeptides are grafted with serum albumins in the serum albumin layer through biotin-avidin specific binding. The amino acid sequence of the polypeptides is selected from one or more of amino acid sequences shown in SEQ ID NO: 1-SEQ ID NO: 3. The targeted delivery system iscapable of specifically targeting tissue which is inappropriately expressed by the placenta-like chondroitin sulfate A.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
Bio-capsule for treating secondary hyperlipemia and preparation method
InactiveCN106109946APromote decompositionPurify the bloodMetabolism disorderAmide active ingredientsDiseaseLiver and kidney
The invention relates to a bio-capsule for treating secondary hyperlipemia and a preparation method. Raw materials of active ingredients of the bio-capsule include commom banana pericarp, oenanthe javanica, radix clerodendri philippini, lotus petiole, biondia henryi, henbit, radix dipsaci, common seseli herb, crepis rigescens, crataegus root, all grass of anderson cirrhopetalum, tilia, twig and leaf of java bishopwood, lettuce seeds, chondroitin sulfate a, pantethine and lifibrate. The bio-capsule for treating the secondary hyperlipemia has the advantages that traditional Chinese medicines with efficacy of clearing heat, detoxifying, dispersing blood stasis, dredging collateral, removing food retention, harmonizing stomach, invigorating spleen, promoting digestion, nourishing liver and kidney, strengthening muscles and bones, conditioning blood vessels and the like are combined reasonably and used to cooperate with western medicine ingredients, by the aid of combination of the medicines, metabolic waste in blood can be cleared, the blood can be purified, lipid metabolism of a human body can be improved, decomposition course of lipid can be accelerated, fat accumulation in the human body can be decreased, blood lipid level can be lowered, complications like cardiovascular and cerebrovascular diseases and the like can be prevented, and proved by clinical verification, the bio-capsule is free of toxic, side or untoward effect and is applicable to wide popularization and application.
Owner:肖琳
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com