Method of extensive culture of antigen-specific cytotoxic T cells
a cytotoxic t cell and culture method technology, applied in the field of inducing, maintaining and expanding cytotoxic t cells, can solve the problems of inability to obtain the necessary cell count, inability to proliferate t cells to 10sup>9 /sup>to 10 and eventually decrease the cell coun
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example 1
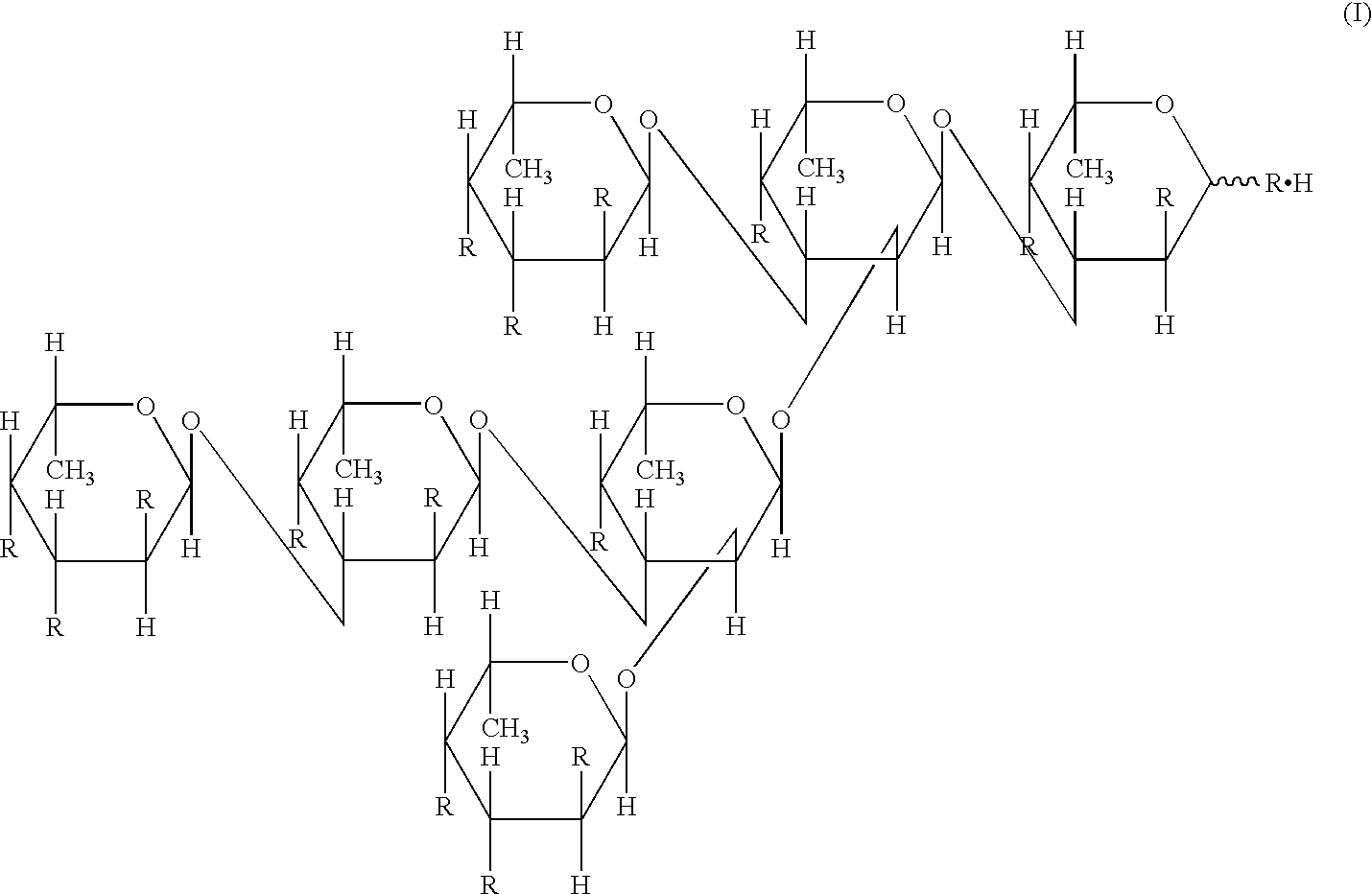
[0094]Kjellmaniella crassifolia was sufficiently dried, and thereafter 20 kg of the dried product was powdered with a free mill (manufactured by Nara Kikai Seisakusho). In 900 liters of tap water was dissolved 7.3 kg of calcium chloride dihydrate (manufactured by Nippon Soda Co., Ltd.), and 20 kg of the powdered product of Kjellmaniella crassifolia was then mixed therewith. The resulting mixture was heated for 40 minutes by blowing steam until the liquid temperature was raised from 12° to 90° C. Thereafter, the mixture was kept at 90° to 95° C. for 1 hour under stirring, and then cooled, to give 1100 liters of a cooled product. Subsequently, the cooled product was subjected to solid-liquid separation with a solid-liquid separator (manufactured by West Farrier Separator, Model: CNA), to give about 900 liters of supernatant after solid-liquid separation. The amount 360 liters of the supernatant after solid-liquid separation was concentrated up to a volume of 20 liters with FE10-FC-FUS...
preparation example 2
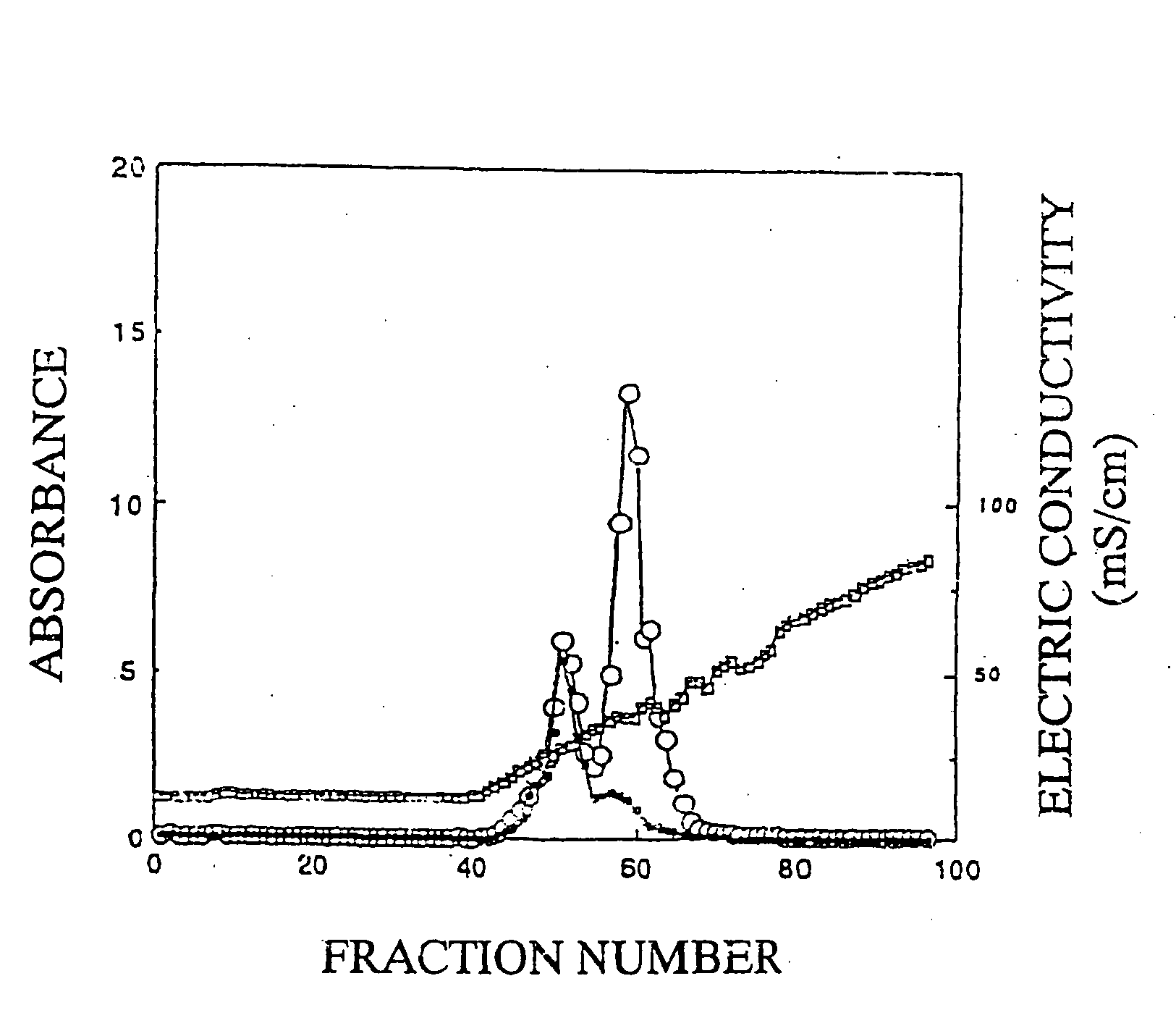
[0095]Seven grams of the dried product of the fucoidan described in Preparation Example 1 was dissolved in 700 ml of a 20 mM imidazole buffer (pH 8) containing 50 mM sodium chloride and 10% ethanol, and insoluble substances were removed by centrifugation. The supernatant after centrifugation was applied onto a DEAE-Cellulofine A-800 column (φ11.4 cm×48 cm) (manufactured by Seikagaku Corporation) equilibrated with the same buffer, and then washed with the same buffer. The elution was carried out with a concentration gradient of from 50 mM to 1.95 M sodium chloride (250 ml per fraction). A total sugar content and an uronic acid content were determined by the phenol-sulfuric acid method and the carbazole-sulfuric acid method, to give Fractions 43 to 49, Fractions 50 to 55, and Fractions 56 to 67, in the order of elution. Next, these fractions were desalted by electrodialysis, and thereafter lyophilized, to give each of Fraction I (340 mg) from Fractions 43 to 49, Fraction II (870 mg) f...
preparation example 3
[0096](1) A 2-liter Erlenmeyer flask was charged with 600 ml of a culture medium comprising an artificial sea water (manufactured by Jamarin Laboratory), pH 8.2, containing 0.25% glucose, 1.0% peptone, and 0.05% yeast extract, and then sterilized (at 120° C. for 20 minutes). Alteromonas sp. SN-1009 (FERM BP-5747) was inoculated into the culture medium, and cultured at 25° C. for 26 hours, to give a seed culture medium. A 30-liter jar fermentor was charged with 20 liters of a culture medium comprising an artificial sea water, pH 8.0, containing 1.0% peptone, 0.02% yeast extract, 0.2% sulfated polysaccharide described in item (2) of Example 2 described below, and 0.01% defoaming agent (manufactured by Shin-Etsu Chemical Co., Ltd., KM70), and sterilized at 120° C. for 20 minutes. After cooling, a 30 L jar fermentor was charged with 600 ml of the above-mentioned seed culture medium, and cultured at 24° C. for 24 hours under the conditions of 10 liters of aeration per minute and a stirri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com