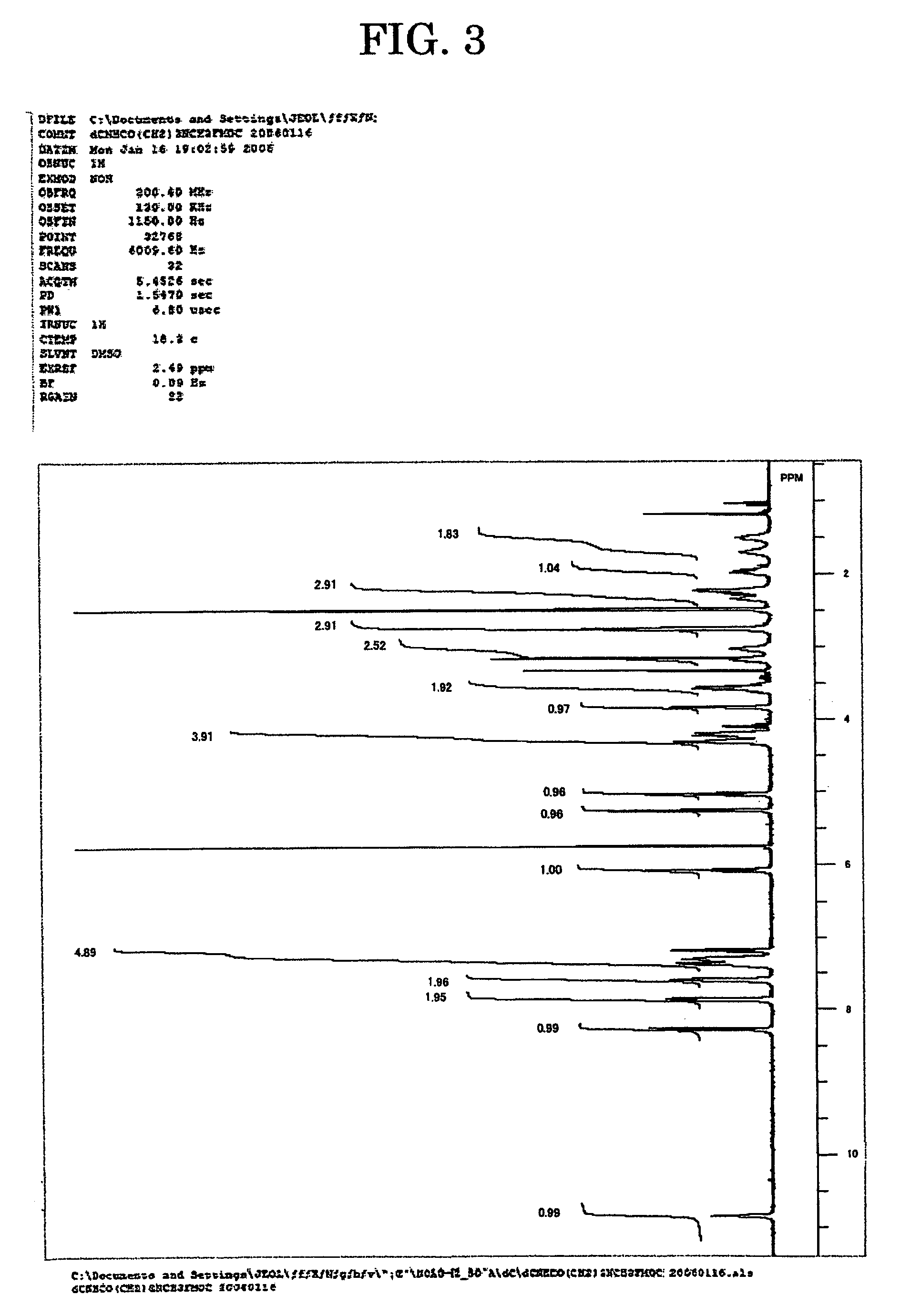
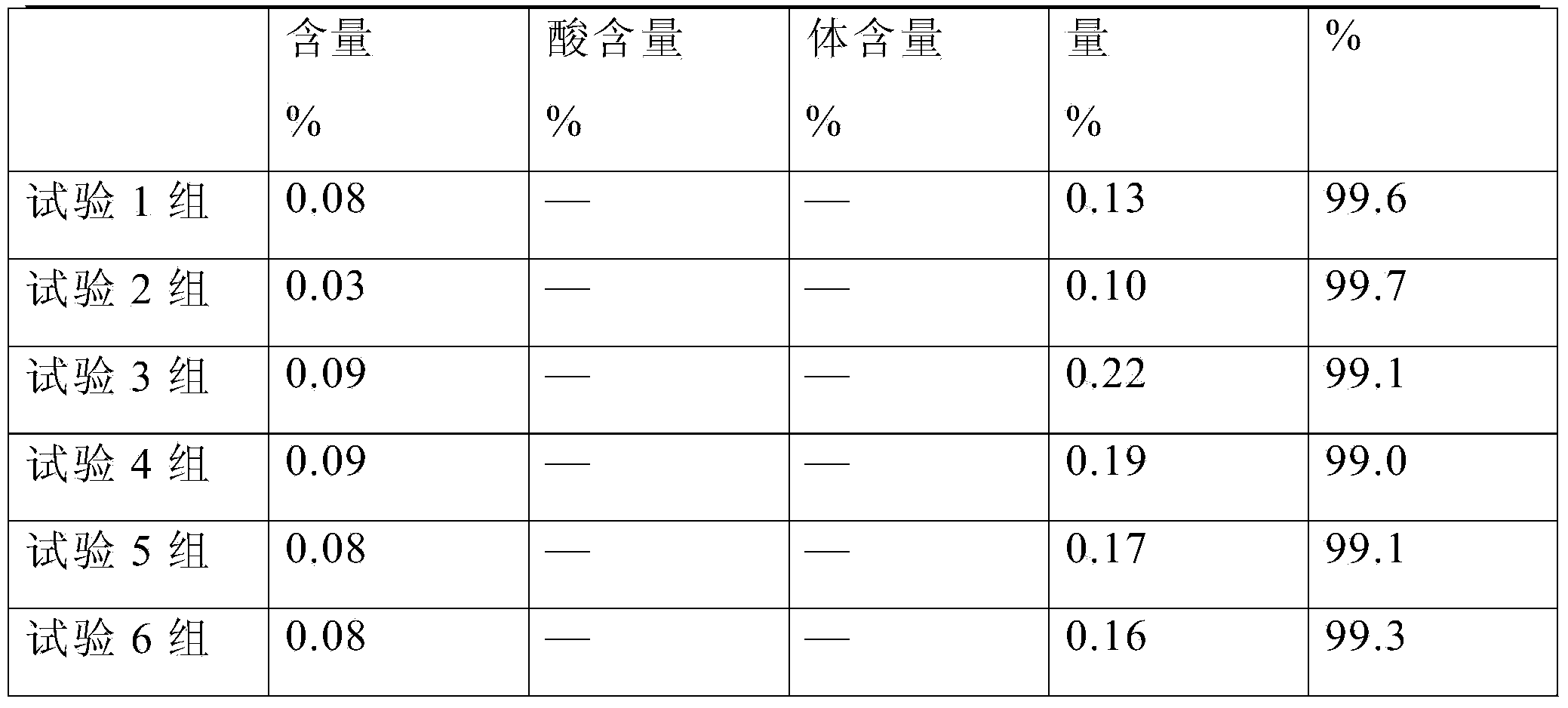
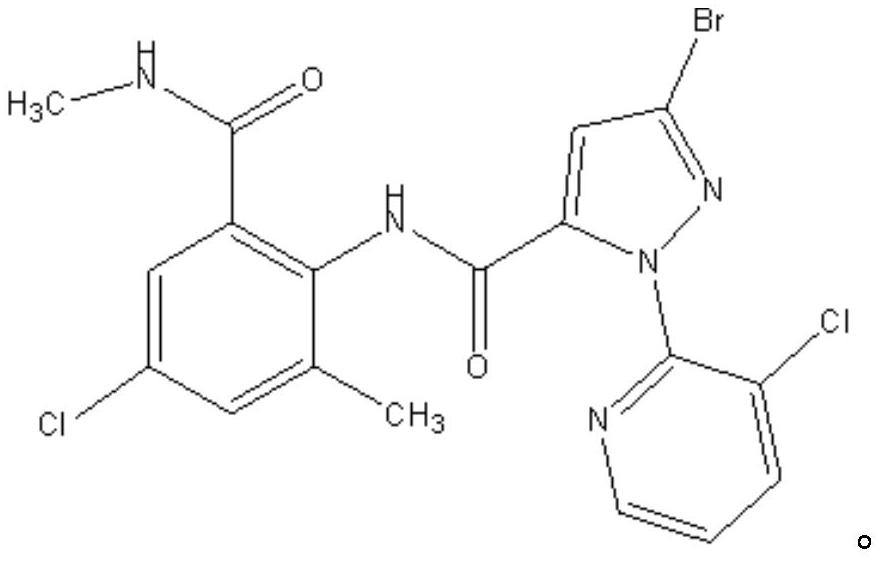
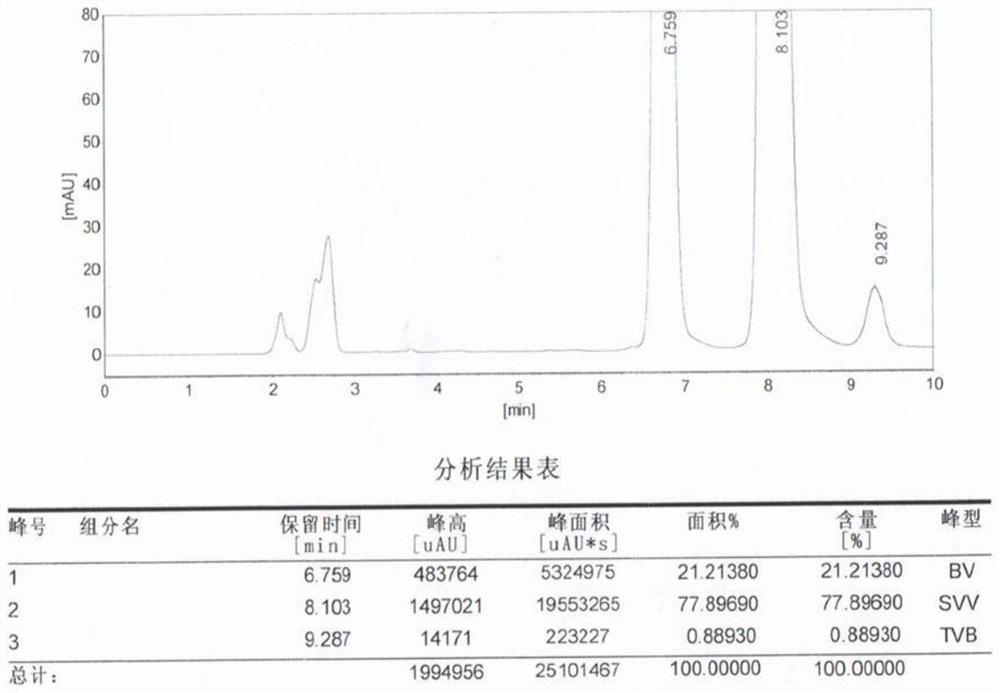
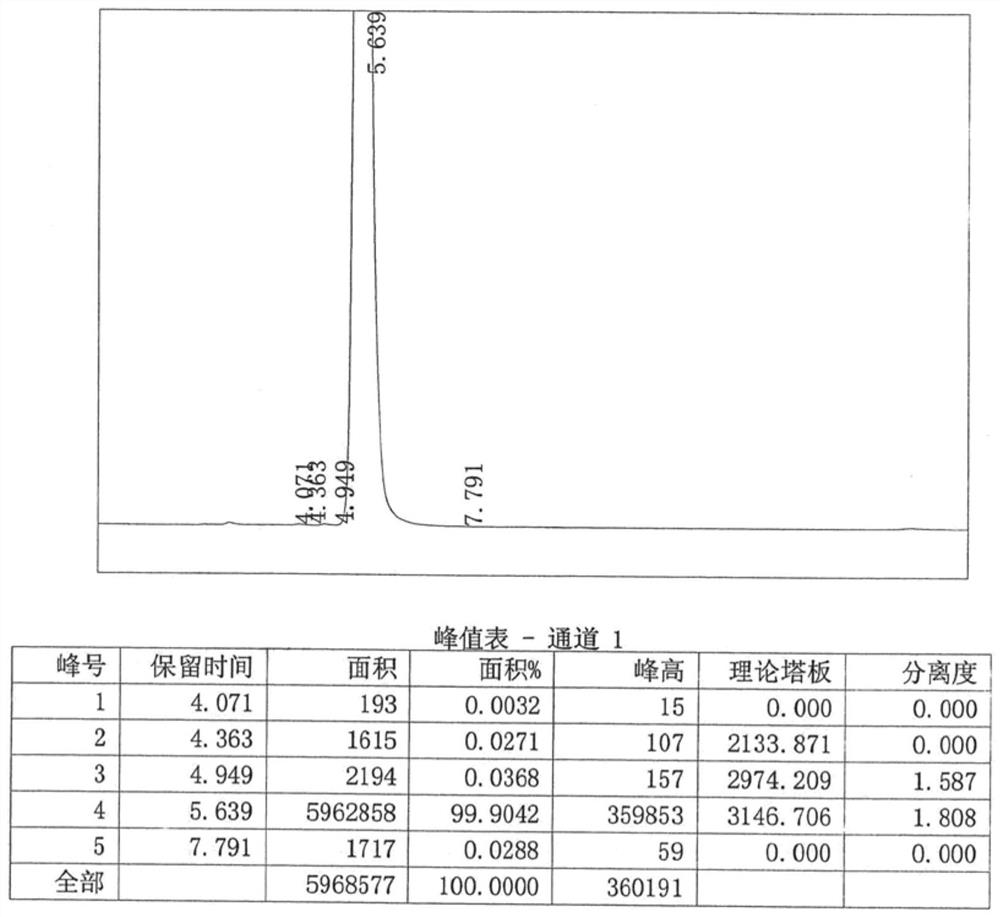
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
92 results about "Aminomethylbenzoic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
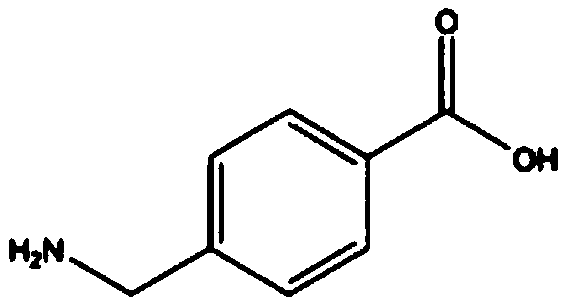
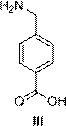
Aminomethylbenzoic acid (more precisely, 4-aminomethylbenzoic acid or p-aminomethylbenzoic acid, PAMBA) is an antifibrinolytic.
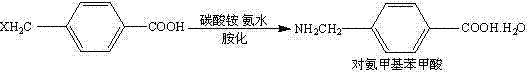
Preparation method of aminomethylbenzoic acid
InactiveCN105037186AMild reaction conditionsRaw materials are cheap and easy to getOrganic compound preparationAmino-carboxyl compound preparationAcid hydrolysisHexamethylenetetramine
The invention discloses a preparation method of aminomethylbenzoic acid. Specifically, p-cyanobenzyl chloride (particularly 4-cyanobenzyl chloride) is taken as a raw material, and then 4-cyanobenzyl chloride is subjected to ammonolysis reaction under the catalysis of urotropine, so that the intermediate 4-cyanobenzylamine is obtained, and acid hydrolysis is carried out to obtain the end product aminomethylbenzoic acid. According to the preparation method of aminomethylbenzoic acid, provided by the invention, the synthetic procedures are short, the raw materials are easy to get, the yield is high, the cost is low, the quality is good, the pollution is less, the operation is simple and convenient and large-scale industrial production is facilitated.
Owner:SUZHOU JINGYE MEDICINE & CHEM
Medical sodium alginate gel microsphere and preparation method and application thereof
InactiveCN103239730AControl releaseReduce erosionAntibacterial agentsPharmaceutical non-active ingredientsWater insolubleMicrosphere
The invention provides a medical sodium alginate gel microsphere and a preparation method and application of the medical sodium alginate gel microsphere. The medical sodium alginate gel microsphere consists of a composite medicine carrier and a water-insoluble medicine; the medicine is coated with the composite medicine carrier; and the composite medicine carrier is an ion crosslinking agent-sodium alginate-divalent metal ion, wherein the ion crosslinking agent is 4-aminomethylbenzoic acid or tranexamic acid. The preparation method comprises the following steps of: (1) mixing ion crosslinking agent aqueous solution with divalent metal ion solution in the same volume to obtain composite solidifying liquid; (2) dispersing medicine powder or an agent into sodium alginate aqueous solution; uniformly mixing; dropwise adding the mixture into the composite solidifying liquid obtained in step (1) through a high-voltage static droplet generating device or a syringe needle, so that the mixture drops are solidified into spheres; and (3) dehydrating gel microspheres which are washed with the distilled water; and drying at normal temperature. The medical sodium alginate gel microsphere can be used for treating tuberculosis, endocrine disease and tumor, and also can be used for treating local acute hemorrhage and chronic errhysis.
Owner:THE 309TH HOSPITAL OF CHINESE PEOPLES LIBERATION ARMY
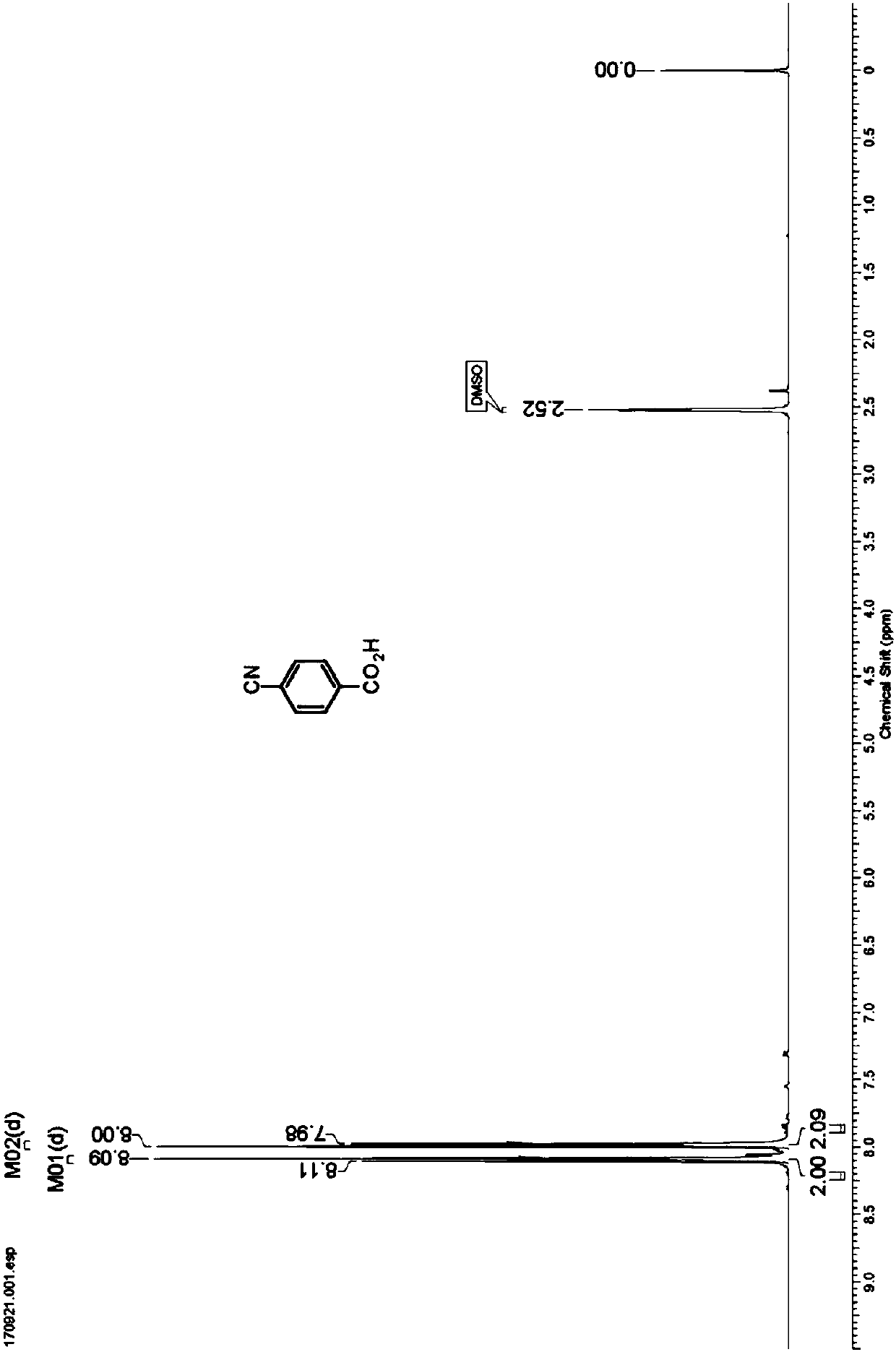
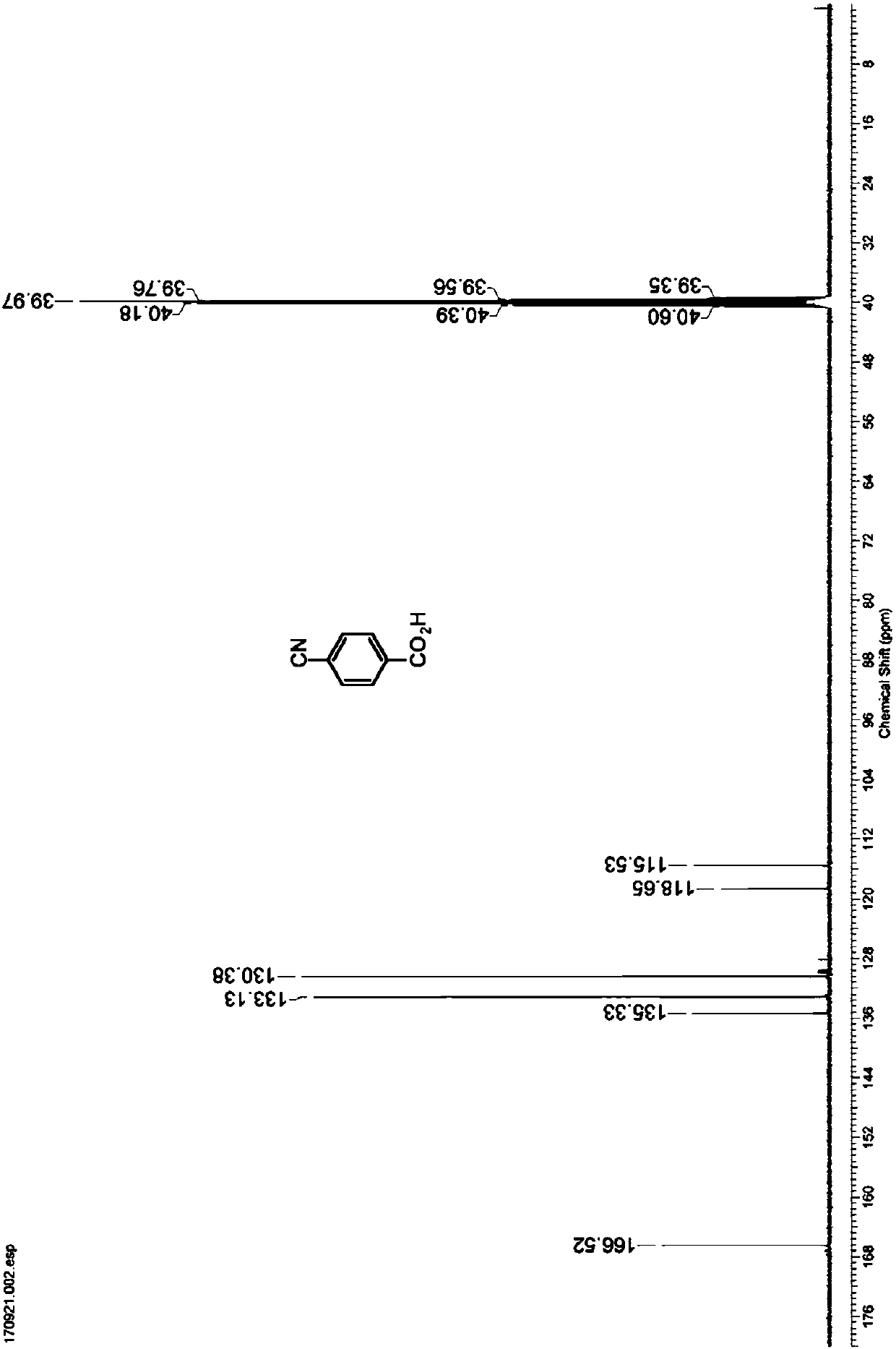
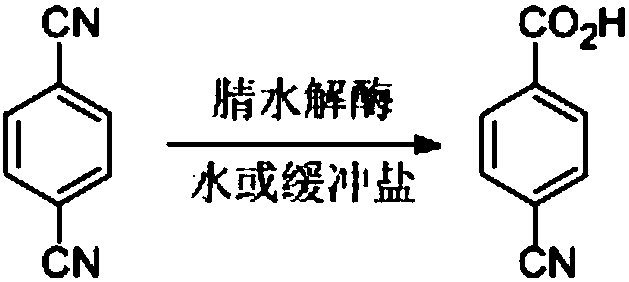
Nitrilase capable of preparing paracyanobenzoic acid by hydrolyzing p-benzenedicarbonitrile
ActiveCN107641622AMild reaction conditionsQuick responseHydrolasesFermentationArabidopsis thalianaPollution
The invention discloses a nitrilase N1 derived from pantoea sp.AS-PWVM4 and a gene thereof, a nitrilase N2 derived from arabidopsis thaliana and a gene thereof, a nitrilase N3 derived from acidovoraxfacilis 72W and a gene thereof, a nitrilase N4 derived from leptolyngbya sp. and a gene thereof, a nitrilase N5 derived from brassica oleracea var.oleracea and a gene thereof and a nitrilase N6 derived from camelina sativa and a gene thereof, and a method for preparing paracyanobenzoic acid as p-aminomethylbenzoic acid intermediate by using the nitrilase as a biological catalyst; resting cells ofthe corresponding nitrilases can be used for catalyzing 100g / L of substrate; the conversion rate is greater than 99%; the method has the obvious characteristics of mild reaction conditions, no pollution and simple process route, and has broad industrial application prospects.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
Method for preparing tranexamicacid
ActiveCN107954887AProcess for reducing acid adjustmentTransposition reaction time shortenedOrganic compound preparationOrganic chemistry methodsHydrogenation reactionTranexamic acid
The invention relates to a method for preparing tranexamicacid. The method comprises the following steps: adding the aminomethylbenzoic acid, water, concentrated sulfuric acid and a catalyst into a reaction vessel for stirring and heating; then introducing hydrogen for carrying out hydrogenation reaction to obtain hydrogenated reaction liquid; adding the hydrogenated reaction liquid and the concentrated sulfuric acid into the reaction vessel, raising the temperature to 180 to 200 DEG C, and carrying out heat-preservation stirring and conversion reaction to obtain the tranexamicacid. The methodfor preparing the tranexamicacid, disclosed by the invention, has the advantages of simplified technology, short reaction time and high synthesis efficiency.
Owner:CHANGZHOU YINSHENG PHARMA
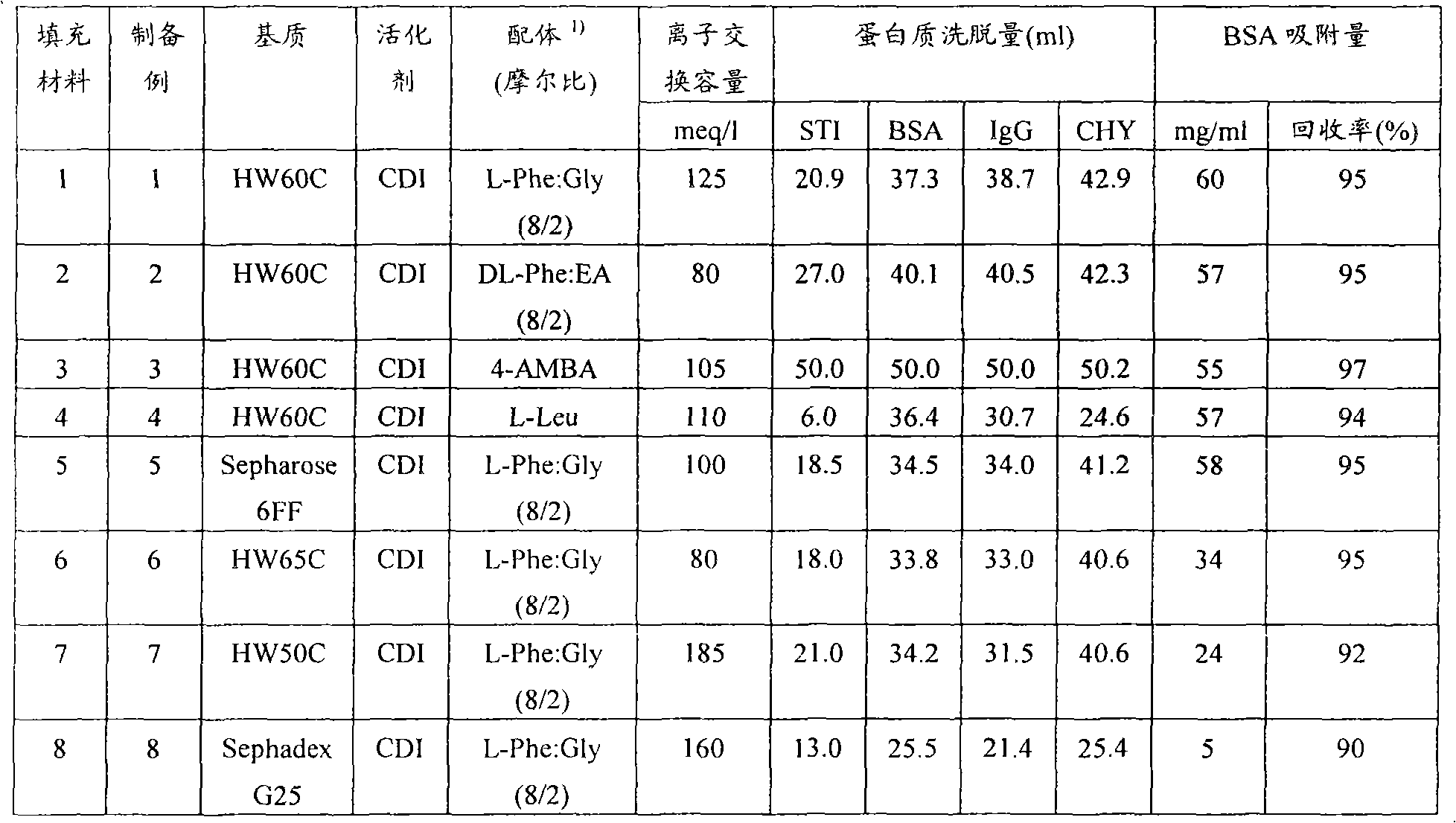
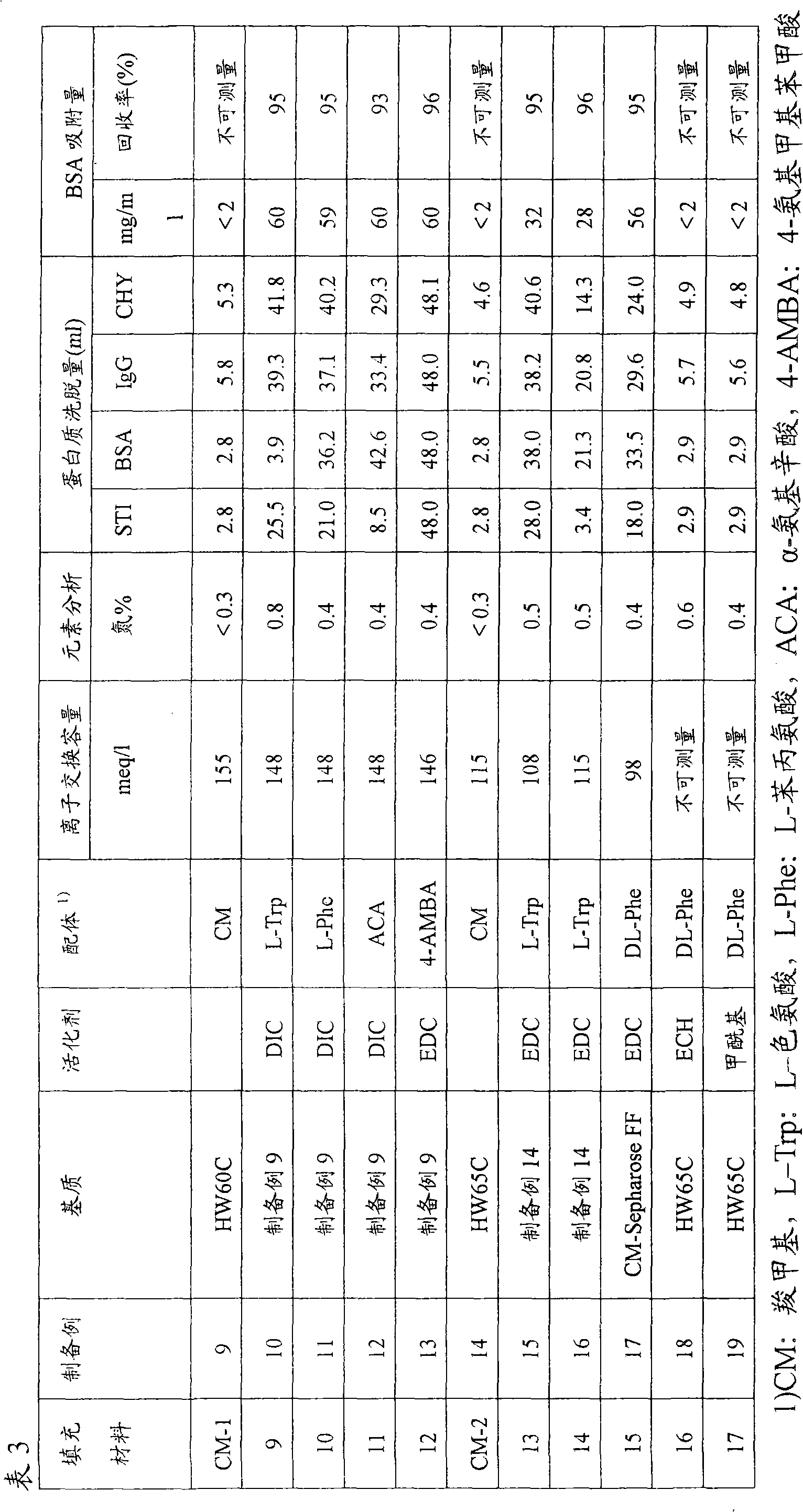
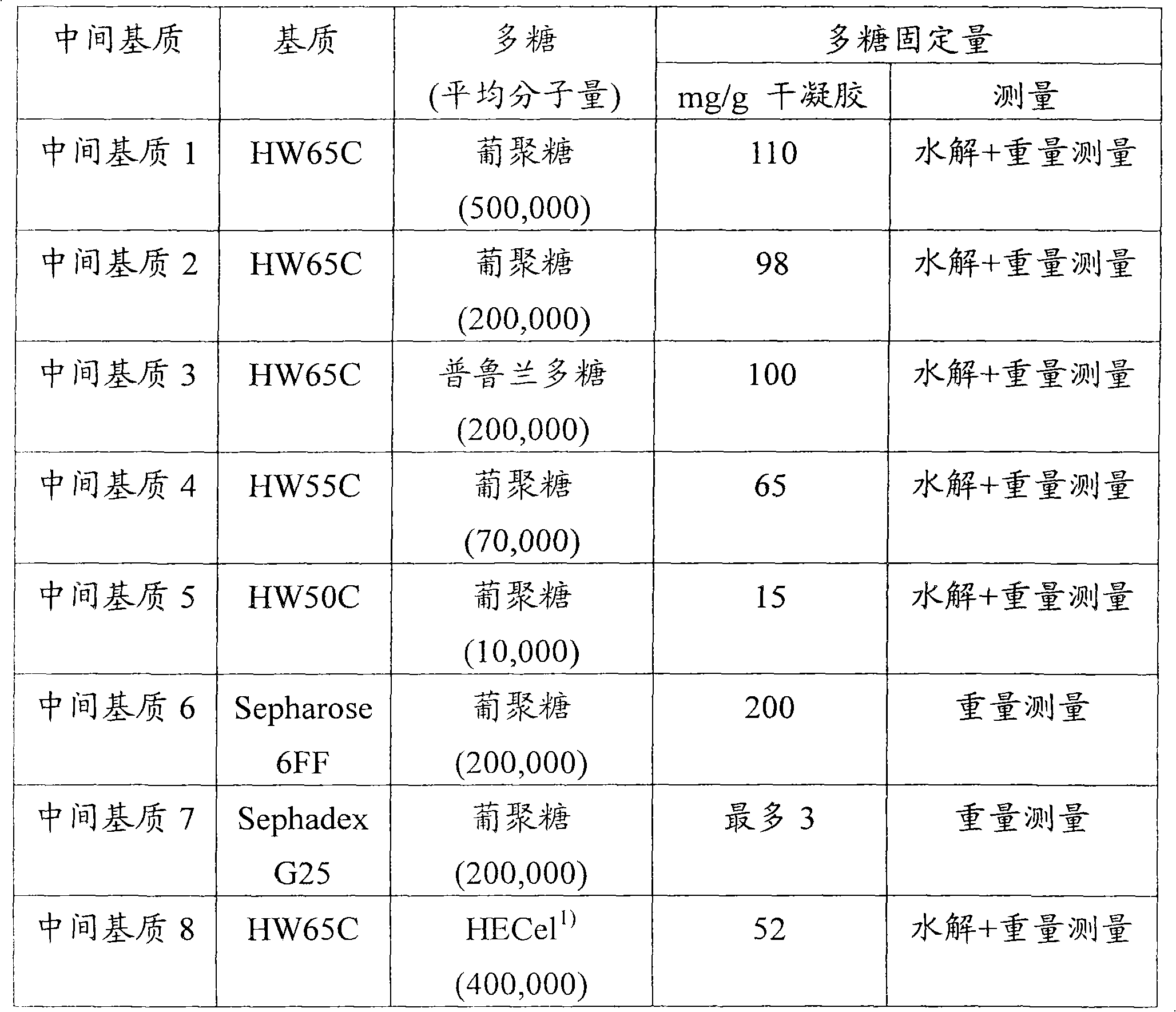
Packing material for liquid chromatography and process for separation and purification of biopolymer by means of the packing material
ActiveCN101791490AHigh binding capacityEasy to separateIon-exchange process apparatusOther chemical processesBenzoic acidDesorption
To provide a novel packing material for liquid chromatography capable of separating and purifying, or collecting and recovering, a biopolymer such as a protein or a peptide by adsorption and desorption by a pH change without being influenced by the isoelectric point of the protein or by the salt concentration in a solvent in which the biopolymer such as the protein is dissolved, and to provide a process for concentrating and recovering a desired biopolymer such as a protein or a peptide from a large amount of dilute cell culture solution by means of such a packing material. Separation and purification, or collection and recovery, of a biopolymer, is carried out by liquid chromatography by means of a packing material for liquid chromatography comprising a base matrix and a ligand immobilized to the base matrix, wherein the base matrix is a hydrophilic base matrix having alcoholic hydroxy groups on its surface, the ligand is at least one ligand selected from the group consisting of an +--amino acid represented by the following formula (1): RCH(NH 2 )COOH (1) wherein R is an aromatic group or a C 5-7 non-ionic aliphatic group, and an aminomethyl benzoic acid, and the ligand is immobilized to the base matrix by an amide bond or an urethane bond via the amino group contained in the compound represented by the formula (1).
Owner:TOSOH CORP
Method for synthesizing aminomethylbenzoic acid
PendingCN108484426AReduce pollutionShort synthetic routeOrganic compound preparationAmino-carboxyl compound preparationPalladium on carbonActivated carbon
The invention discloses a method for synthesizing aminomethylbenzoic acid. The method comprises the following step: with p-eormylbenzoic acid as an initial raw material, performing catalytic hydrogenation amination, thereby obtaining the aminomethylbenzoic acid, wherein the reaction temperature of catalytic hydrogenation amination is 80-150 DEG C; the reaction pressure of catalytic hydrogenation amination is 1.5-5.5MPa; ammonia water or an ammonia gas is adopted as an amination reagent of catalytic hydrogenation amination; a palladium-carbon catalyst is adopted as a catalyst of catalytic hydrogenation amination; the amount of the palladium-carbon catalyst accounts for 1-10% of the weight of the p-eormylbenzoic acid; and a step of refining treatment comprises alkali treatment, activated carbon decoloring and acid neutralization. The method disclosed by the invention is relatively short in synthesis route, relatively small in environment pollution, relatively low in production cost, freeof toxic material such as sodium cyanide or liquid chlorine, relatively high in security, relatively high in yield and applicable to industrial large-scale production.
Owner:CHANGZHOU LANLING PHARMA
Synthetic method for aminomethylbenzoic acid
ActiveCN108623488AReduce pollutionSimple post-processingOrganic compound preparationAmino-carboxyl compound preparationOrganic solventSide reaction
The invention discloses a synthetic method for aminomethylbenzoic acid. The synthetic method specifically comprises the following steps: adding 4-halomethyl alkyl benzoate and triethylamine, dropwiseadding ethanol-water solution in which 2-amino-5-methyl-1,3,4-thiadiazole is dissolved while stirring, after a reaction is completed, evaporating solution until a lot of solids are separated out, cooling, filtering, and drying to obtain 4-aminomethyl alkyl benzoate; adding the 4-aminomethyl alkyl benzoate to acid solution, reacting by stirring, cooling, adding water, and dropwise adding alkali until the solution is alkaline, and a lot of the solids are separated out, filtering, washing, and drying to obtain the aminomethylbenzoic acid. The synthetic method is moderate in reaction conditions, less in side reactions, high in yield, low in cost, short in reaction and post-processing time, low in energy consumption, high in production efficiency, small in environmental pollution, and simple in'three wastes' treatment, and suitable for the industrial production. -CN with strong toxicity and an organic solvent with strong pollution are not used, and reaction raw materials are cheap in priceand easy to obtain.
Owner:HUNAN UNIV OF ARTS & SCI
Hemostatic composition and device
InactiveUS20160346239A1Reduced tendency to swellEasy to useOrganic active ingredientsSurgical adhesivesFibrinolytic inhibitorEpsilon-Aminocaproic Acid
A hemostatic composition comprises calcium alginate, a chitosan, epsilon-aminocaproic acid, an acid selected from aminomethylbenzoic acid and tranexamic acid, and tannin. The method of making the hemostatic composition comprises mixing one or more polysaccharide bases, tannin, a fibrinolytic inhibitor, colloidal silver and a solvent to form a mixture, and drying the mixture at a temperature between 25° C. and 80° C. until residual moisture content is approximately 15 to 20%.
Owner:KOROBOV MAXIM
Application of urotropine as catalyst in aminomethylbenzoic acid synthesis
InactiveCN102816077AReduce pollutionHigh yieldOrganic compound preparationAmino-carboxyl compound preparationHexamethylenetetramineAminomethylbenzoic acid
The invention relates to application of urotropine as a catalyst in aminomethylbenzoic acid synthesis. The method comprises the following steps: at the temperature of 10-30 DEG C, dissolving urotropine in water, stirring until the urotropine is completely dissolved, adding p-chloromethylbenzoic acid or p-bromomethylbenzoic acid, stirring uniformly, slowly introducing ammonia gas within 1 hour until the reaction liquid is completely clear, heating to 40-60 DEG C to react for 3-4 hours, evaporating under reduced pressure to remove ammonia until the pH value is 7-8, cooling to crystallize, filtering, and drying to obtain the aminomethylbenzoic acid, wherein the filtrate can be continuously used repeatedly more than six times. By using the urotropine as the catalyst for aminating aminomethylbenzoic acid, compared with the prior art, the invention can greatly enhance the product yield, wherein the first-use yield of the catalyst is 60%, and the product yield of the repeatedly used mother solution can reach 70-80%, thereby greatly lowering the industrial production cost and reducing the environmental pollution.
Owner:SHANGHAI MODERN HASEN SHANGQIU PHARMA
Packing material for liquid chromatography and process for separation and purification of biopolymer by means of the packing material
ActiveUS20100160605A1High binding capacityIncrease capacityIon-exchange process apparatusPeptide/protein ingredientsBenzoic acidDesorption
To provide a novel packing material for liquid chromatography capable of separating and purifying, or collecting and recovering, a biopolymer such as a protein or a peptide by adsorption and desorption by a pH change without being influenced by the isoelectric point of the protein or by the salt concentration in a solvent in which the biopolymer such as the protein is dissolved, and to provide a process for concentrating and recovering a desired biopolymer such as a protein or a peptide from a large amount of dilute cell culture solution by means of such a packing material.Separation and purification, or collection and recovery, of a biopolymer, is carried out by liquid chromatography by means of a packing material for liquid chromatography comprising a base matrix and a ligand immobilized to the base matrix, wherein the base matrix is a hydrophilic base matrix having alcoholic hydroxy groups on its surface, the ligand is at least one ligand selected from the group consisting of an α-amino acid represented by the following formula (1):RCH(NH2)COOH (1)wherein R is an aromatic group or a C5-7 non-ionic aliphatic group, and an aminomethyl benzoic acid, and the ligand is immobilized to the base matrix by an amide bond or an urethane bond via the amino group contained in the compound represented by the formula (1).
Owner:TOSOH CORP
Amidite for nucleic acid synthesis and nucleic acid synthesizing method
To provide an amidite for nucleic acid synthesis, which enables a protective group therein to be removed under moderate conditions and can be practically used, and a nucleic acid synthesizing method using the amidite for nucleic acid synthesis. Specifically, the present invention relates to an amidite for nucleic acid synthesis represented by General Formula (I) below, and a nucleic acid synthesizing method using the amidite for nucleic acid synthesis:where X denotes a base; Y denotes a protective group formed of any one of a 4-aminobutyric acid derivative, an o-aminomethylbenzoic acid derivative, an o-aminophenylacetic acid derivative, an o-aminoethylbenzoic acid derivative, an o-aminomethylphenylacetic acid derivative, an o-aminophenylpropionic acid derivative and a 5-aminovaleric acid derivative; and Q denotes one of a hydrogen atom and a hydroxyl group.
Owner:APTA BIOSCI
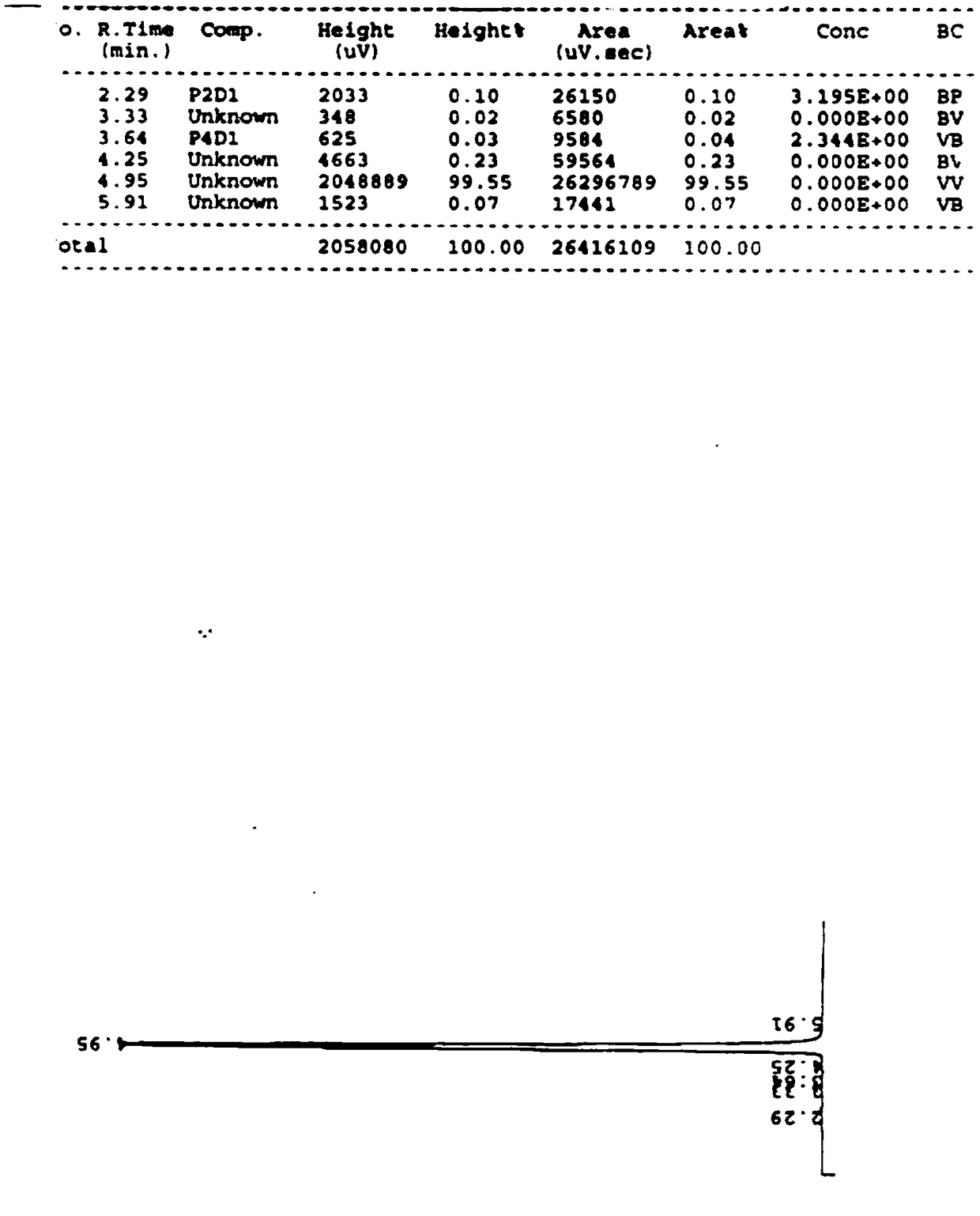
Aminomethylbenzoic acid sample impurity detection device
ActiveCN104198610AImprove weighing accuracyImprove accuracyComponent separationBenzoic acidPhysical chemistry
The invention discloses an aminomethylbenzoic acid sample impurity detection device which comprises a liquid chromatograph, a weighing feeding machine, a mixed quantitative conveying bottle and a simple substance quantitative conveying bottle, wherein the weighing feeding machine consists of an aminomethylbenzoic acid sample weighing feeding machine, a monosodium phosphate weighing feeding machine, a lauryl sodium sulfate weighing feeding machine, a methyl benzoic acid weighing feeding machine and a p-chloromethyl benzoic acid weighing feeding machine. The weighing is all performed in a quantitative manner, the weighing accuracy is improved, the errors are reduced, the measurement accuracy is improved, measurement errors caused by artificial factors are avoided, qualitative and quantitative analysis is achieved, and the repeatability is good.
Owner:DEZHOU BOCHENG PHARMA
Tranexamic acid preparation method
ActiveCN108689870AReduced organic aminesReduce iron contentOrganic compound preparationOrganic chemistry methodsHydrogenation reactionFiltration
The invention discloses a tranexamic acid preparation method, and belongs to the technical field of drug synthesis. The preparation method includes the steps: (1) mixing aminomethylbenzoic acid and pure water, slowly adding concentrated sulfuric acid under the condition of stirring, heating mixture to preset temperature and performing cooling crystallization and filtration to obtain pretreated aminomethylbenzoic acid; (2) adding sulfuric acid solution and catalysts, performing hydrogenation reaction on the pretreated aminomethylbenzoic acid and removing residual sulfuric acid to obtain a hydrogenated product; (3) adding alkali and the pure water into the hydrogenated product, controlling the weight ratio of the hydrogenated product to the alkali to be 1:(3-6), heating the mixture to set temperature, and performing transposition reaction on the hydrogenated product to obtain tranexamic acid. According to the tranexamic acid preparation method, the aminomethylbenzoic acid is pretreated to decrease the content of organic amine and ferrum in raw materials, the service life of the catalysts is prolonged, and production cost is greatly reduced. The adding proportion of the alkali is properly increased, so that the proportion of trans-tranexamic acid in a transposition product, and transposition time is shortened.
Owner:周道平
Aminomethylbenzoic acid freeze-dried powder injection medicine composition for injection
ActiveCN104721153AInhibition of adsorptionAchieve hemostasisPowder deliveryPeptide/protein ingredientsGlycineHepatic Diseases
The invention relates to an aminomethylbenzoic acid freeze-dried powder injection medicine composition for injection. Specifically, on one hand, the invention relates to a freeze-dried powder injection medicine composition. The freeze-dried powder injection medicine composition contains aminomethylbenzoic acid, a freeze-drying excipient such as mannitol, and an optional acid-base regulator; the freeze-drying excipient is selected from saccharose, glucose, mannitol, lactose, sorbitol and glycine, and the weight ratio of the aminomethylbenzoic acid to the freeze-drying excipient is 100: (50 to 500). On the other hand, the invention further relates to a method for preparing the aminomethylbenzoic acid freeze-dried powder injection medicine composition, wherein the method is carried out according to a conventional freeze-dried powder injection medicine preparation method. The aminomethylbenzoic acid freeze-dried powder injection medicine composition for injection disclosed by the invention can be used for treating such bleeding caused by excessive primary fibrinolysis as acute and chronic, local or general high fibrinolytic bleeding which is common in carcinoma, leukaemia, gynaecology and obstetrics accidences, serious liver disease bleeding and the like, and anticipated good properties can be achieved.
Owner:山东北大高科华泰制药有限公司
Preparation method of aminomethylbenzoic acid
InactiveCN108912002AHigh purityHigh yieldOrganic compound preparationAmino-carboxyl compound preparationBenzoic acidDrugs synthesis
The invention discloses a preparation method of aminomethylbenzoic acid, which belongs to the technical field of drug synthesis. The preparation method comprises the following steps: dissolving 4-chloromethyl-benzoic acid in methanol, and obtaining a mixed solution; and adding a concentrated ammonia water into the mixed solution, controlling the temperature at 40 to 80 DEG C, reacting in a reaction container, enabling the 4-chloromethyl-benzoic acid to be converted to 4-aminomethylbenzoic acid, performing the solid-liquid separation, and obtaining aminomethylbenzoic acid. According to the preparation method of the aminomethylbenzoic acid, the methanol is used as the solvent, the 4-aminomethylbenzoic acid with high purity can be prepared without adding a catalyst, fewer byproducts are produced in the reaction process, and the product yield is relatively high; the consumption of the ammonia water is greatly reduced, the recovery cost of the ammonium water is reduced, and the environmental pollution caused by the ammonium can be reduced; and the requirement for equipment is not high, the cost is relatively low, the production process is simple, and mass popularization can be realized.
Owner:周道平
Preparation method of aminomethylbenzoic acid
InactiveCN112047849AHigh purityHigh yieldOrganic compound preparationAmino-carboxyl compound preparationChemical synthesisBenzoic acid
The invention belongs to the technical field of chemical synthesis, and particularly relates to a preparation method of aminomethylbenzoic acid. The method comprises the following steps: carrying outchlorination reaction on p-toluic acid and a chlorination reagent under the condition of a catalyst I to obtain an intermediate p-chloromethylbenzoic acid, and carrying out ammonolysis on the intermediate p-chloromethylbenzoic acid and ammonia water under the condition of a catalyst II to prepare aminomethylbenzoic acid. The purity of the obtained product aminomethylbenzoic acid is greater than 99.9%, the single impurity content is less than 0.1%, and the overall yield of the two-step reaction is greater than 63%. The method is short in synthetic route, free of highly toxic reagents or precious metals, low in production cost, less in environmental pollution, high in overall yield and suitable for large-scale industrial production.
Owner:山东诚汇双达药业有限公司
Aminomethylbenzoic acid and injection composition and quality control method thereof
ActiveCN105929098AInhibition of adsorptionAchieve hemostasisOrganic active ingredientsPeptide/protein ingredientsBenzoic acidTest sample
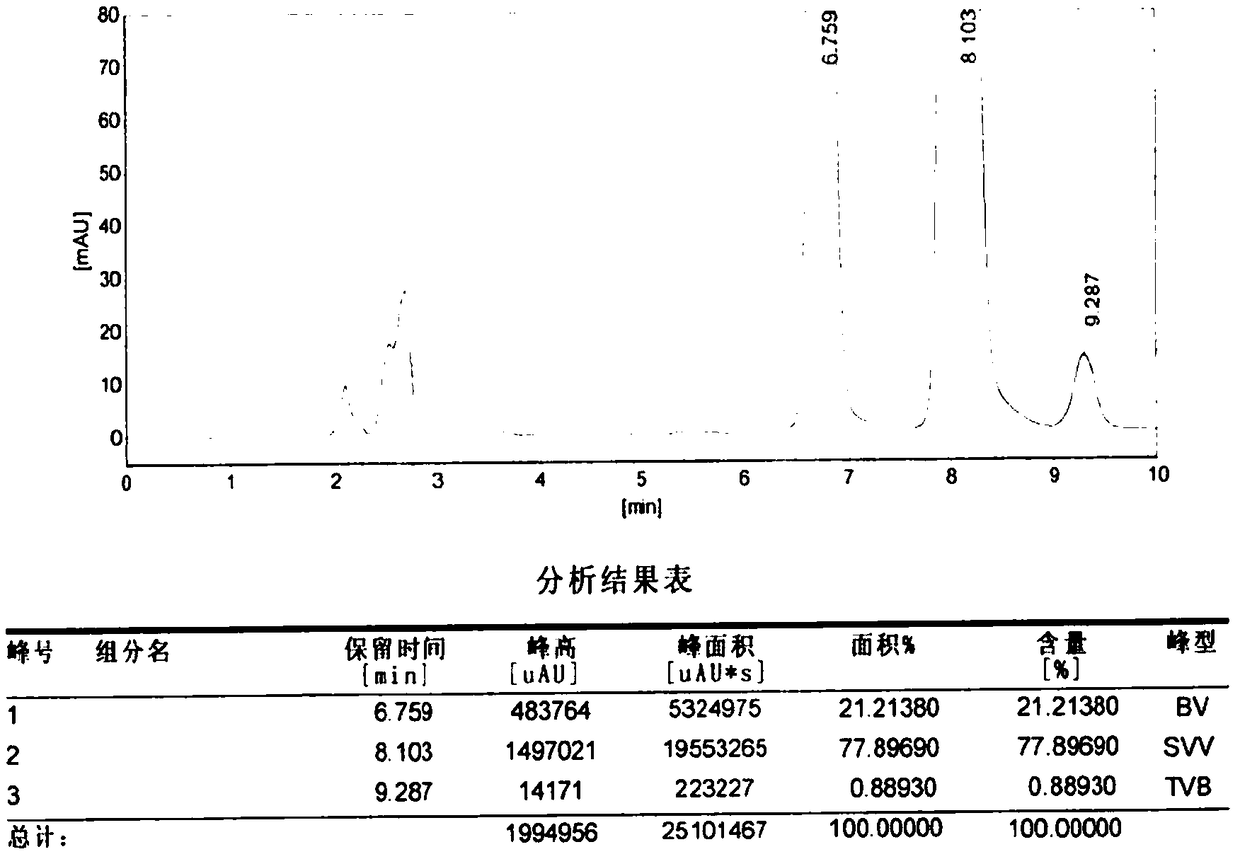
The invention relates to an aminomethylbenzoic acid and injection composition and a quality control method thereof. Particularly, on one hand, the invention relates to a quality control method for an aminomethylbenzoic acid bulk drug or an aminomethylbenzoic acid injection. The method comprises the step of determining the contents of p-methyl benzoic acid and p-carboxyl benzyl chloride in a test sample by virtue of a high performance liquid chromatography. In another embodiment, the method further comprises the step of determining the content of aminomethylbenzoic acid in the test sample by virtue of the high performance liquid chromatography. On the other hand, the invention relates to aminomethylbenzoic acid as a pharmaceutical bulk drug and an injection including aminomethylbenzoic acid and injection water. Quality control on p-methyl benzoic acid and p-carboxyl benzyl chloride in the test sample is realized in a content determination manner by virtue of the high performance liquid chromatography. The method provided by the invention has excellent performances as shown in the specification.
Owner:HUNAN DONGTING PHARMA
Preparation method of 4-methyl-3-[[4-(3-pyridyl)-2-pyrimidyl]amino]benzoic acid, related intermediate and application thereof
ActiveCN101928277ASimple processLow costOrganic chemistryOrganic compound preparationBenzoic acidMethyl palmoxirate
The invention provides a preparation method of 4-methyl-3-[[4-(3-pyridyl)-2-pyrimidyl]amino]benzoic acid. The preparation method comprises the following steps of: carrying out a guanidine-forming reaction on 3-amino-4-methyl toluic acid and cyanamide under the acidic condition of hydrochloric acid to generate 3-[(aminoiminomethyl)amino]-4-methyl-benzoic acid-hydrochloride, and then carrying out a cyclization reaction on the 3-amino-4-methyl toluic acid and cyanamide and 3-(dimethylamino)-1-(3-pyridyl)-2-propylene-1-one to generate the 4-methyl-3-[[4-(3-pyridyl)-2-pyrimidyl]amino]benzoic acid, wherein a structural formula of the 3-[(aminoiminomethyl)amino]-4-methyl-benzoic acid-hydrochloride is shown as the description. The method has short route, simple operation, safe and environmentally-friendly process, repeatability, low cost, high yield, high stability and safety of a guanidine hydrochloride intermediate, suitability for large-scale industrial production and higher economic benefit and social benefit.
Owner:ZHEJIANG JIUZHOU PHARM CO LTD
Method for building small pig model for research on lower limb deep venous thrombus formation and pulmonary embolism
InactiveCN102940537AAccelerates blood clotting processImprove toughnessIn-vivo testing preparationsSurgical veterinaryDiseaseSurgical operation
The invention belongs to the field of animal medical models and particularly relates to a method for building a small pig model for researches on lower limb deep venous thrombus formation and pulmonary embolism. The method is characterized in that the method comprises the steps of firstly forming small pig posterior limb deep venous thrombus, cutting a small pig anterior limb cephalic vein open for intubation, injecting the in-vitro prepared thrombus into a superior vena cava and delivering the thrombus to a pulmonary artery through cardiac circulation to cause pulmonary artery embolism to build the small pig model for researches on lower limb deep venous thrombus formation and pulmonary embolism, wherein aminomethylbenzoic acid injection in appropriate proportion being 1: (15-20) is added during the preparation of the in-vitro thrombus. By building the small pig model for researches on lower limb deep venous thrombus formation and pulmonary embolism, a good animal model can be provided for pathophysiologic researches on diseases, clinical drug researches and surgical practices. The method provided by the invention has the advantages of simple anesthesia operation, clear effect, simple and rapid surgical operation, high pulmonary embolism formation success rate, low fatality rate, convenient inspection means and the like.
Owner:JINHUA MUNICIPAL CENT HOSPITAL
Care medicine composition used before operative anesthesia and preparation method thereof
InactiveCN105535489AImprove immunityReduce tensionAntibacterial agentsNervous disorderAdditive ingredientCefuroxime
The invention discloses a care medicine composition used before operative anesthesia and a preparation method thereof. The medicine composition is composed of Chinese herbal medicinal ingredients and Western medicinal ingredients. The Chinese herbal medicinal ingredients mainly comprise fruits of Celastrus rosthornianus Loes, sow thistle flower and seeds, rhapis excelsa, the root of Laxleaf Beautifulflower Millettia, all-grass of auriculate dichrocephala, pine among the Indian Bread, fructus terminaliae billericae, aizoon stonecrop herb, valerian, sandalwood extract and crowndaisy chrysanthemum extract. The Western medicinal ingredients mainly comprise aminomethylbenzoic acid, diaminocaproic acid, taurine and cefuroxime. The care medicine composition is scientifically matched according to different medicine effects and characteristics of Chinese and western medicine, is applied to according to indications, has the effects of soothing nerves and calming heart, relieving pain and removing swelling, resisting bacteria and diminishing inflammation, stopping bleeding, enhancing human body immunocompetence and resisting virus infection, and effectively eases preoperative tension, anxiety, fear and other psychic reactions of a patient. The care medicine composition used before operative anesthesia is simple in preparation technology, remarkable in treatment effect and gentle in medicine effect and has the good medical value.
Owner:张宝英
Amidite for nucleic acid synthesis and nucleic acid synthesizing method
To provide an amidite for nucleic acid synthesis, which enables a protective group therein to be removed under moderate conditions and can be practically used, and a nucleic acid synthesizing method using the amidite for nucleic acid synthesis. Specifically, the present invention relates to an amidite for nucleic acid synthesis represented by General Formula (I) below, and a nucleic acid synthesizing method using the amidite for nucleic acid synthesis:where X denotes a base; Y denotes a protective group formed of any one of a 4-aminobutyric acid derivative, an o-aminomethylbenzoic acid derivative, an o-aminophenylacetic acid derivative, an o-aminoethylbenzoic acid derivative, an o-aminomethylphenylacetic acid derivative, an o-aminophenylpropionic acid derivative and a 5-aminovaleric acid derivative; and Q denotes one of a hydrogen atom and a hydroxyl group.
Owner:APTA BIOSCI
Preparation method of nano anti-tissue-adhesion fiber membrane having anti-tumor function
InactiveCN107596457AReduce the pain of radiotherapy and chemotherapyExtend the life cycleMonocomponent protein artificial filamentSurgeryFiberUpconversion luminescence
The invention discloses a preparation method of a nano anti-tissue-adhesion fiber membrane having an anti-tumor function. Soluble nano-fibers, which have hemostasis effect, are prepared through electro-spinning technology in a compounding manner from raw materials including: up-conversion luminescent nano-particles, such as NaYF4:Er / Yb, NaYF4:Tm / Yb, YVO4:Er / Yb, NaYF4:Sm / Nd, which have anti-cancereffects, and a hemostasis medicine, such as Baquting, hemocoagulase and aminomethylbenzoic acid, etc. During a process of excision of cancer lesions, quick hemostasis is achieved, and tissue adhesionis prevented during postoperation. Through the optical-thermal physical therapy function of the up-conversion luminescent nano-particles, in-situ recurrence of cancer tissue is inhibited.
Owner:DALIAN JIAOTONG UNIVERSITY
A kind of method for preparing tranexamic acid
ActiveCN107954887BProcess for reducing acid adjustmentTransposition reaction time shortenedOrganic compound preparationOrganic chemistry methodsHydrogenation reactionToluene
The invention relates to a method for preparing tranexamicacid. The method comprises the following steps: adding the aminomethylbenzoic acid, water, concentrated sulfuric acid and a catalyst into a reaction vessel for stirring and heating; then introducing hydrogen for carrying out hydrogenation reaction to obtain hydrogenated reaction liquid; adding the hydrogenated reaction liquid and the concentrated sulfuric acid into the reaction vessel, raising the temperature to 180 to 200 DEG C, and carrying out heat-preservation stirring and conversion reaction to obtain the tranexamicacid. The methodfor preparing the tranexamicacid, disclosed by the invention, has the advantages of simplified technology, short reaction time and high synthesis efficiency.
Owner:CHANGZHOU YINSHENG PHARMA
Preparation method of high-strength and high-water absorption craft woven paper
InactiveCN110016832AImprove writing effectImprove efficiencySpecial paperMicroorganism/enzyme additionPotato starchBran
The invention belongs to the technical field of art crafts, and particularly relates to a preparation method of high-strength and high-water absorption craft woven paper. The preparation method comprises the following specific steps: (1) weighing raw materials, (2) mixing cotton shells with soybean straw, performing crushing, performing steam treatment, freezing treatment and steam treatment, andconducting cooling to room temperature so as to obtain a straw-shell material, (3) soaking corn bran in an acetic acid aqueous solution, performing cold storage treatment, mixing the obtained corn bran with gumbo leaf fermentation broth, performing crushing and pulping, adding the straw-shell material, and performing even mixing so as to obtain a pulp material, (4) adding algal polysaccharides, Sargassum fusiforme polysaccharides, modified potato starch and modified lotus root starch to the pulp material, performing heating with gentle fire, adding aminomethylbenzoic acid, sodium sulfamate andnano zinc oxide, performing homogenizing treatment, and performing paper making and drying. The water absorption performance of the paper can be ensured effectively, the paper is flexible and elastic, and has high structural stability and resistance to cracking and tearing, and smooth printing and writing can also be maintained effectively, so that the efficiency and performance of application ofthe craft woven paper are improved greatly.
Owner:阜南县金源柳木工艺品有限公司
Pharmaceutical composition of tranexamic acid
ActiveCN103405384APeptide/protein ingredientsPharmaceutical delivery mechanismOrganic acidAminomethylbenzoic acid
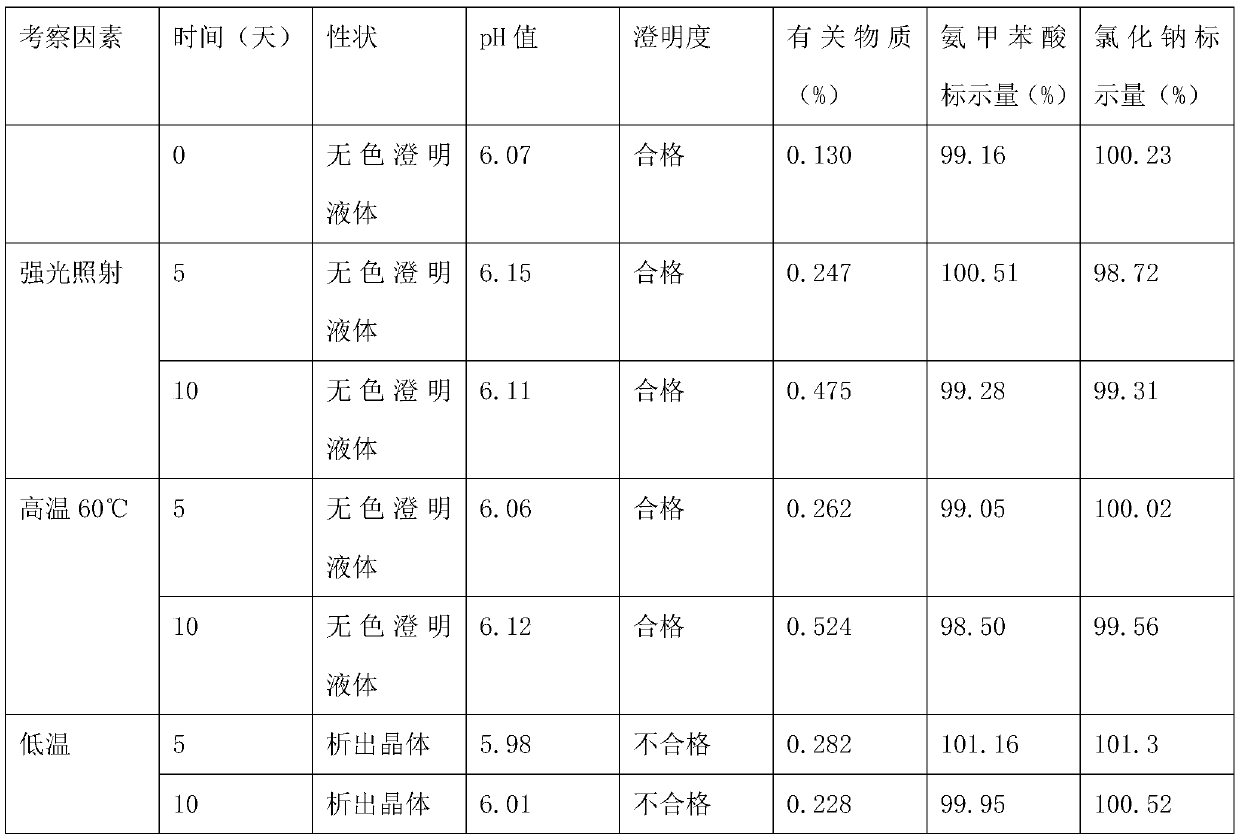
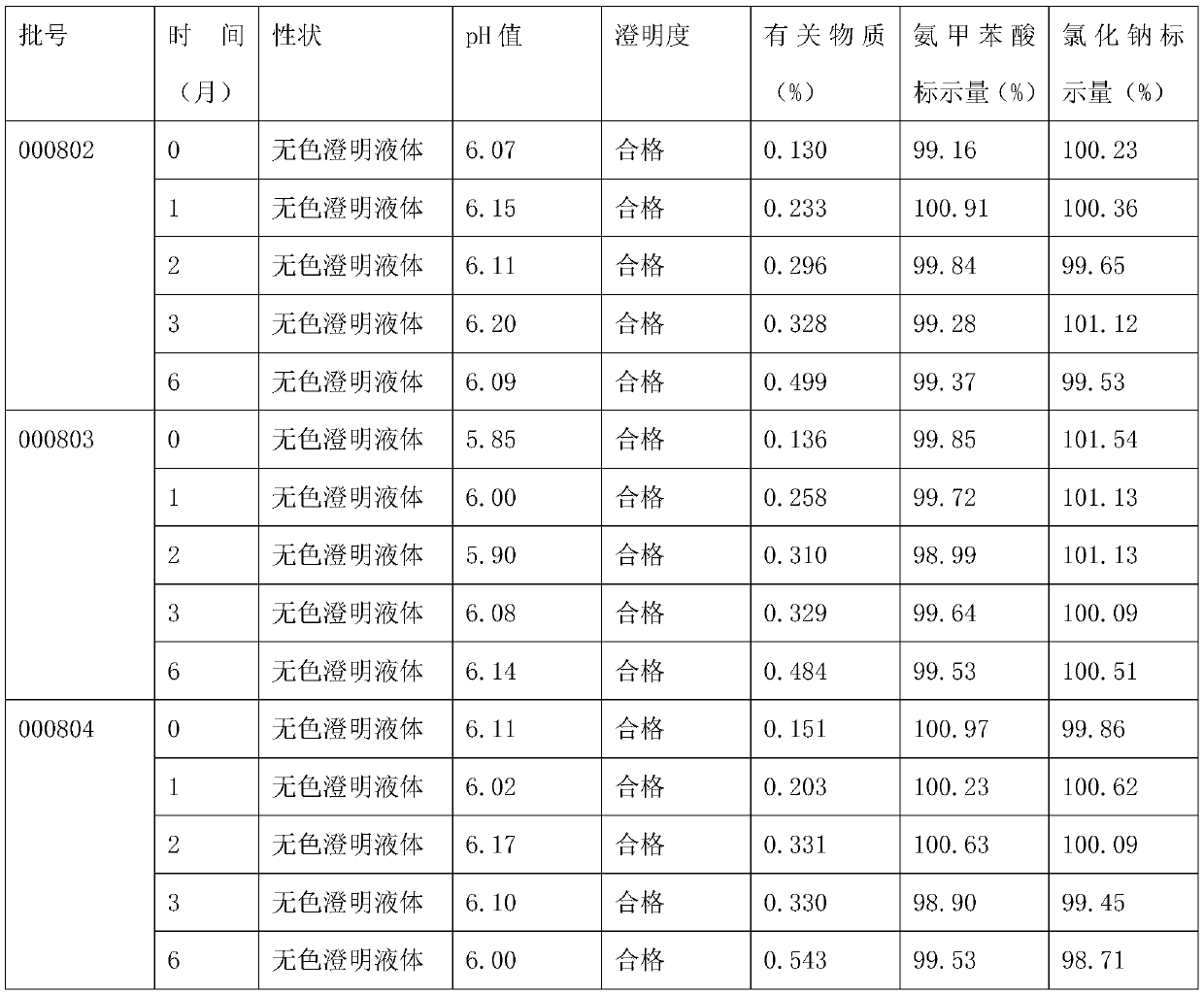
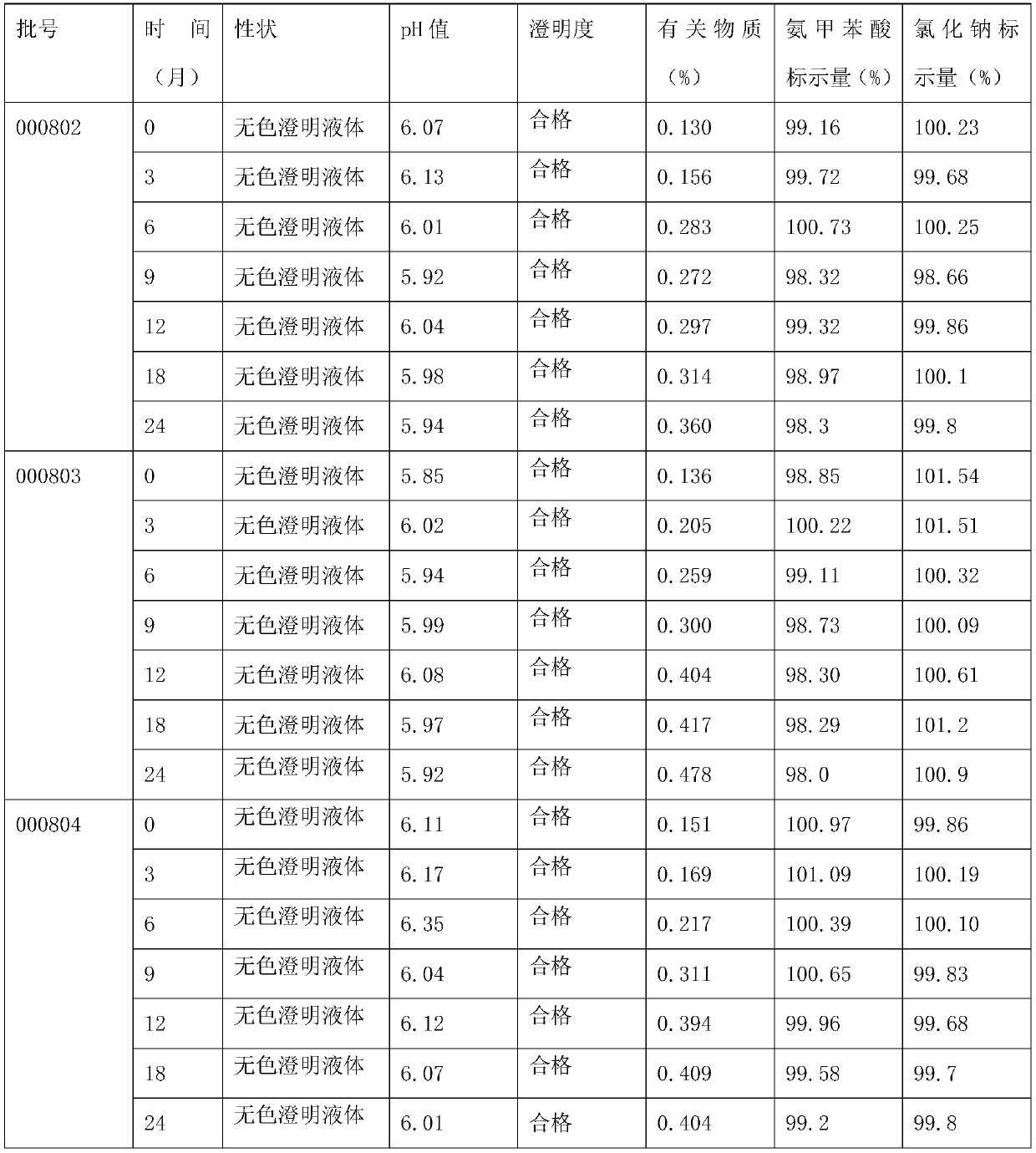
The invention belongs to the technical field of medicines, discloses a pharmaceutical composition of tranexamic acid, and particularly relates to a pharmaceutical composition containing tranexamic acid and organic acid. The pharmaceutical composition can be prepared into injection; the high-temperature and high-light tests show that the impurities, namely, cycloolefin, aminomethylbenzoic acid and a Z-isomer, and the total impurities of the injection meet the quality requirements. A preparation prepared from the pharmaceutical composition has the advantages of high stability and low impurity content.
Owner:乐信乐美(海南)生物科技有限公司
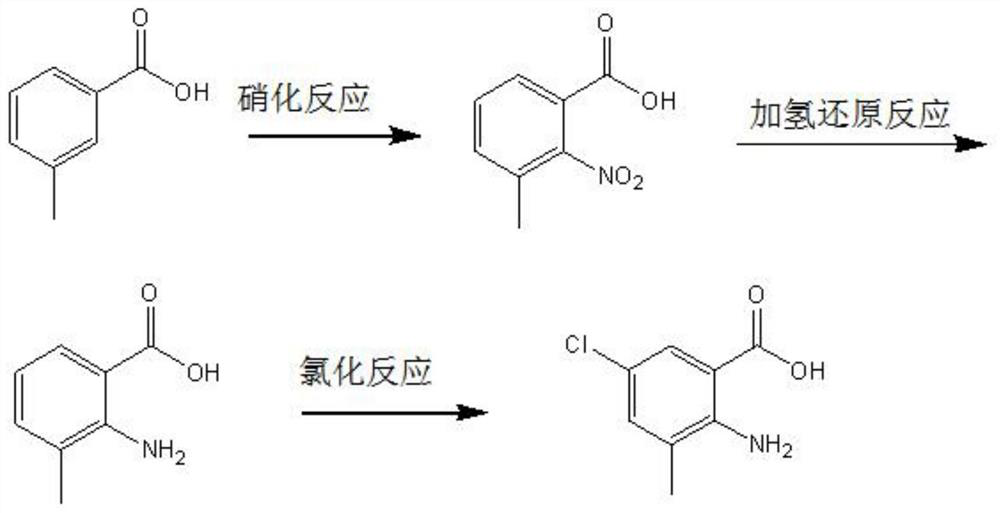
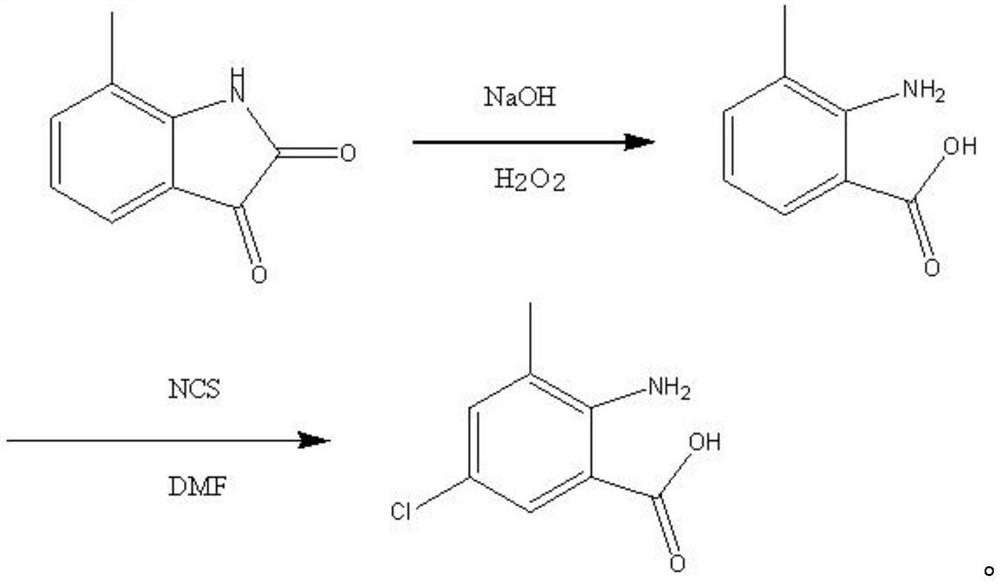
Preparation method of 2-amino-3-methyl-5-chlorobenzoic acid
ActiveCN112778147AHigh yieldImprove qualityOrganic compound preparationAmino-carboxyl compound preparationBenzoic acidBenzoyl peroxide
The invention belongs to the technical field of organic synthesis, and particularly relates to a preparation method of 2-amino-3-methyl-5-chlorobenzoic acid. The preparation method provided by the invention comprises the steps of mixing m-toluic acid and nitric acid, and carrying out nitration reaction to obtain 2-nitro-3-methyl benzoic acid, wherein the mass concentration of the nitric acid is 60-75%; mixing the 2-nitro-3-methyl benzoic acid, a hydrogenation reduction reaction solvent and a hydrogenation catalyst, and carrying out hydrogenation reduction reaction in a hydrogen atmosphere to obtain 2-amino-3-methyl benzoic acid; and mixing the 2-amino-3-methyl benzoic acid, a chlorination reagent, benzoyl peroxide and a chlorination reaction solvent, and carrying out a chlorination reaction to obtain the 2-amino-3-methyl-5-chlorobenzoic acid. The preparation method provided by the invention has the advantages of cheap and easily available reaction raw materials, high product yield and high purity, and is easy for industrial production.
Owner:锦州三丰科技有限公司
Aminomethylbenzoic acid sodium chloride injection and preparation method thereof
InactiveCN110215432AOrganic active ingredientsPeptide/protein ingredientsActivated carbonSodium Chloride Injection
The invention discloses an aminomethylbenzoic acid sodium chloride injection and a preparation method thereof. Raw materials of the injection are composed of: 5-7 kg of aminomethylbenzoic acid, 26-28kg of sodium chloride, 1.1-1.3 kg of activated carbon, and water for injection and the total being 3000000 ml. The invention provides the stable and safe aminomethylbenzoic acid sodium chloride injection, solves the technical problem that aminomethylbenzoic acid sodium chloride is stable and sensitive to light, and provides the safe aminomethylbenzoic acid sodium chloride injection for a clinicalapplication.
Owner:辽宁海神联盛制药有限公司
A kind of preparation method of tranexamic acid
ActiveCN108689870BReduced organic aminesReduce iron contentOrganic compound preparationOrganic chemistry methodsPtru catalystHydrogenation reaction
The invention discloses a tranexamic acid preparation method, and belongs to the technical field of drug synthesis. The preparation method includes the steps: (1) mixing aminomethylbenzoic acid and pure water, slowly adding concentrated sulfuric acid under the condition of stirring, heating mixture to preset temperature and performing cooling crystallization and filtration to obtain pretreated aminomethylbenzoic acid; (2) adding sulfuric acid solution and catalysts, performing hydrogenation reaction on the pretreated aminomethylbenzoic acid and removing residual sulfuric acid to obtain a hydrogenated product; (3) adding alkali and the pure water into the hydrogenated product, controlling the weight ratio of the hydrogenated product to the alkali to be 1:(3-6), heating the mixture to set temperature, and performing transposition reaction on the hydrogenated product to obtain tranexamic acid. According to the tranexamic acid preparation method, the aminomethylbenzoic acid is pretreated to decrease the content of organic amine and ferrum in raw materials, the service life of the catalysts is prolonged, and production cost is greatly reduced. The adding proportion of the alkali is properly increased, so that the proportion of trans-tranexamic acid in a transposition product, and transposition time is shortened.
Owner:周道平
Method for converting 4,4'-dicarboxyl dibenzylamine into p-methylbenzoic acid and p-aminomethylbenzoic acid by debenzylation
InactiveCN107188796ASimple processReduce processing costsOrganic compound preparationAmino-carboxyl compound preparationBenzoic acidAminomethylbenzoic acid
In a hemostatic tranexamic acid production process, the chlorination single-pass conversion rate of p-methylbenzoic acid is normally controlled in a range of 65-70% in order to control impurity generation, which causes that 30-35% of p-methylbenzoic acid does not participate in reaction; further, impurities such as p-dichloromethyl benzoic acid are inevitably generated in a chlorination process; in an ammoniation process, di-substituted and tri-substituted side reactions are generated besides generation of p-aminomethylbenzoic acid, and thus a great quantity of solid wastes are generated, wherein by analysis, the solid wastes include 5% of p-aminomethylbenzoic acid, 13% of p-formylbenzoic acid, 35% of p-methylbenzoic acid, 28% of 4,4'-dicarboxyl dibenzylamine, 3% of 4,4',4''-tricarboxyl tribenzylamine, 11% of N-(p-aminomethylbenzoyl)-aminomethylbenzoic acid and 5% of others. The invention provides a method for converting 4,4'-dicarboxyl dibenzylamine into p-methylbenzoic acid and p-aminomethylbenzoic acid by hydrogenation debenzylation in presence of catalysts, and the recovery rate is not lower than 90%.
Owner:CHANGZHOU UNIV
Preparation method of aminomethylbenzoic acid injection
InactiveCN104224696AGuaranteed sterilization effectAdequate sterility assurancePeptide/protein ingredientsPharmaceutical delivery mechanismNitrogenBottle
The invention discloses a preparation method of an aminomethylbenzoic acid injection. The method comprises the following steps: 1, adding 50% of newly boiled injection water to a thick mixing tank, adding aminomethylbenzoic acid, stirring and dissolving; 2, adding medicinal carbon and stirring evenly; 3, adding injection water to full dose, and stirring for 15 minutes; 4, filtering by a Suzhou sand rod and a polyether sulfone folding filter of 0.33 micron; 5 sampling and detecting visible foreign particles; 6 filtering a liquid medicine in a storage tank by a polyether sulfone folding filter of 0.41 micron, sampling and detecting the visible foreign particles to be qualified, and then inputting into a base bottle for encapsulating; 7 introducing nitrogen into the base bottle for encapsulating; and 8 sterilizing at the temperature of 121 DEG C for 15 minutes. According to the preparation method, dual sterile filtration is adopted, and the sterilization effect is ensured.
Owner:SHANGHAI XINYI JINZHU PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
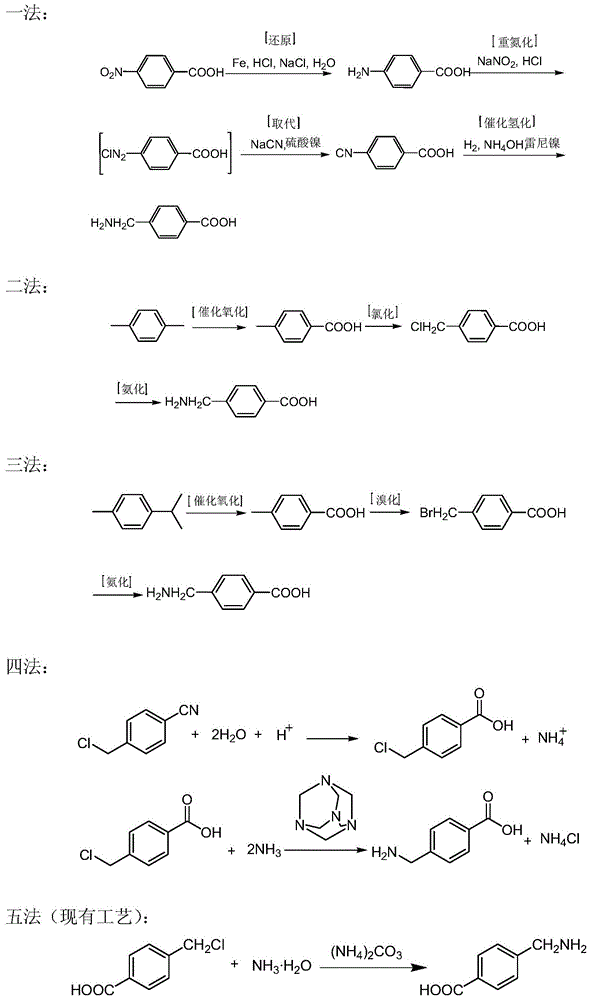

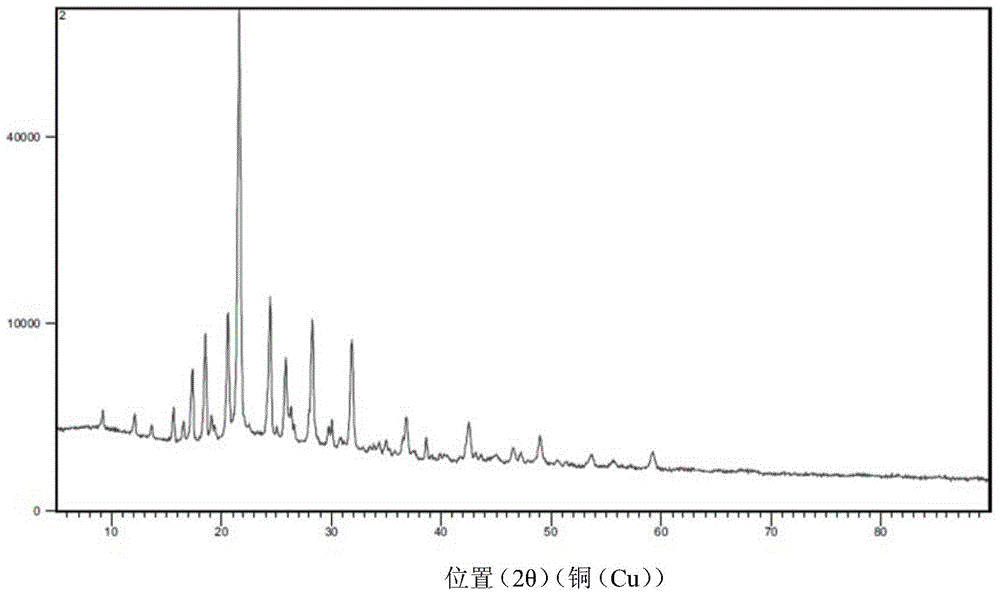













![Preparation method of 4-methyl-3-[[4-(3-pyridyl)-2-pyrimidyl]amino]benzoic acid, related intermediate and application thereof Preparation method of 4-methyl-3-[[4-(3-pyridyl)-2-pyrimidyl]amino]benzoic acid, related intermediate and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a80a22c8-4078-4726-ba46-b0c4d0b99c59/000001.png)
![Preparation method of 4-methyl-3-[[4-(3-pyridyl)-2-pyrimidyl]amino]benzoic acid, related intermediate and application thereof Preparation method of 4-methyl-3-[[4-(3-pyridyl)-2-pyrimidyl]amino]benzoic acid, related intermediate and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a80a22c8-4078-4726-ba46-b0c4d0b99c59/000002.png)
![Preparation method of 4-methyl-3-[[4-(3-pyridyl)-2-pyrimidyl]amino]benzoic acid, related intermediate and application thereof Preparation method of 4-methyl-3-[[4-(3-pyridyl)-2-pyrimidyl]amino]benzoic acid, related intermediate and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a80a22c8-4078-4726-ba46-b0c4d0b99c59/000003.png)