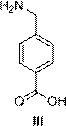
Preparation method of aminomethylbenzoic acid
A technology of aminotoluic acid and chloromethylbenzoic acid is applied in the preparation of carboxylates, the preparation of organic compounds, the preparation of cyanide reactions, etc., and can solve the problem that the reaction system is difficult to handle, the chlorine chlorination efficiency is low, and the overall yield is low. and other problems, to achieve the effect of low production cost, low production cost and high overall yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Synthesis of intermediate p-chloromethylbenzoic acid (II):
[0040] Dissolve p-toluic acid (490g) and azobisisobutyronitrile (8.52g) in dichloroethane (2200g), raise the temperature of the reaction system to 50-60°C, then add dropwise at a temperature of 30-100°C Sulfonyl chloride (1000g), a large amount of gas will be released after the reaction is triggered, and the dropping rate should be controlled to prevent material flushing. After the reaction system is reacted at 50-60°C for 3-4 hours, less than 5% of the raw materials remain. The reaction system was concentrated to dryness under reduced pressure, and the obtained fractions were recovered and used mechanically (the recovery rate was more than 70%), and the obtained solids were directly used in the next step without purification.
[0041] (2) Synthesis of aminomethylbenzoic acid (III)
[0042] Dissolve the solid (612g) obtained in step (1) in a mixed solvent of methanol (1200g) and water (270g), add urotrop...
Embodiment 2
[0044] (1) Synthesis of intermediate p-chloromethylbenzoic acid (II):
[0045] Dissolve p-toluic acid (100 kg) and azobisisobutyronitrile (1.74 kg) in dichloroethane (450 kg), raise the temperature of the reaction system to 50-60°C, and then control the temperature to 30-100°C Add sulfuryl chloride (204 kg) dropwise, a large amount of gas will be released after the reaction is initiated, and the dropping rate should be controlled to prevent material flushing. After the reaction system is reacted at 50-60°C for 4-6 hours, less than 5% of the raw materials remain. The reaction system was concentrated to dryness under reduced pressure, and the obtained fractions were recovered and used mechanically (the recovery rate was more than 75%), and the obtained solids were directly used in the next step without purification.
[0046] (2) Synthesis of aminomethylbenzoic acid (III)
[0047] Dissolve the solid obtained in step (1) in a mixed solvent of methanol (245 kg) and water (55 kg), a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com