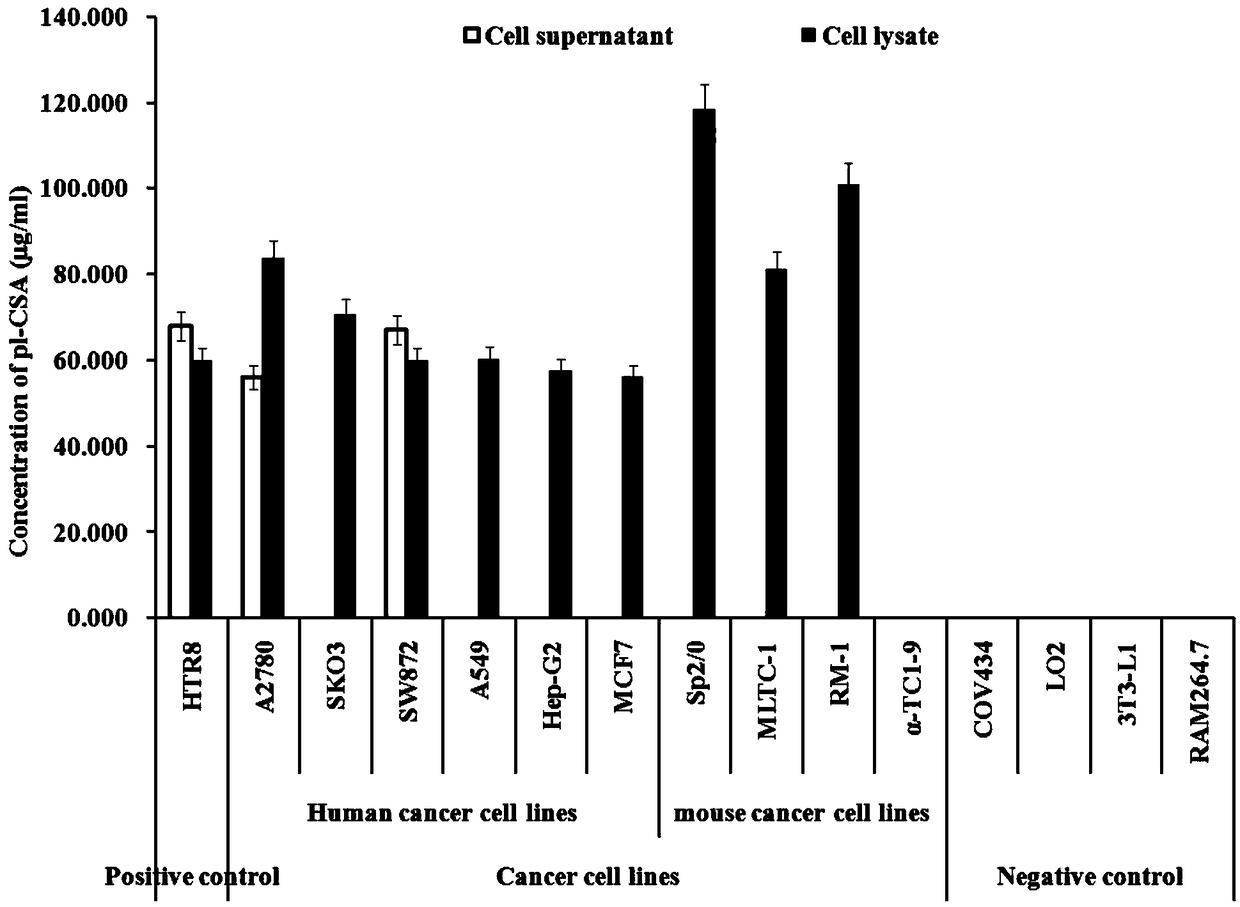
Placental chondroitin sulfate A based cancer screening and early stage diagnosis reagent and method
A technology of chondroitin sulfate and placenta, which is applied in the field of biotechnology detection, can solve the problems of few verification methods and difficulty in diagnosing cancer, and achieve the effect of simple method, low detection limit and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
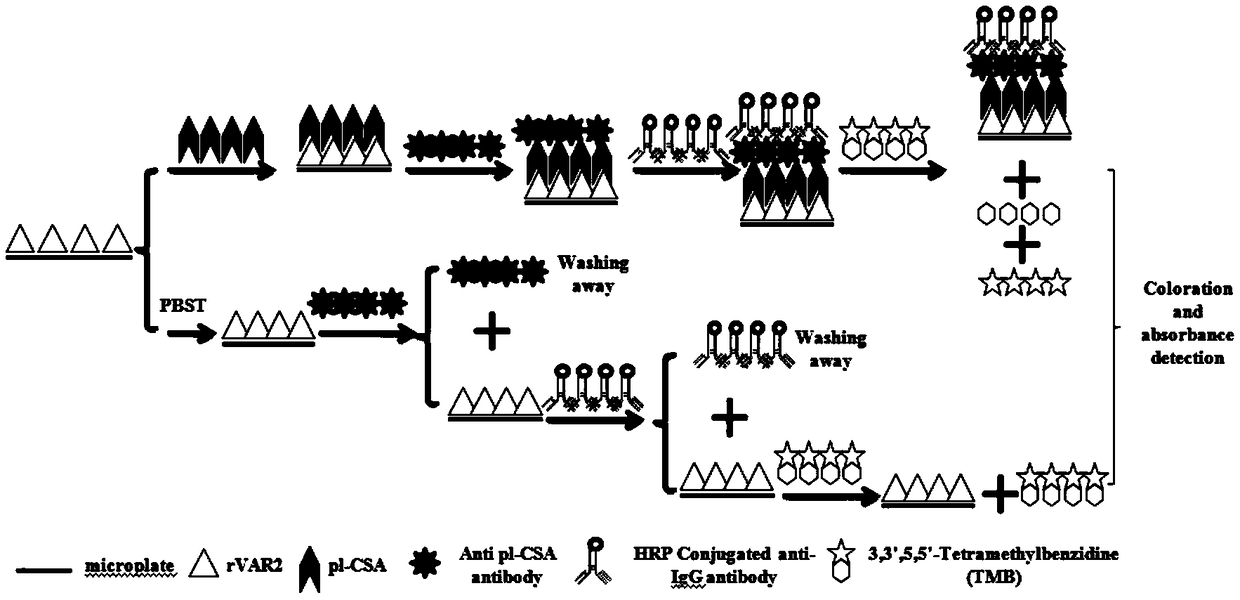
[0061] Example 1. Preparation of ELISA capture protein
[0062] The minimal binding peptide EDVKDINFDTKEKFLAGCLIVSFHEGKC of VAR2CSA was chemically synthesized and named as pl-CSA-BP as the capture protein of ELISA method.
Embodiment 2
[0063] Example 2. Preparation of purified pl-CSA
[0064] pl-CSA can be prepared by commercial or chemical methods, and the present invention adopts the prior patented method of our laboratory to prepare pl-CSA. For the preparation method, please refer to the preparation in the application number: 201710966913.2. details as follows,
[0065] Chromatographic purification of placenta-like chondroitin sulfate A or its derivatives by means of affinity chromatography, wherein the affinity chromatography column material is a conjugate of recombinant Plasmodium-infected erythrocyte surface antigen protein and an affinity chromatography matrix, The chromatographic purification method is to load the crude product of placental-like chondroitin sulfate A or its derivatives on the affinity chromatography column, and wash with the washing liquid until no impurities flow out, then elute with the eluent and Collect the pure product of placenta-like chondroitin sulfate A or its derivatives....
Embodiment 3
[0068] Example 3. Preparation of pl-CSA Antibody
[0069] 1) Immunization of Balb / c mice
[0070] 50 μg of immunogen pl-CSA was dissolved in 200 μL of PBS solution, mixed with an equal volume of complete Freund’s adjuvant, and fully emulsified by a micro-antigen emulsification device for 1 hour. Six-week-old female Balb / c mice were then injected at multiple points on the back of the neck. Freund's complete adjuvant was used for the initial immunization, and it was repeated once. The booster immunization was Freund's incomplete adjuvant, and the immunization interval was 2 weeks. Three days before fusion, 100 μg of pl-CSA immunogen dissolved in 200 μL of PBS solution was injected intraperitoneally, without adjuvant agent. 7 days to 10 days after the third immunization, blood was collected from the tail of the mice, about 15 μL per mouse, for titer detection.
[0071] 2) Potency determination
[0072] The titer was determined by indirect non-competitive ELISA. Dissolve 1 μg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com