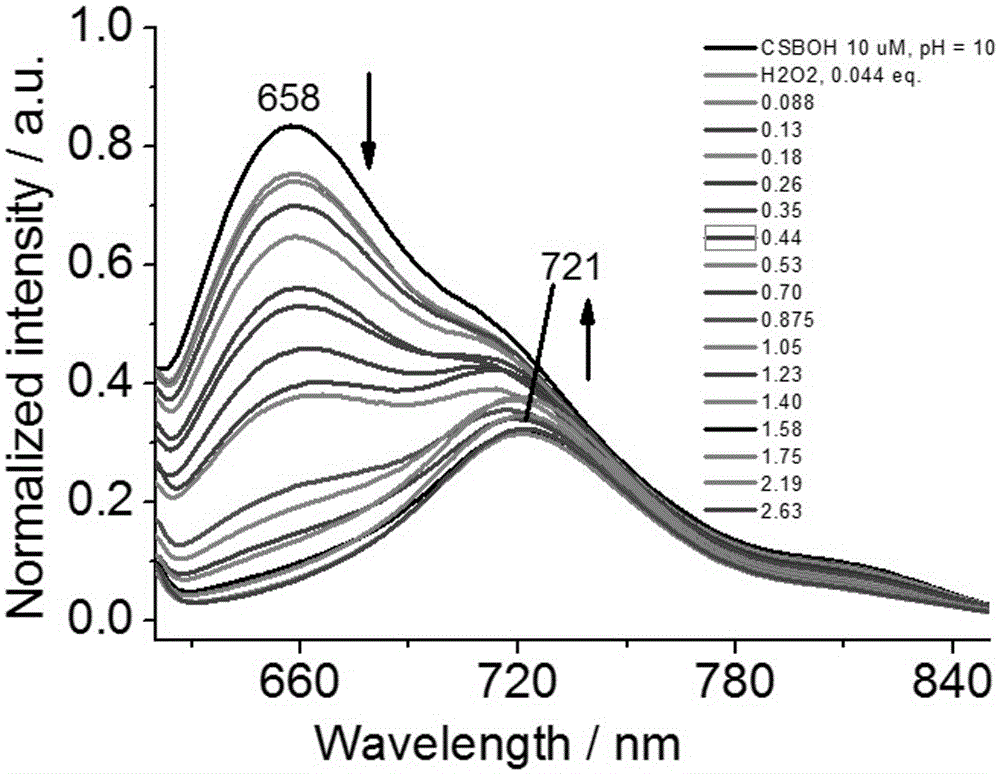
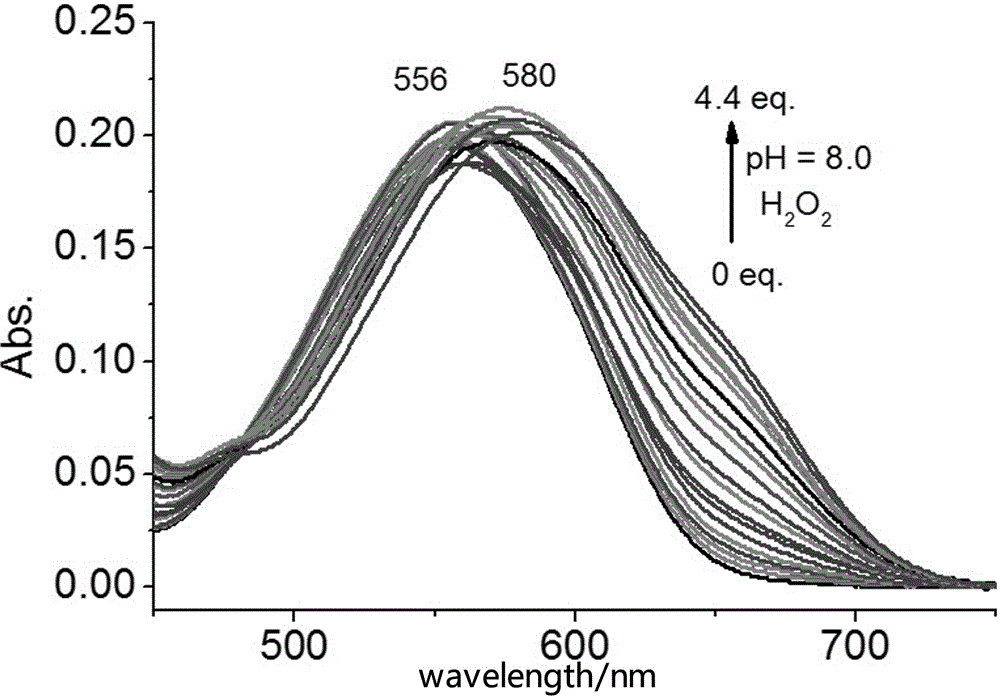
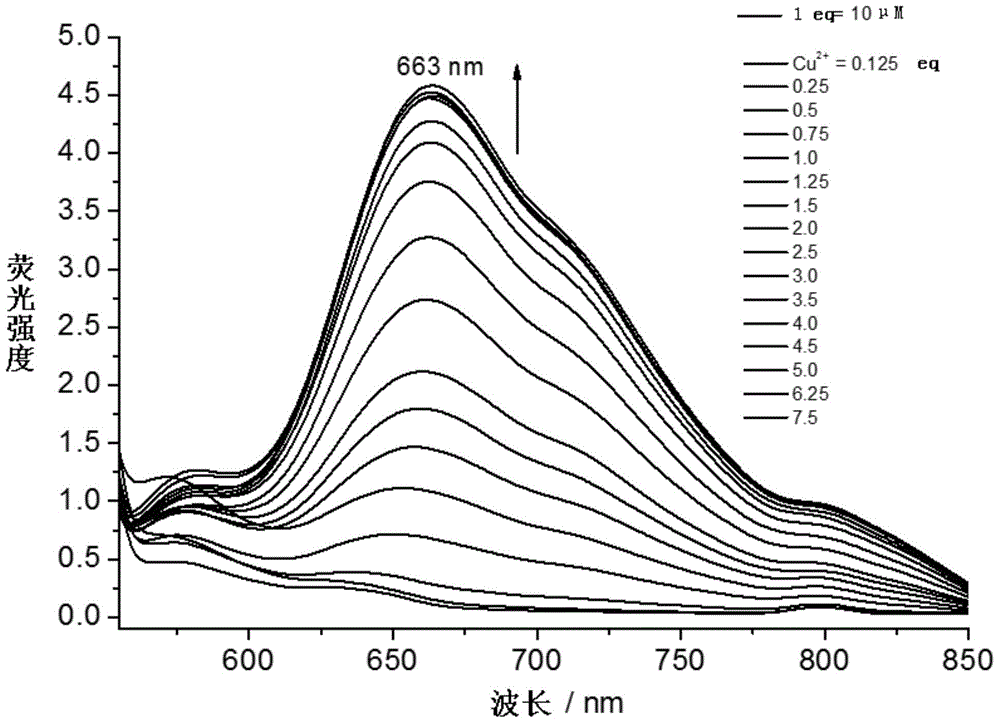
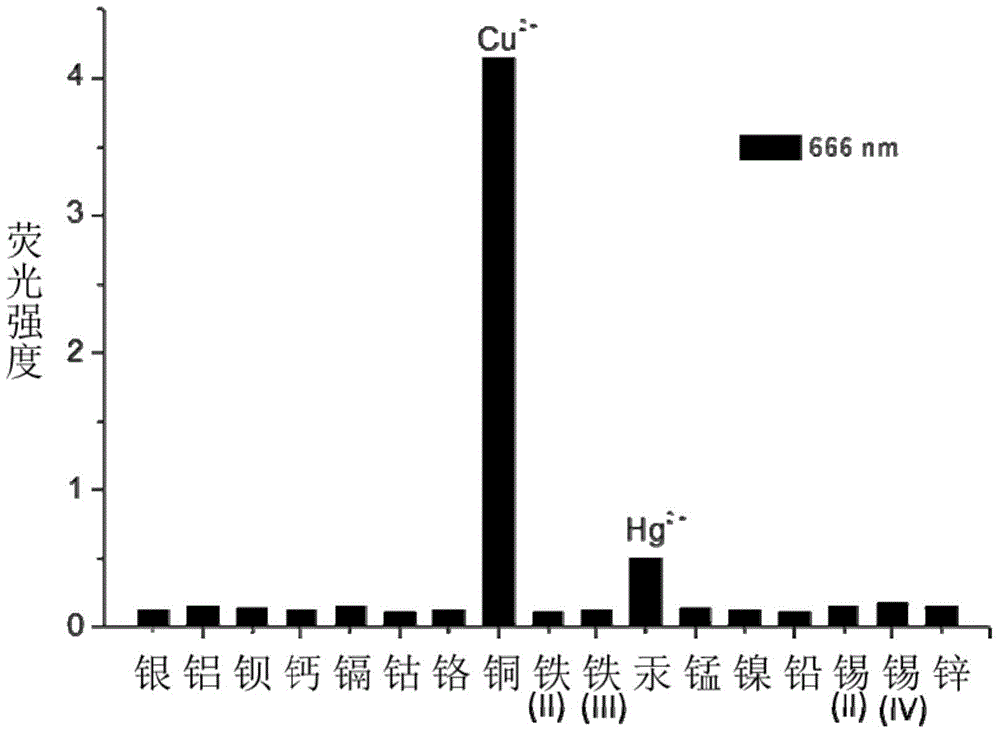
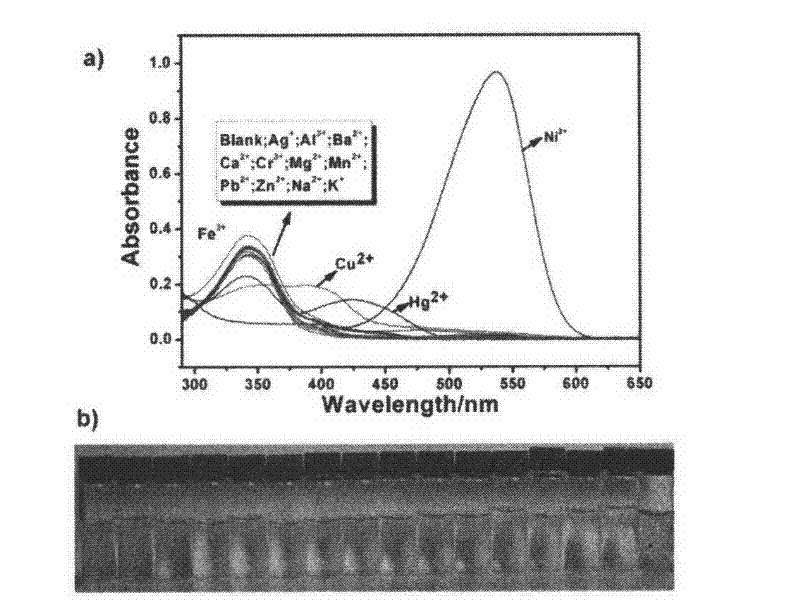
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
194results about How to "Obvious phenomenon" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Novel fluorescence probe for detecting hydrogen peroxide in alkaline environment and preparation method and biological application thereof
InactiveCN105001858AHigh selectivityHigh detection sensitivityGroup 3/13 element organic compoundsFluorescence/phosphorescenceOrganic solventMolecular probe
The invention discloses a novel fluorescence probe for detecting hydrogen peroxide in an alkaline environment and a preparation method thereof, and belongs to the technical field of molecular probes. The structural formula of the probe is as follow (please see the formula in the specification), and the preparation method of the hydrogen peroxide fluorescence probe is simple. The probe detects the hydrogen peroxide in the mode of fluorescence enhancement and obvious color change and can recognize the hydrogen peroxide high selectively in a water system or an organic solvent system or an organism; the fluorescence of the probe itself is weaker, an obtained solution is purple after the probe is added in water or organic solvent, and the fluorescence of the solution is obviously enhanced and the color of the solution is changed into green after the probe acts with the hydrogen peroxide. The selectivity to hydrogen peroxide detection is high, the detection sensitivity is high, and a phenomenon is obvious and convenient to recognize.
Owner:UNIV OF JINAN
Failure point positioning method for semiconductor power device failure analysis
InactiveCN102854429AConvenient to judge timeViolent reactionElectrical testingElectrical resistance and conductanceLight beam
The invention provides a failure point positioning method for semiconductor power device failure analysis. The failure point positioning method comprises the following steps of: performing chemical corrosion on a metal aluminum layer covering the surface of a power device by utilizing a chemical corrosion stripping technology, completely removing the aluminum layer, and completely keeping a barrier layer below the metal aluminum layer; positioning the front side of the power device by utilizing a micro light microscope and a light beam induced resistance variation technology, simulating electric conditions under the failure conditions, electrifying by using a point needle method, simulating the electric conditions, and finding possible failure points; and performing physical verification of electronic package assembly failure analysis by utilizing the positioning result of an electronic package assembly failure analysis tool on the previous steps, and finding the final physical failure point. The failure point positioning method has the advantages that the metal aluminum layer is effectively stripped, and the integrity of the barrier layer is kept; and moreover, the positioning speed and efficiency of the semiconductor power device failure point are greatly improved, and an extremely high precision is kept.
Owner:SHANGHAI FALAB TEST
Polythymine template, fluorescent copper nano-cluster based on same, preparation method of fluorescent copper nano-cluster and ATP detection method
InactiveCN105087765ATightly boundHigh fluorescence stabilityMicrobiological testing/measurementDNA preparationFluorescenceSingle strand dna
The invention provides a polythymine template, a fluorescent copper nano-cluster based on the same, a preparation method of the fluorescent copper nano-cluster and an ATP detection method. The polythymine template has a single-chain DNA sequence and comprises at least one polythymine sequence which is (T)n, wherein n refers to natural number ranging from 15 to 40. The polythymine template can be used for preparing the fluorescent copper nano-cluster, preparation cost is low, and operation is simple and convenient. The ATP detection method is low in cost, simple and easy-to-implement in detection step, obvious in phenomenon, easy for observation and capable of quickly detection concentration of ATP in a sample in a high-sensitivity manner.
Owner:SHENZHEN INST OF ADVANCED TECH
Inorganic/organic hybrid photochromic material, preparation method and application
ActiveCN102965095AChange color quicklyObvious phenomenonTenebresent compositionsBenzyl ViologenPhotochromic sunglasses
The invention relates to an inorganic / organic hybrid photochromic material, a preparation method and an application, which belongs to the inorganic / organic hybrid photochromic material field, and relates to a synthetic method and an application of a benzyl viologen bismuth chloride inorganic / organic hybrid photochromic compound. The benzyl viologen bismuth chloride inorganic / organic hybrid photochromic compound has excellent photochromism performance, is fast to change color under the irradiation of UV and visible light, and also fast to fade while being heated and placed in a darkroom, the material can be used in the fields of light information storage, protection decoration, fake resistance and authentic identification, secrecy, optical switch device, light information converter, photochromic sunglass lens used for radiation protection, diaphragm material and the like.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
Novel ratio type sulfite fluorescent probe as well as preparation method and biological application thereof
InactiveCN106674183AHigh selectivityHigh detection sensitivityOrganic chemistryFluorescence/phosphorescenceOrganic solventFluorescence
The invention discloses a novel ratio type sulfite fluorescent probe as well as a preparation method and biological application thereof. The structural formula of the probe is shown as a formula in the description. The probe has very high fluorescent light; after the probe is added into water or an organic solvent, an obtained solution is blue; after the probe acts with sulfite, the color of the solution is gradually faded and the color of the solution becomes green fluorescent. A synthesis process of the probe is simple and the yield of a prepared product is high. The novel ratio type sulfite fluorescent probe can be applied to a water body system, an organic solvent system or a biological system, and the sulfite is detected in a manner of enhancing and weakening fluorescent light of two wave bands and obviously changing the color; the novel ratio type sulfite fluorescent probe is high in selectivity and high in sensitivity.
Owner:UNIV OF JINAN
Supermolecular sensor based on host-guest self-assembly and preparation and application thereof
InactiveCN109187472AQuick checkHave obvious phenomenonFluorescence/phosphorescenceFluorescencePerylene derivatives
The invention discloses a supermolecular sensor based on host-guest self-assembly, which is formed from a complexing of functional pillar [5] arenes as host, and 2-pyridine derivative DP as object inan H2O-MSO system. The supermolecular sensor can coordinate with Ag+ to form a supermolecular sensor complex PQDP-Ag, accompanied by fluorescence enhancement, so the supermolecular sensor can be usedfor single selective fluorescence recognition of Ag+, with a lowest limit of detection of 6.04x10-9 M. If added I-, fluorescence of the sensor complex PQDP-Ag is redshifted and enhanced. As a result,PQDP-Ag can identify I- efficiently, continuously and selectively, with a lowest limit of detection of 4.40x10-9 M. The invention also prepares a test paper carrying the supermolecular sensor, which can detect Ag+ ions in solution well, and has the advantages of portability, obvious phenomenon and rapid detection.
Owner:NORTHWEST NORMAL UNIVERSITY
Fluorescent probe for detecting hydrogen sulfide and preparation method and application thereof
InactiveCN107418556AHigh selectivityHigh detection sensitivityOrganic chemistryFluorescence/phosphorescenceOrganic solventFluorescence
The invention discloses a fluorescent probe for detecting hydrogen sulfide and a preparation method and application thereof. The fluorescent probe has a molecular formula of C21H21N4O+ and has a structure shown in the description. The fluorescent probe self has very weak fluorescence; when the fluorescent probe is added into water or an organic solvent, an obtained solution is purple; when the fluorescent probe reacts with hydrogen sulfide, the color of the solution gradually fades away, and fluorescence enhancement is shown. The fluorescent probe for detecting the hydrogen sulfide disclosed by the invention has high selectivity in hydrogen sulfide detection, high detection flexibility, an obvious phenomenon and convenience in recognition. The fluorescent probe for detecting the hydrogen sulfide disclosed by the invention is simple in preparation method, high in prepared product yield and suitable for large-scale popularization and application.
Owner:UNIV OF JINAN
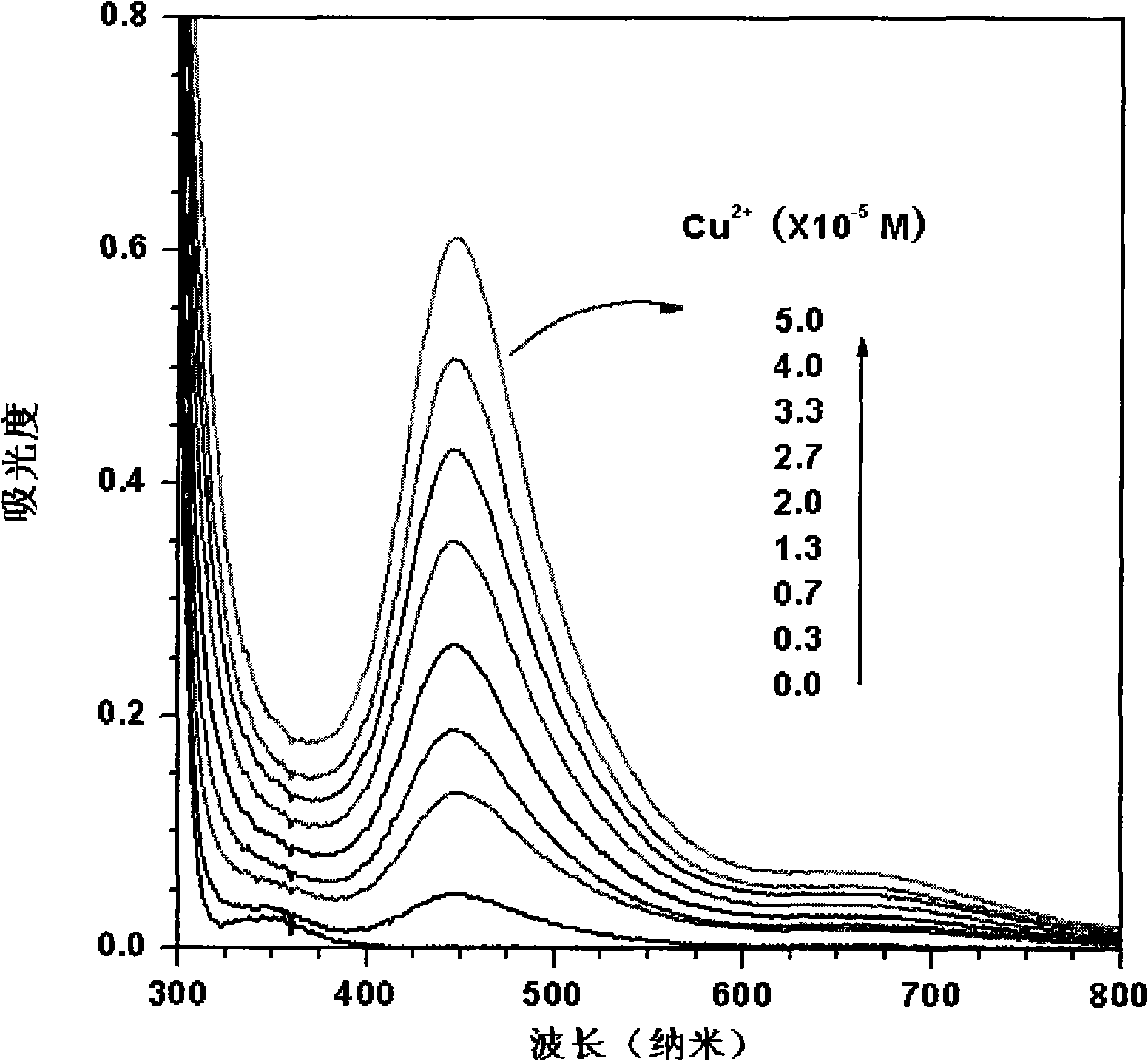
Novel high-selectivity fluorescence probe for bivalent copper ions as well as preparation method and biological application of novel high-selectivity fluorescence probe
InactiveCN104893712ASensitive detectionHigh selectivityOrganic chemistryFluorescence/phosphorescenceFluorescenceCopper
The invention discloses a novel high-selectivity fluorescence probe for bivalent copper ions as well as a preparation method and biological application of the novel high-selectivity fluorescence probe. The fluorescence probe has a structure as shown in the formula (I) in the specification. The invention also discloses a preparation method and application of the fluorescence probe. The fluorescence probe can be used for selectively detecting bivalent copper ions in a water system and is changed from colorlessness to a dark color when the bivalent copper ions exist, and the fluorescence is remarkably enhanced. Due to the characteristics, the fluorescence probe disclosed by the invention has a remarkable advantage when being used for detecting the content of heavy metal ions in an environment and an organism and has a potential application value in the fields of fluorescence labeling and bioimaging.
Owner:UNIV OF JINAN
Method and kit for quick qualitative testing chlorine dioxide in milk
InactiveCN101231245AObvious phenomenonHigh detection sensitivityMaterial analysis by observing effect on chemical indicatorTesting dairy productsChlorine dioxideChemical composition
The invention relates to a test method, in particular to a rapid qualitative test method of chlorine dioxide in milk, and also relates to a rapid qualitative detection reagent kit of chlorine dioxide. The invention belongs to the technical field of the chemical composition detection. A qualitative adulteration test method of the chlorine dioxide performs the detection according the following procedures that: a test paper strip is immerged into a milk sample, whether the chlorine dioxide is remained or not in the milk sample is judged according to the color change of a test paper strip after being taken out, and the detection limit of the method is 0.0002 percent.
Owner:INNER MONGOLIA MENGNIU DAIRY IND (GRP) CO LTD
Preparation of carbon quantum dots having catalysis performance, and applications of kit based on carbon quantum dots
The present invention discloses preparation of carbon quantum dots having catalysis performance, and applications of a kit based on the carbon quantum dots. The carbon quantum dot preparation mainly comprises: carrying out oil bath heating on polyethyleneimine, cysteine, ethylene glycol, distilled water and solid phosphoric acid to obtain a pale yellow gel, dissolving with water, and naturally cooling to a room temperature; and treating the obtained brown gel product to obtain the product. The applications of the kit based on the carbon quantum dots mainly comprise: the characteristic that the carbon quantum dots catalyze and decompose hydrogen peroxide to organically combine two nanometer materials such as the carbon quantum dots and the gold nanoparticles, and the carbon quantum dots have biological enzyme property and the antibody and the antigen are specifically bound to form a sandwich structure so as to achieve the detection of breast cancer antigen and the hypersensitivity visual detection of ice drug. According to the present invention, the phenomenon is obvious, the operation is easy, the sensitivity is ultra-high, and the breast cancer antigen concentration in the serum and the ice drug content in human serum, saliva and urine can be effectively and accurately determined.
Owner:HUNAN UNIV OF SCI & TECH
Method for controlling Pichia pastoris to ferment to produce antibacterial peptide by utilizing dissolved oxygen parameters
ActiveCN101979649AReduce usageSufficient antibacterial activityMicroorganism based processesFermentationPichia pastorisBio engineering
The invention relates to a method for controlling Pichia pastoris to ferment to produce an antibacterial peptide by utilizing dissolved oxygen parameters, and belongs to the technical field of biological engineering. The method comprises the following steps of: (1) calibrating the percent of dissolved oxygen (DO) to obtain a fermentation medium; (2) simultaneously marking the percent as a 0 pointand starting to ferment; (3) when the value of the DO is up to above 50 percent from 10 percent below, starting to feed methanol until the value of the DO falls back to 10 to 30 percent; stopping feeding the methanol; when the value of the DO is stably up to over 50 percent again, starting to feed the methanol again until the value of the DO falls back to 10 to 30 percent; stopping feeding the methanol; operating circularly; and controlling the value of the DO to be maintained between 10 and 30 percent all the time by feeding the methanol, adjusting ventilation and controlling a rotation speed; and (4) when the value of the DO does not fall back after feeding the methanol, finishing fermenting. The method of the invention has a short fermentation period and less dosage of the methanol; and compared with conventional dissolved oxygen value regulation and control, the method is easier to operate, and conditions are easier to grasp.
Owner:山东华辰制药有限公司
Preparation method and application of rapid detection kit for copper-containing nanorod compound
InactiveCN109239064AEasy to manufactureNot easy to inactivateMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsConcentrations glucoseAbsorbance
The invention discloses a preparation method and an application of a rapid detection kit for a copper-containing nanorod compound. The method comprises the following steps of preparing the copper-containing nanorod compound; adding glucose solutions with different concentrations into a centrifuge tube, adding glucose oxidase, adding the copper-containing nanorod compound and a color developing agent, adjusting the pH value, obtaining a standard curve with a good linear relation according to a relation between an absorbance value of the color developing agent at the wavelength of 652nm and theglucose concentration, and based on this, performing calculation to obtain the glucose content in a to-be-detected sample; and adding the copper-containing nanorod compound connected with an INS-aptamer into the centrifuge tube, adding insulin with different concentrations, adding a hydrogen peroxide solution and the color developing agent (TMB), obtaining a standard curve with a good linear relation according to a relation between the absorbance value of the color developing agent at the wavelength of 652nm and the insulin concentration, and based on this, performing calculation to obtain theinsulin content in the to-be-detected sample. The kit also can be used for distinguishing type 1 diabetes and type 2 diabetes. The method is obvious in phenomenon, easy and convenient in operation, high in sensitivity, and accurate and effective.
Owner:HUNAN UNIV OF SCI & TECH
Lower alcohol detecting probe, preparation method and application thereof
InactiveCN103320116AGood choiceThe synthetic route is simpleOrganic chemistryAnalysis by material excitationAlcoholHydrogen atom
The invention discloses a lower alcohol detecting probe, and a preparation method and application of the detecting probe, and belongs to the technical field of analytical chemistry detection. The lower alcohol detecting probe has a general formula shown in the description, in which R1 represents a hydrogen atom, R2 represents a nitrogen atom or a group shown in the description and R3 represents a hydrogen atom or a methoxy group. Probe molecules of the lower alcohol detecting probe are made into a detecting plate, the detecting plate turns red or pink after being reacted with lower alcohol, and the phenomenon is obvious and can be recognized by naked eyes; the lower alcohol detecting probe is convenient for use and low in cost, can be directly used for detecting lower alcohol in a solution, can detect lower alcohol rapidly and in real time, is suitable for being used in large scale actual production, and has a good application prospect in the environmental protection field; and the lower alcohol detecting probe avoids the problem that professionals need to use professional tools and software for a long time to obtain results by means of large-scale equipment.
Owner:ZHENGZHOU UNIV
Quick detecting method for formaldehyde content in aquatic product
InactiveCN103217419AGuaranteed food safetyEasy to operateMaterial analysis by observing effect on chemical indicatorShake upAquatic product
The invention discloses a quick detecting method for formaldehyde content in an aquatic product. The quick detecting method comprises the following steps: (1) preparing a Schiff reagent; (2) making a standard color chart, namely respectively adding 5mL formaldehyde solutions with different concentrations to 8 different colorimetric tubes, respectively adding a 0.5mL Schiff reagent to the colorimetric tubes, adjusting the pH of the solutions to 2.5, shaking up the solutions, stewing and developing for 10 minutes, and making the color chart by taking colors displayed by the solutions as standard colors; and (3) detecting, namely extracting a 5mL soak solution of an aquatic product sample to be detected, adding a 0.5mL Schiff reagent to the soak solution, adjusting the pH of the solution to 2.5, shaking up the solution, stewing and developing for 10 minutes, and comparing the color displayed by the solution with standard colorimetric splines. The quick detecting method disclosed by the invention has the characteristic that the formaldehyde and the Schiff reagent react at a normal state to generate a purple-red compound, and the shade of the compound is in direct proportion to formaldehyde content so as to realize colorimetric assay. The quick detecting method has the advantages of simple and quick operation, high sensitivity, simple conditions, low reagent price, and the like.
Owner:SHAANXI UNIV OF TECH
Novel hydrazine fluorescence probe for coumarin derivative
ActiveCN109988560AObvious phenomenonEasy to observeOrganic chemistryFluorescence/phosphorescenceFluorescenceHEPES
The invention discloses a novel fluorescence probe for detecting hydrazine as well as a preparation method and application of the novel fluorescence probe. The probe has a molecular formula: C27H29NO5and has a structure as shown in the specification. The probe has very strong fluorescence and shows a very strong solubilization color rendering phenomenon. The fluorescence probe shows luminous yellow fluorescence in a mixed solution of DMSO and HEPES in the ratio of 4:1, but is turned into blue fluorescence after interacting with hydrazine. The fluorescence probe disclosed by the invention hasexcellent selectivity, relatively high sensitivity and obvious phenomenon when being used for detecting hydrazine, and the effect of identifying hydrazine by using the probe is still not affected whenthe pH value in a detection environment is 3-12. The preparation method of the novel fluorescence probe is simple, and the prepared product is high in yield.
Owner:UNIV OF JINAN
Application of ratio type fluorescent probe in detection of peroxynitrite anions
ActiveCN111423423AHigh selectivityHigh detection sensitivityOrganic chemistryFluorescence/phosphorescenceFluoProbesOrganic solvent
The invention discloses an application of a ratio type fluorescent probe in detection of peroxynitrite anions, and the structural formula of the ratio type fluorescent probe is as follows: when the fluorescent probe does not react with peroxynitrite anions, intramolecular carboxyl can form an intramolecular spiral ring, a conjugated structure of a fluorophore is destroyed so that fluorescence quenching is caused, when the fluorescent probe reacts with peroxynitrite anions, the spiro ring can be opened to recover the conjugated structure of the fluorophore again, and strong fluorescence is displayed under the illumination condition. The preparation method of the fluorescent probe is simple and has a high product yield, the probe is simple in structure and suitable for large-scale promotionand application, peroxynitrite anions can be recognized and detected in a water system and an organic solvent system in a high-selectivity mode, the ratio of two fluorescence signals serves as an output signal through the ratio type fluorescence probe, interference of background factors can be well eliminated, interference of other factors is reduced, and the detection sensitivity and accuracy areimproved.
Owner:QILU UNIV OF TECH
Naked eye visual colorimetric method-based probe for detecting nickel ions and preparation method and applications thereof
InactiveCN102519949AThe synthetic route is simpleLow costOrganic chemistryMaterial analysis by observing effect on chemical indicatorHigh selectivityImine
The invention discloses a naked eye visual colorimetric method-based probe {7-hydroxy-3-[(3-pyridine methylene) imine]-2H-1-benzopyran} for detecting nickel ions in an aqueous medium and a probe-based nickel ion detection kit and test paper. The nickel ion probe can be synthetized only by a three-step reaction; a detection mechanism is based on a complexing reaction; and the nickel ions in a solution can be detected by using a naked eye visual colorimetric method. The nickel ion detection kit provided by the invention comprises a reagent stock solution 1 and a reagent stock solution 2, wherein the reagent stock solution 1 is used as a buffer solution, and the reagent stock solution 2 is used as a nickel ion probe solution. The nickel ion detection test paper provided by the invention comprises the 7-hydroxy-3-[(3-pyridine methylene) imine]-2H-1-benzopyran and a solid carrier. The naked eye visual colorimetric method-based probe for detecting the nickel ions in the aqueous medium is capable of realizing the high-sensitivity and high-selectivity detection on trace nickel ions in the aqueous medium, has convenience in detection and visual judgment, and has a huge application prospect in the environmental-protection field.
Owner:JIANGNAN UNIV
Novel iron ion fluorescence probe and preparation method thereof
ActiveCN108129435AHigh selectivityEnhancement phenomenonOrganic chemistryFluorescence/phosphorescenceAluminum IonMolecular Probe Techniques
The invention discloses a Nnovel iron ion fluorescence probe and a preparation method thereof, and belongs to the technical field of molecular probes. The structural formula of the probe is as follows: the preparation method of the iron ion fluorescence probe is simple. The probe detects iron ions by a mode of enhancing fluorescence and obviously changing color, the iron ions can be identified ina water system highly selectively, and other metal ions (silver ions, aluminum ions, calcium ions, cobalt ions, copper ions, nickel ions, potassium ions, magnesium ions, sodium ions, nickel ions and zinc ions) are not responded. The solution obtained by adding the probe in the water system is orange; after action of the solution and the iron ions, the fluorescence of the solution is obviously enhanced, the color is changed into yellow, the selectivity on the iron ions is high, the phenomenon is obvious and identification is facilitated.
Owner:UNIV OF JINAN
Method for the detection of cyanide ion
InactiveCN101571531ALow costObvious phenomenonAnalysis using chemical indicatorsAqueous solutionCyanide ion
The invention relates to a method for the detection of cyanide ion, comprising the steps of: adding copper ions into a solution to be tested to obtain a copper ion-containing solution having the concentration of 5.0*10-5.0*10mol / L, then adding copper reagent of sodium diethyldithiocarbamate into the copper ion-containing solution, wherein, if the color of the solution is not changed after the addition of the copper reagent, the fact that the solution to be tested contains cyanide ion is represented, and if the color of the solution is changed after the addition of the copper reagent, the solution to be tested contains cyanide ion having the concentration less than 6.0*10mol / L or contains no cyanide ion. The invention is applicable to aqueous solution system, visible to naked eyes, low in price and detection limit, and reversible in phenomenon. Change of the color of the system is observable to naked eyes by means of the addition of cyanide ion of 1.0*10, the larger the concentration is, the more obvious the change of the color takes place, and the color is gradually changed from dark yellow into transparent color. With good reproducibility when pH is equal to 6-10, the solution can be used for the detection of minim-cyanide ion and can be practicably applied in aspects of drinking water, food, environmental protection, etc.
Owner:WUHAN UNIV
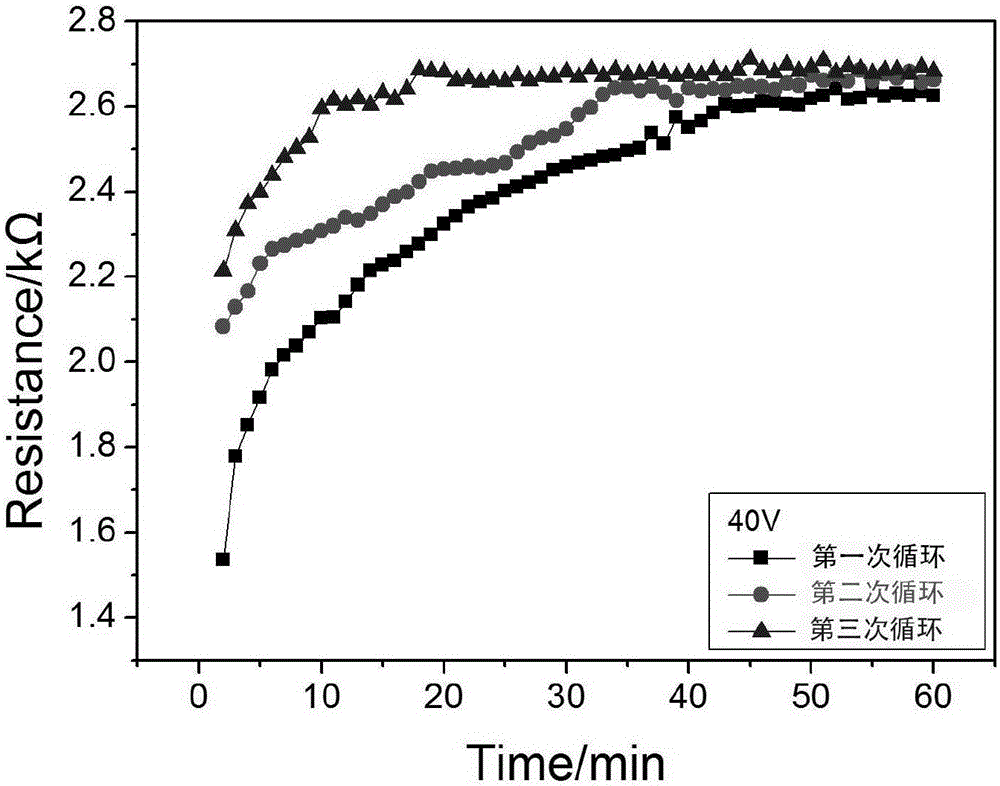
Preparation method for pure metal bismuth nanoparticles
InactiveCN105908214AEasy accessSimple preparation processPhotography auxillary processesNanotechnologySilver pasteChloride salt
The invention provides a preparation method for pure metal bismuth nanoparticles and belongs to the technical filed of metal nano material preparation. The preparation method comprises the following steps that bismuth elements in chloride salt containing bismuth are uniformly deposited in hole channels of a porous alumina mold plate, so that pure metal bismuth nanowire arrays are obtained; the porous alumina mold plate fully deposited with pure metal bismuth nanowires is connected with a wire with conductive silver paste, so that an electrode is made; then the electrode is connected into an experimental circuit in series, the direct current voltage is set to be 40 V, the experiment time is set to be 60 min, and the experiment process is repeated for two to three times; and a measured sample cross section is selected to be observed through a scanning electron microscope, and thus it can be found that the particle refinement phenomenon appears on the pure metal bismuth nanowires along with increasing of the times of the repeated experiments. According to the preparation method for the pure metal bismuth nanoparticles, the preparation process is simple, and the raw materials are easy to obtain; the voltage and the time of experimental reaction are controllable; and in addition, the prepared materials are widely applied to thermoelectricity, lubricating oil additives and other aspects.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Test device for bearing pressure of egg shell structure
InactiveCN102445388ASimple structureIncrease visibilityMaterial strength using tensile/compressive forcesEngineering
Owner:王富民
Sodium sulfocyanate adulteration qualitative detection method for material milk
InactiveCN101126720AObvious phenomenonHigh detection sensitivityMaterial analysis by observing effect on chemical indicatorTesting dairy productsChemical compositionSodium sulfocyanate
The utility model relates to a test method, in particular to a test method for adulteration of sodium sulfocyanate in raw milk, belonging to the technical filed of chemical composition test. In the test method for adulteration of sodium sulfocyanate in raw milk, the test is conducted according to the following steps: adding in the ferric salt solution of certain concentration in raw milk sample and judging whether the adulteration of sodium sulfocyanate exists in the raw milk sample according to the color change of the contact surface of ferric salt and raw milk sample. The test limit of the method is 0.01%.
Owner:INNER MONGOLIA MENGNIU DAIRY IND (GRP) CO LTD
Method for distinguishing pearl powders from shell powders
InactiveCN101620176BEasy to distinguishSimple methodPreparing sample for investigationColor/spectral properties measurementsAragonitePotassium bromide
The invention relates to a method for distinguishing pearl powders from shell powders, comprising the following steps: the pearl powders and the shell powders are calcined and then respectively mixed with potassium bromide to be made into transparent flakes, then the infrared spectrum of the transparent flakes is measured under the conditions of resolution of 4 cm<-1> and scanning for 16 times with the scanning range of 400-4000 cm <-1>, the pearl powders and the shell powders are distinguished according to the characteristic peak of 701.0 cm <-1>, 1082.1 cm <-1>, 2919.3 cm<-1> and 3468.5 cm <-1>; as organic substances in the calcined pearl powders are partially decomposed, the crystal type of calcium carbonate changes from the aragonite type to the calcite type, and the calcium carbonatestarts to decompose; the organic substances in the shell powders are almost totally decomposed, the aragonite type calcium carbonate is totally converted into the calcite type calcium carbonate, and the calcium carbonate does not start to decompose into calcium oxide yet, therefore, the method can be used for distinguishing the pearl powders from the shell powders. Compared with the prior distinguishing method, the method can simply, rapidly and effectively distinguish the pearl powders from the shell powders with obvious phenomenon.
Owner:钱国英 +2
Full optical fiber type wave-particle dualism measuring apparatus
The invention relates to a device for measuring light wave-particle duality and realizes verification and observation for wave-particle duality. The invention provides an observation device of the wave-particle duality which has the advantages of simple structure, easy construction, strong noise immunity, and easily observing measuring result. Single photon source, a light ring-shaped device and an X-shaped optical splitter / combiner are connected in turn to form the device. Moreover, the device is arranged two single photon detecting counter. The physical basis of the device is: when measuring corpuscular property, the device makes use of a characteristic of route random selection when the photons pass the X-shaped optical splitter / combiner, and a characteristic of prominently displaying classical particle motional orbits and locus; when measuring the wave character, the device makes use of Sagnac interferometer, a superposition characteristic when the photons meet at the X-shaped optical splitter / combiner, and an interference characteristic of prominently displaying classical wave.
Owner:BEIJING UNIV OF POSTS & TELECOMM
Fluorescent probe for detecting biological mercaptan in water-soluble environment and preparation method and application thereof
InactiveCN110128440AHigh selectivityHigh detection sensitivityOrganic chemistryFluorescence/phosphorescenceWater solubleSulfonyl
The invention belongs to the technical field of organic small molecule fluorescent probes, and provides a fluorescent probe for detecting biological mercaptan in a water-soluble environment and a preparation method and application thereof. The molecular formula of the fluorescent probe is C32H26N3O10S<+>, the name of the probe compound is 4-(2-carboxyphenyl)-7-(diethylamino)-2-(4-(((2,4-dinitrophenyl) sulfonyl) oxy) phenyl) benzopyran, which is abbreviated as PA-SN, and the structural formula is shown in the specification. The preparation method of the fluorescent probe is simple. The probe has large fluorescence enhancement multiple and obvious color change, and can recognize the biological mercaptan with high selectivity in a water system, an organic solvent system and organisms. The fluorescence of the probe is weak and almost colorless in the organic system. After the probe is reacted with the biological mercaptan, the fluorescence of a solution increases significantly, and the color changes to purple obviously. The detection of the biological mercaptan is highly selective, sensitive and obvious.
Owner:SHANXI UNIV
Bernoulli principle experiment instrument
A Bernoulli principle experiment instrument is characterized by comprising a long strip-shaped air duct, wherein the air duct is jointly defined by two oppositely arranged support plates and two oppositely arranged rubber membranes; each support plate is made of a transparent material; the rubber membranes are flatly sealed and glued on the support plates; a horn-shaped air inlet is fixed in one end of the air duct; an air outlet is fixed in the other end of the air duct. The Bernoulli principle experiment instrument has the benefits as follows: an electric hair drier faces the horn-shaped air inlet to blow air, airflow rapidly flows through in the air duct, the pressure intensity is reduced, the rubber membranes are sunken inwards under the action of external pressure intensity, and the phenomenon is remarkable. The support plates are made of the transparent material, so that the observation is facilitated. The Bernoulli principle experiment instrument has the advantages of convenience in operation and repeatability.
Owner:曾颖
Novel hydrodynamic lubrication demonstrator
The invention discloses a novel hydrodynamic lubrication demonstrator. The novel hydrodynamic lubrication demonstrator comprises a movable ring, a stationary ring, an oil tank filled with silicone oil, a motor and a transmission device, the movable ring and the stationary ring are arranged in the oil tank, the stationary ring is arranged above the movable ring, and the movable ring and the stationary ring are performed with oil seal; the center of the movable ring is connected with a spindle through a flat key, and the spindle is in transmission connection with the motor shaft of the motor through the transmission device; when rotating, an oil film is formed between the movable ring and the stationary ring; wedge-shaped bumps are arranged on the lower surface of the stationary ring at intervals along the circumferential direction, and the lower surface of the stationary ring is matched with the upper surface of the movable ring to form wedge-shaped gaps of the oil film; through holes are formed in the upper surface of the stationary ring and communicated with an oil pressure display pipe and the wedge-shaped gaps, and the upper end of the oil pressure display pipe is communicated with the external environment.
Owner:ZHEJIANG UNIV OF TECH
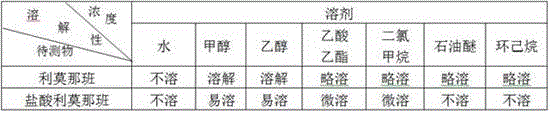
Rapid detection method for rimonabant
ActiveCN105548161AImprove solubilityImprove extraction efficiencyMaterial analysis by observing effect on chemical indicatorAmount of substanceChemistry
The invention discloses a rapid detection method for rimonabant. As to-be-detected samples undergo three steps, i.e., alkalization and dispersion, extraction and separation, and acidification and precipitatioin, whether the samples contain rimonabant can be preliminarily determined according to results of a precipitation reaction; and suspected positive samples are further subjected to an alkaloid precipitation reaction so as to confirm whether the samples contain rimonabant or not. The method utilizes different dissolvability of different substances to remove interferent in three steps and more accurately determines whether the samples contain rimonabant or not by using two precipitation reactions of different mechanisms. The detection method provided by the invention is simple to operate, low in analysis cost, strong in specificity, high in sensitivity and applicable to on-site rapid detection of illegal addition of rimonabant in fat-reducing Chinese patent medicines, health food and foodstuffs.
Owner:SHANDONG INST FOR FOOD & DRUG CONTROL
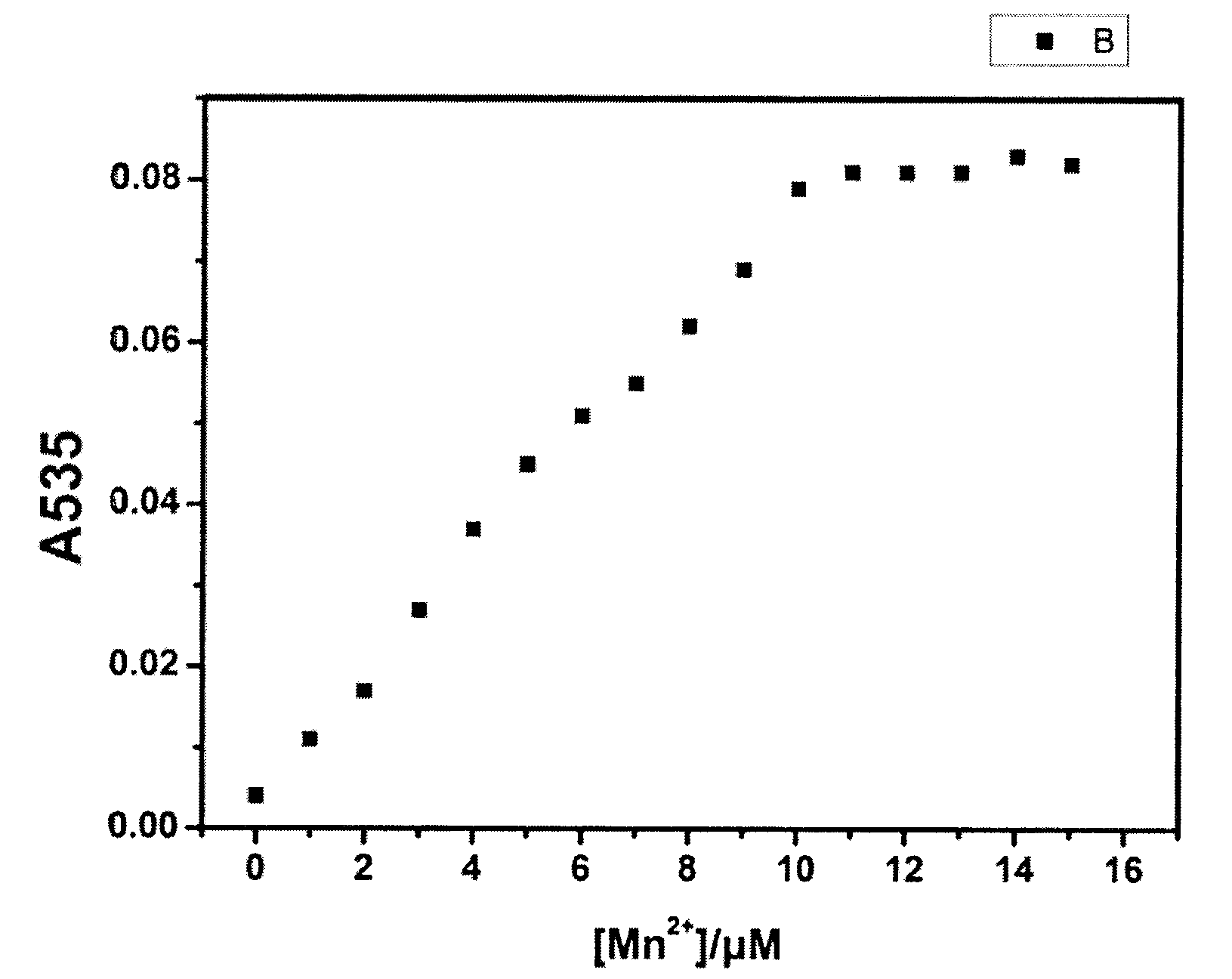
Detection of preparation of manganese ions probe based on naked eye visual colorimetry and application
InactiveCN102967596AGood choiceDo not cause disturbanceMaterial analysis by observing effect on chemical indicatorEthylenediamineManganese
The invention discloses a probe-N,N-dis(4-diethylamine salicylidene)ethylenediamine for detecting manganese ions in water quality based on a naked eye visual colorimetry and a manganese ions detection kit and a test paper based on the probes. The manganese ions probe can be synthesized by one step reaction, a detection mechanism is based on the complexing reaction, and the naked eye visual colorimetry is used for detecting the manganese ions in a liquid to be measured. The manganese ions detection kit comprises a reagent storage liquid 1 and a reagent storage liquid 2, wherein the reagent storage liquid 1 is a HEPES buffer, and the reagent storage liquid 2 is a solution of N,N-dis(4-diethylamine salicylidene)ethylenediamine. The manganese ions detection test paper is composed of the N,N-dis(4-diethylamine salicylidene)ethylenediamine and a solid carrier. The invention can realize a rapid quantitative or semi-quantitative detection with high sensitivity and high selectivity for microscale manganese ions in the water quality. The probe has the advantages of simple synthesis route and low cost, and has large application prospect in the environmental protection field.
Owner:JIANGNAN UNIV
Interferometric optical fiber humidity sensor based on graphene and manufacturing method of interferometric optical fiber humidity sensor
PendingCN110006847ACompact structureEasy to manufacturePhase-affecting property measurementsCouplingGraphene
The invention relates to an interferometric optical fiber humidity sensor based on graphene. The interferometric optical fiber humidity sensor comprises a glass slide pasted with a metal cylinder, andtwo single-mode optical fibers; the two single-mode optical fibers pass through the two sides of the metal cylinder correspondingly and are bent oppositely to form two crossed coupling points; and the two ends of the single-mode optical fibers are fixed to the glass slide, tapering is conducted on the middles of the single-mode optical fibers, tapered lumbar areas are located between the two crossed coupling points, the tapered lumbar area of one of the single-mode optical fibers is covered with a reduced graphene oxide film layer, and the two ends of the single-mode optical fiber covered with reduced graphene oxide serve as the input end and the output end correspondingly. The interferometric optical fiber humidity sensor is simple and compact in structure and easy to prepare, mutual fusion welding of optical fibers of special types is not needed, and thus the cost is low; and an optical fiber interference structure is suitable for sensing of gas of other types and biological and chemical sensing as well.
Owner:JINAN UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com