Tranexamic acid preparation method
A technology of tranexamic acid and aminotoluic acid, which is applied in the preparation of organic compounds, organic chemical methods, cyanide reaction preparation, etc., can solve the problems of reducing the service life of platinum catalysts, increasing production costs, and reducing production efficiency. The effect of shortening the indexing time, reducing the pressure on environmental protection and improving the service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A preparation method of tranexamic acid in the embodiment of the present invention, comprising:
[0034] (1) Mix 50 kg of aminotoluic acid with 300 L of pure water, slowly add 10 L of concentrated sulfuric acid under stirring conditions, heat to 100 ° C, dissolve the aminotoluic acid, cool and crystallize, and filter to obtain the pretreated aminotoluic acid;
[0035] (2) add sulfuric acid solution and metallic platinum catalyst, adjust pH to 1~3, carry out hydrogenation reaction to the pretreated aminotoluic acid, remove redundant sulfuric acid, obtain hydrogenation product;
[0036] (3) Add barium hydroxide and pure water to the hydrogenation product, control the mass ratio of the hydrogenation product to barium hydroxide to 1:3, raise the temperature to 200°C, and perform high-temperature transposition reaction on the hydrogenation product. The reaction time is 10h, to obtain tranexamic acid.
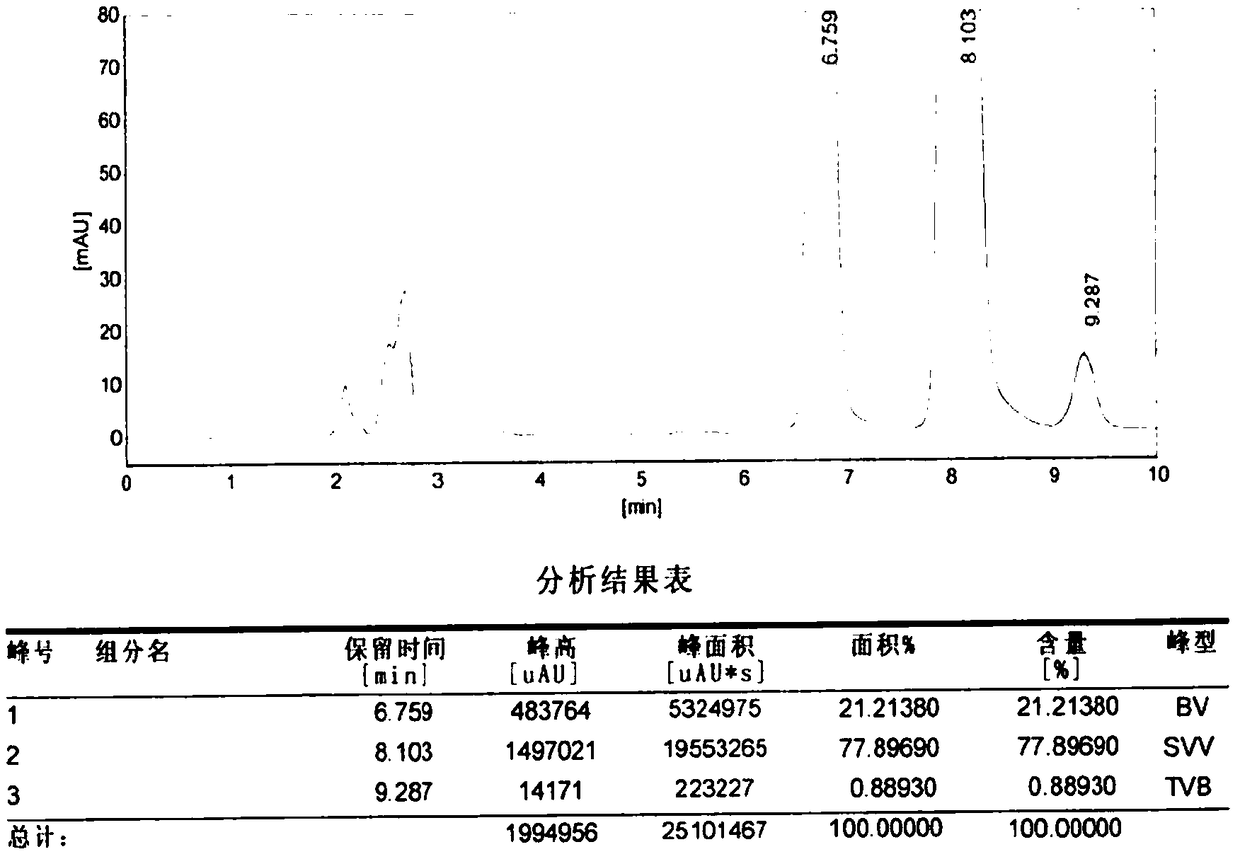
[0037] Table 1 is the high performance liquid chromatography (HPLC) resu...
Embodiment 2
[0045] A preparation method of tranexamic acid in the embodiment of the present invention, comprising:
[0046] (1) Mix 50 kg of aminotoluic acid with 300 L of pure water, slowly add 10 L of concentrated sulfuric acid under stirring conditions, heat to 105° C., dissolve the aminotoluic acid, cool and crystallize, and filter to obtain the pretreated aminotoluic acid;
[0047] (2) add sulfuric acid solution and metallic platinum catalyst, adjust pH to 1~3, carry out hydrogenation reaction to the pretreated aminotoluic acid, remove redundant sulfuric acid, obtain hydrogenation product;
[0048] (3) Add barium hydroxide and pure water to the hydrogenation product, control the mass ratio of the hydrogenation product to barium hydroxide to 1:4, raise the temperature to 190°C, and perform high-temperature transposition reaction on the hydrogenation product. The reaction time is 10h, to obtain tranexamic acid.
[0049] Aminotoluic acid raw material is through pretreatment, and the hi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com