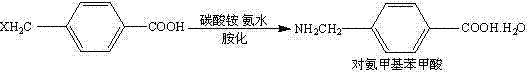
Application of urotropine as catalyst in aminomethylbenzoic acid synthesis
A technology for synthesizing aminotoluic acid and catalysts, applied in the preparation of organic compounds, cyanide reaction preparation, organic chemistry, etc., can solve the problems of high industrial production costs and low product yields, and achieve increased product yields and reduced production costs , the effect of reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] At 10°C, 33.6g (0.24mol) of urotropine and 54.0g (3.0mol) of water were put into the reactor, stirred until completely dissolved, then 34.2g (0.2mol) of p-chloromethylbenzoic acid was added, stirred Evenly, pass ammonia gas until the reaction solution is completely dissolved, then heat to 40 ° C for 4 hours, and finally evaporate the ammonia under reduced pressure to pH 7-8, cool and crystallize, filter, and dry to obtain 21.1 g of aminotoluic acid (mol Yield 62.4%).
Embodiment 2
[0026] At 22°C, 44.9g (0.32mol) of urotropine and 61.2g (3.4mol) of water were put into the reactor, stirred until completely dissolved, then 34.2g (0.2mol) of p-chloromethylbenzoic acid was added, stirred Evenly, pass ammonia gas until the reaction solution is completely dissolved, then heat to 50°C and react for 4 hours, and finally evaporate the ammonia under reduced pressure to pH 7-8, cool and crystallize, filter, and dry to obtain 22.3 g of aminotoluic acid (mol Yield 65.9%).
Embodiment 3
[0028] At 30°C, 56.1g (0.40mol) of urotropine and 64.8g (3.6mol) of water were put into the reactor and stirred until it was completely dissolved, then 34.2g (0.2mol) of p-chloromethylbenzoic acid was added and stirred Evenly, pass ammonia gas until the reaction solution is completely dissolved, then heat to 60°C and react for 3 hours, and finally evaporate the ammonia under reduced pressure to pH 7-8, cool and crystallize, filter, and dry to obtain 21.8 g of aminotoluic acid (mol Yield 64.4%).
[0029] The results of the first six applications are shown in the table below:
[0030]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com