Method for the detection of cyanide ion
A technology of cyanide ion and copper ion, which is applied in the field of detection of cyanide ion, can solve the problems of many interference factors, cumbersome measurement procedures, narrow measurement range, etc., and achieve the effects of cost reduction, obvious phenomenon and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
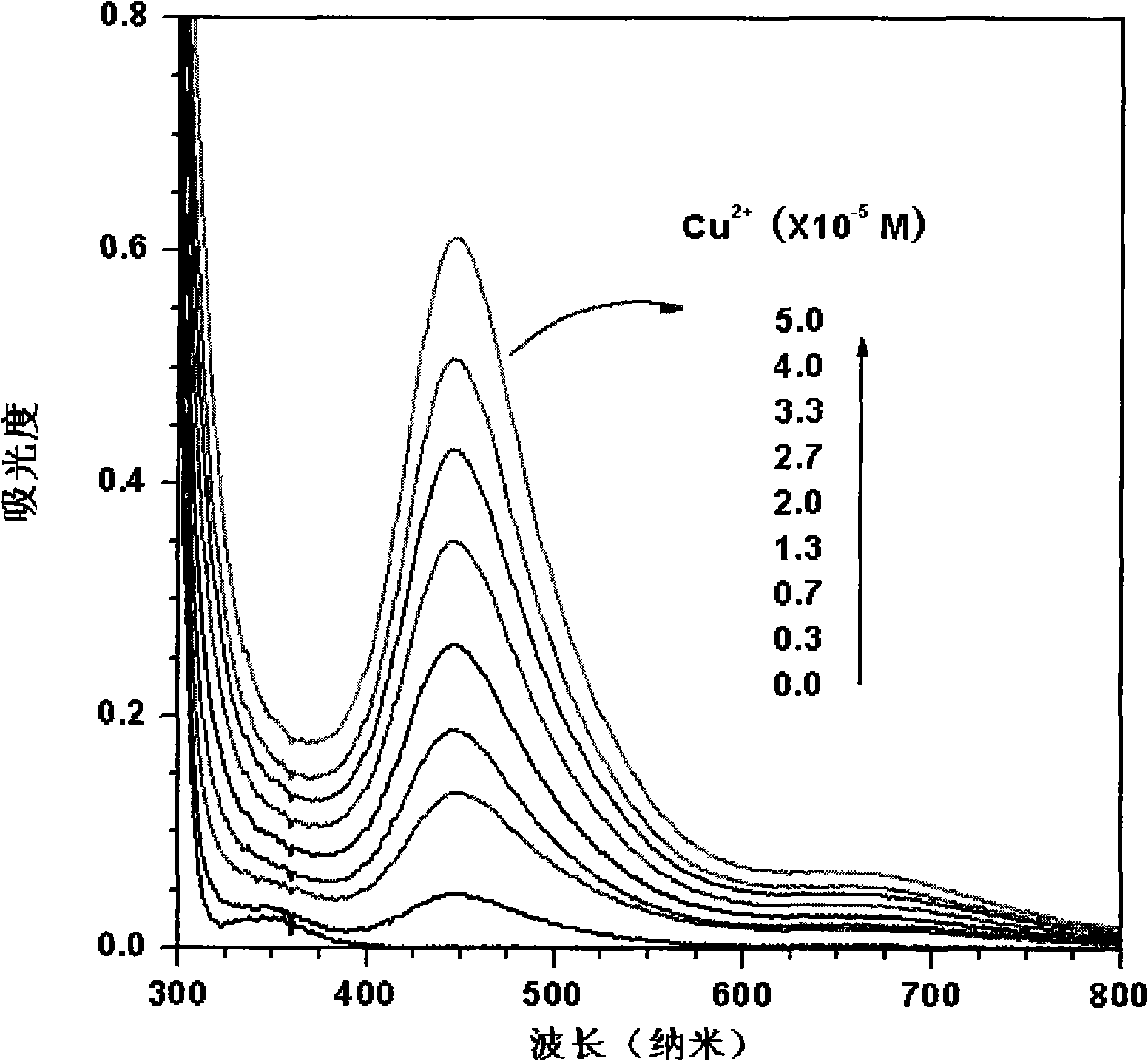
[0023] (1) Add the copper reagent sodium diethylenedithiocarbamate in twice-distilled water of pH=6.8 to prepare a concentration of 5.0×10 -4 mol / L sodium diethylenedithiocarbamate aqueous solution, and then add different amounts of the concentration of 1.0 × 10 -3 mol / L copper nitrate solution, the concentration of copper ions in the solution after adding is 0, 0.5×10 -5 , 1.0×10 -5 , 1.5×10 -5 , 2.0×10 -5 , 2.5×10 -5 , 3.0×10 -5 , 3.5×10 -5 , 4.0×10 -5 , 4.5×10 -5 , 5.0×10 -5 mol / L, the prepared solution is poured into a 10 mm wide quartz cell respectively, and the respective spectrograms are obtained in an ultraviolet spectrometer (Shimadzu UV-2550 spectrometer), figure 1 is the UV-visible spectrum after changing the concentration of copper ions.
[0024] (2) Prepare 5.0×10 in twice distilled water with pH=6.8 -5 mol / L copper nitrate solution, the concentration of adding different amounts is 1.0×10 -2 mol / L sodium cyanide solution, the concentration of cyanide i...
Embodiment 2
[0026] (1) Add 1.0×10 -2 mol / L copper reagent sodium diethylenedithiocarbamate makes the concentration of sodium diethylenedithiocarbamate 5.0×10 -4 mol / L, the concentration of adding different amounts is 1.0×10 -3 mol / L copper nitrate solution, the concentration of copper nitrate in the solution after adding is 0, 0.3×10 -5 , 0.7×10 -5 , 1.3×10 -5 , 2.0×10 -5 , 2.7×10 -5 , 3.3×10 -5 , 4.0×10 -5 , 5.0×10 -5 mol / L, the prepared solution is poured into a 10 mm wide quartz cell respectively, and the respective spectrograms are obtained in an ultraviolet spectrometer (Shimadzu UV-2550 spectrometer), image 3 is the UV-visible spectrum after changing the concentration of copper ions.
[0027] (2) Prepare 5.0×10 in the sodium hydroxide aqueous solution of pH=9.1 -5 mol / L copper nitrate solution, the concentration of adding different amounts is 1.0×10 -2 mol / L sodium cyanide solution, the concentration of cyanide ion in the solution after adding is 0, 1.0×10 -4 , 2.0×10 ...
Embodiment 3
[0029] (1) Add copper reagent sodium diethylenedithiocarbamate to tap water so that the concentration of sodium diethylenedithiocarbamate is 5.0×10 -4 mol / L, the solution after adding copper reagent is colorless and transparent, add 1.0×10 -3 mol / L copper nitrate solution, the concentration of copper ions in the solution after adding is 5.0×10 -4 mol / L, the color of the solution after adding copper nitrate is dark yellow.
[0030] (2) Prepare 5.0×10 in tap water -4 mol / L copper nitrate solution, the solution after adding copper nitrate is colorless and transparent, and the concentration is 1.0×10 -2 mol / L sodium cyanide solution so that the cyanide ion concentration of the added solution is 7.0×10 -4 mol / L, the solution after adding sodium cyanide is colorless and transparent, then add copper reagent sodium diethylenedithiocarbamate, the concentration of copper reagent mother liquor is 1.0×10 -2 mol / L, so that the copper reagent concentration in the solution after adding i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com