Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31results about How to "Avoid Biosecurity Risks" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vero cell serum-free culture medium and application thereof
ActiveCN111733126AClear chemical compositionLow costCulture processArtificial cell constructsBiotechnologyAluminium chloride
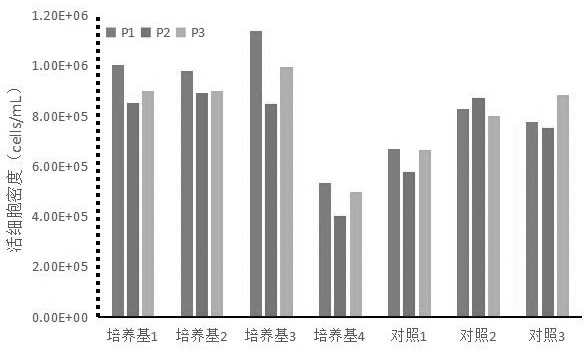
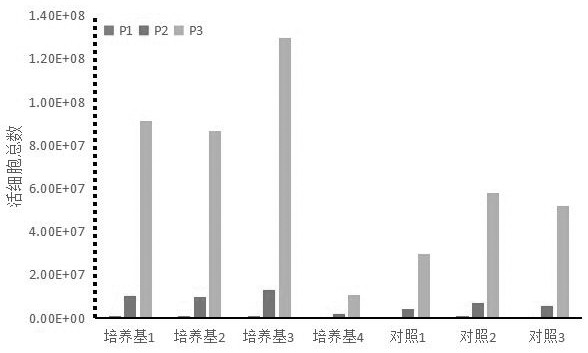
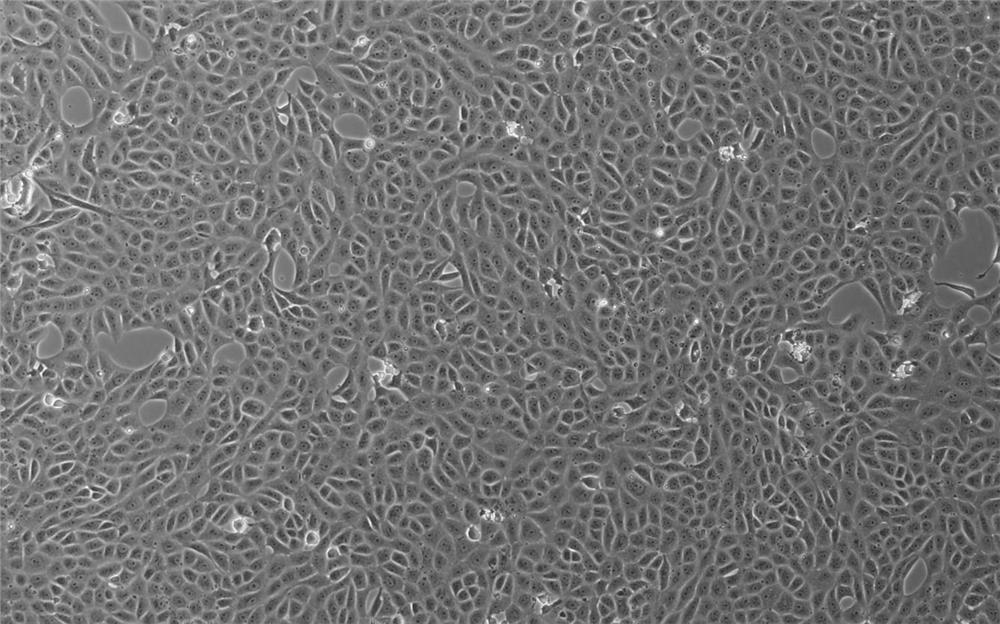
The invention relates to a Vero cell serum-free culture medium and an application thereof. The Vero cell serum-free culture medium comprises an amino acid component, a vitamin component, an inorganicsalt component, trace element components, carbohydrate and other molecular compound components, wherein the trace element components include the following components: zinc sulfate heptahydrate, coppersulfate pentahydrate, ferric nitrate nonahydrate, ferrous sulfate heptahydrate, sodium selenite, nickel chloride, stannous chloride, silver nitrate, cobalt chloride and aluminum chloride. The serum-free culture medium provided by the invention has the advantages of definite chemical components and low cost, avoids the biological safety risk caused by the use of serum when the serum is not needed,and provides convenience for the large-scale production of biological products, especially vaccines. Vero cells grow well in the culture medium, and the serum is not used, so that the stability of the production process of the biological products and the stability of the quality of finished products are improved, and particularly, the research, development and production of novel coronavirus (SARS-CoV-2) vaccines are accelerated.
Owner:苏州依科赛生物科技股份有限公司
Antibacterial hydrocolloid and preparation method of antibacterial hydrocolloid
InactiveCN102716509AAvoid Biosecurity RisksPrevent wound infectionAbsorbent padsBandagesChemistryMetal
The invention relates to antibacterial hydrocolloid and a preparation method of the antibacterial hydrocolloid and belongs to the technical field of medicine. Through the fiber superfine pulverization technology, hydroxypropyl trimethylammonium chloride chitosan fiber with high antibacterial performance is processed into superfine powder bodies with the average particle diameter being 1 to 5mum, the superfine powder bodies, hot sol substances, moisture absorption macromolecules and tackifiers are sequentially added in different process stages, and the hydrocolloid is formed, so the potential biosafety risk caused by the use of nanometer metal as antibacterial agents is avoided. The traditional preparation process of the hydrocolloid is changed through the preparation method of the antibacterial hydrocolloid provided by the invention. When the antibacterial hydrocolloid prepared by the invention acts on wound, bacteria on the surfaces of the chronic wound can be killed, the wound infection can be prevented, meanwhile, a large amount of wound transudate can be absorbed, micro gel is formed after the transudate absorption, the humid environment is provided for the wound, and the wound healing is promoted.
Owner:WUHAN TEXTILE UNIV
Bacillus coagulans as well as microbial preparation, preparation method and application thereof
ActiveCN107400637AImproved communityInhibition of growth and reproductionGas treatmentBacteriaFecesSide effect
The invention discloses bacillus coagulans Daoduo 4 as well as a screening method thereof and further discloses a microbial preparation containing bacillus coagulans Daoduo 4 as well as a preparation method and an application of the microbial preparation. The microbial preparation is simple to culture, stable in structure, non-toxic, harmless, free of side effects and capable of effectively inhibiting breeding and growth of harmful microorganisms such as putrefying bacteria, pathogenic bacteria and the like, has relatively high stress resistance to the environment, has the advantages of high temperature resistance, acid resistance, choline resistance and the like and can inhibit growth of harmful bacteria in garbage, feces and sewage under complicated conditions, remove odor, reduce pollution and improve the environment. Besides, the bacillus coagulans can effectively remove VOCs (volatile organic compounds) and remarkably reduce harm of decoration pollution to human health after being applied to newly decorated houses and other places.
Owner:上海道多生物科技有限公司
Preparation and application of enhanced serum avian adenovirus type 4 subunit vaccine
ActiveCN111840537AStrong immune efficiencyReduce volumeViral antigen ingredientsAntiviralsEscherichia coliAvian adenovirus
The invention relates to preparation and application of an enhanced serum avian adenovirus type 4 subunit vaccine. According to the invention, a baculovirus expression system is used for expressing avian adenovirus type 4 fibrin (Fiber-2); an escherichia coli expression system is used for constructing and expressing two cytokines, namely interleukin 2 (IL-2) and interferon gamma (IFN-gamma); and the Fiber-2, the interleukin 2 (IL-2), and the interferon gamma (IFN-gamma) are mixed for application. The vaccine prepared by using the method disclosed by the invention is low in cost, good in immuneeffect, and capable of effectively preventing the infection of the avian adenovirus type 4.
Owner:EAST CHINA UNIV OF SCI & TECH
Biological sample transferring box and method
InactiveCN110817140AReduce heat exchangeReduce the rate at which the temperature risesDomestic cooling apparatusLighting and heating apparatusThermodynamicsProcess engineering
The invention provides a biological sample transferring box and method. The transferring box is provided with a multilayer protection structure and comprises a sample protection box, an inner heat preservation box and an outer heat preservation box. The sample protection box is used for containing a sample container containing a biological sample. The sample protection box is arranged in the low-temperature negative-pressure inner heat preservation box. The inner heat preservation box is arranged in the heat preservation box with a heat preservation function. The inner heat preservation box comprises an inner box with an inner heat preservation layer and an inner box cover matched with an opening in the upper end of the inner box. A gas channel assembly communicating with the interior of the inner box is arranged on the inner box cover. The biological sample transferring box is convenient to use, safe and reliable; by arranging two heat preservation layers, heat exchange between the outside and the interior of the box is reduced; and by placing an ice box in the inner heat preservation box, the interior of the inner box is vacuumized through the gas channel assembly arranged on theinner box cover, the biological sample is in a low-temperature negative-pressure condition, and it is avoided that biological factors possibly with biological safety risks are diffused to the outsideto pollute the environment.
Owner:JINYUBAOLING BIO PHARMA CO LTD
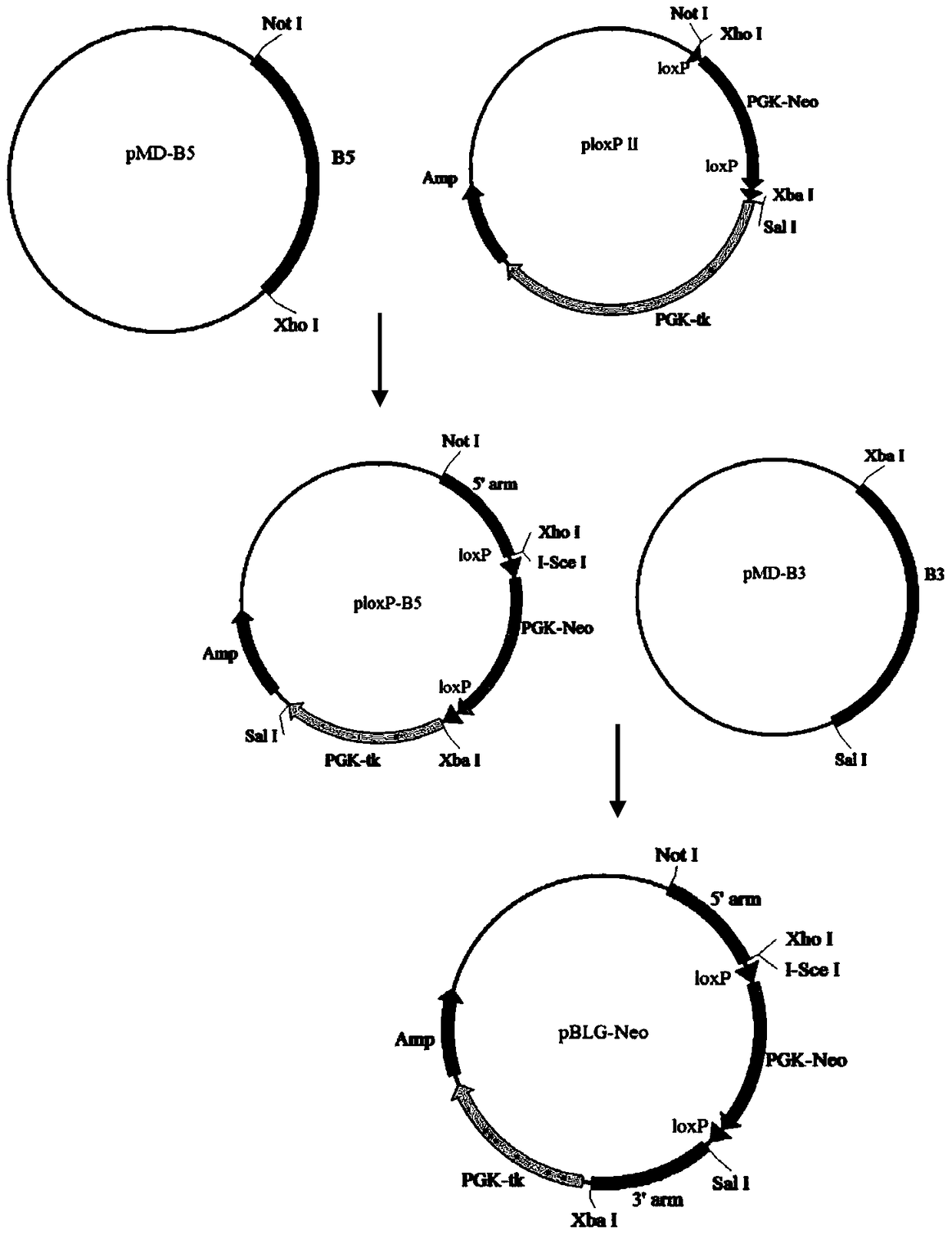
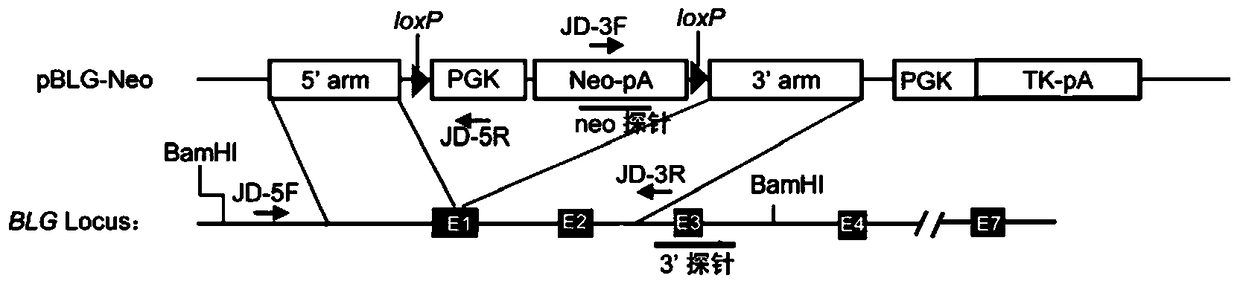
TALEN-mediated vector for knocking out goat BLG through gene targeting and recombinant cell
ActiveCN104726495AKnockout precisionKnockout achievedFermentationVector-based foreign material introductionBeta-lactoglobulinIn vitro transcription
The invention discloses a TALEN-mediated vector for knocking out goat BLG through gene targeting and a recombinant cell. A TALEN eukaryotic expression vector aiming at a first exon of a goat beta-lactoglobulin gene and a BLG gene knockout vector containing short homologous arms are designed and constructed according to the goat beta-lactoglobulin gene and are jointly transfect goat fetal fibroblast together with mRNAs obtained by in vitro transcription based on TALEN expression vector as a template, and a BLG gene knockout cell is obtained after drug screening and identification. According to the invention, the gene targeting efficiency is greatly improved by utilizing a TALEN technology; meanwhile, the utilization of the short homologous arms facilitates more convenient and more efficient screening of the targeted cells; random integration of the TALEN expression vector in genomes is avoided by utilizing mRNAs.
Owner:NORTHWEST A & F UNIV +1
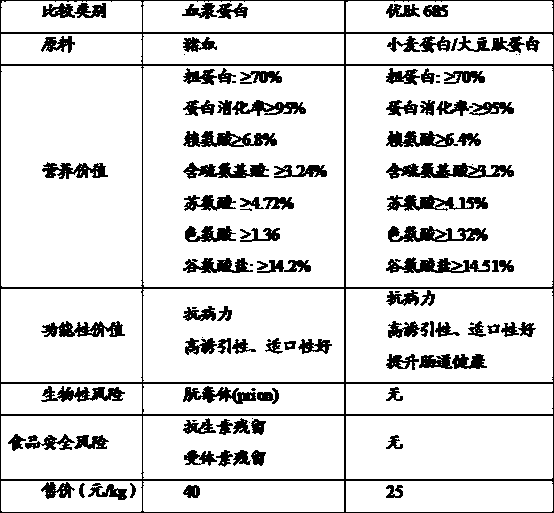
Milk replacer for baby pigs using Youtai 685 instead of plasma protein powder
The invention provides a milk replacer for baby pigs using Youtai 685 instead of plasma protein powder, which is prepared from the following raw materials: basic feed for suckling pigs, fish meal, whey powder, lactose, lactoprotein powder, calcium hydrogen phosphate, emulsified oil powder, rock powder, methionine, choline chloride, acidifier, glucose, frankincense type masking agent, complex vitamins for suckling pigs, lysine, probiotics, Youtai 685 and Yiningyi. Since the milk replacer uses the Youtai 685 instead of plasma protein powder, the feed cost is obviously reduced, but the productivity of baby pigs is kept unchanged; and a series of biological safety risks caused by the plasma protein powder are avoided. Besides, the milk replacer can effectively reduce the conditions of poor appetite, digestion disorders, diarrhea, growth retardation, low feed utilization efficiency and the like after baby pigs are weaned, thus providing a guarantee for quick growth of weanling baby pigs.
Owner:ANYOU BIOTECH GRP
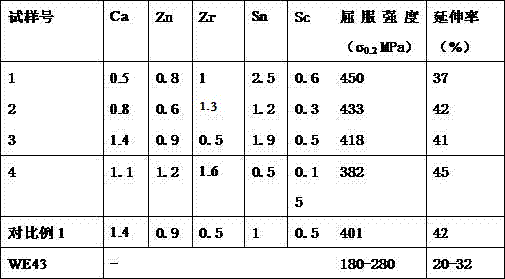
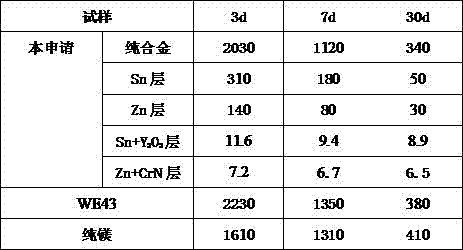
Biodegradable metal vascular scaffold
InactiveCN107158479AGrain refinementImprove mechanical propertiesSurgeryCoatingsMetalMaterials science
The invention relates to a biodegradable vascular scaffold, which comprises: a tubular biodegradable metal structure, optionally a biodegradable metal layer covering at least one part of the surface of the metal structure, and optionally a compound layer covering at least one part of the surface of the metal structure and / or the metal layer, wherein the thickness of the metal layer is 0-20 [mu]m, preferably 5-15 [mu]m, and the thickness of the compound layer is 0-5 [mu]m, preferably 1-4 [mu]m.
Owner:青岛市即墨区人民医院
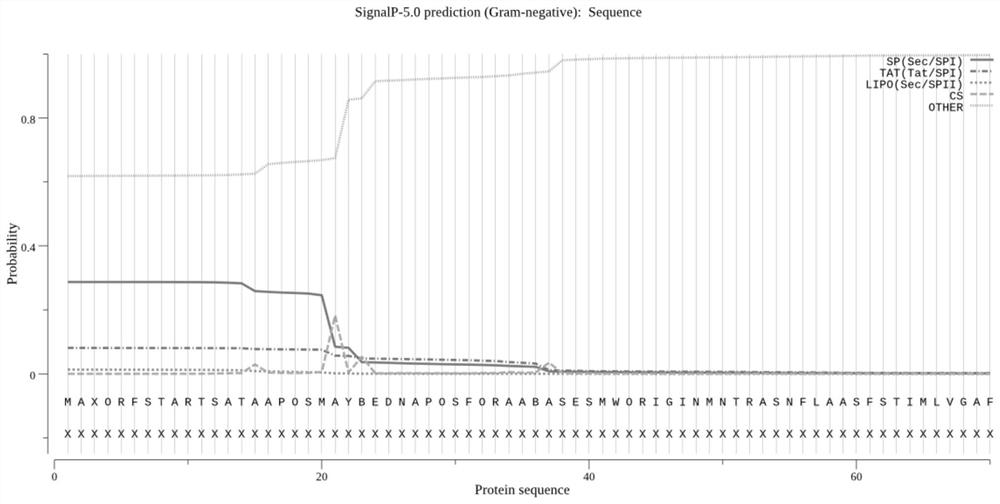
Fusion protein of brucella outer membrane protein OMP25 and periplasmic protein BP26 as well as expression and application of the fusion protein
PendingCN111978410AGood antigenicityShort timeAntibacterial agentsBacterial antigen ingredientsMolecular biologyCell Membrane Proteins
The invention discloses a fusion protein of brucella outer membrane protein OMP25 and periplasmic protein BP26 as well as expression and application of the fusion protein. An N-terminal signal peptidesequence of the OMP25 protein and an N-terminal signal peptide sequence of the BP26 protein are deleted, and an antigenic determinant sequence of the OMP25 protein and the BP26 protein is reserved; the fusion protein is formed by connecting a Linker sequence GGAGGCGGGGGTTCTGGAGGCGGGGGTTCT in the middle; the N-terminal signal peptide sequence of the OMP25 protein is the first aa-29th aa, and the N-terminal signal peptide sequence of the BP26 protein is the first aa-24th aa. Compared with single protein, the protein is more economical, convenient and better in antigenicity. The protein is obtained with less time consumption and high yield, the culture and biological safety risks of viable bacteria are avoided, the specificity is strong, and the positioning of the Brucella cell epitope is more beneficial to reveal the essence of humoral immunity.
Owner:SHANDONG BINZHOU ANIMAL SCI & VETERINARY MEDICINE ACADEMY
Brucellosis and foot-and-mouth disease bivalent vaccine and preparation method and application thereof
ActiveCN111773384AOvercome side effectsLess side effectsAntibacterial agentsSsRNA viruses positive-senseAntigenDisease
The invention provides a brucellosis and foot-and-mouth disease bivalent vaccine and a preparation method and application thereof, and relates to the field of biotechnology. According to the brucellosis and foot-and-mouth disease bivalent vaccine provided by the invention, a brucella bacterial ghost antigen is used as a carrier to load foot-and-mouth disease virus-like particles, so as to achievethe purpose that one vaccine can prevent two diseases; additionally, biological safety risks in the use of brucellosis live vaccines are eliminated, and the problem of side effects during the use of foot-and-mouth disease vaccines is overcame.
Owner:天康生物制药有限公司
System and method for carrying out whole-process management on samples
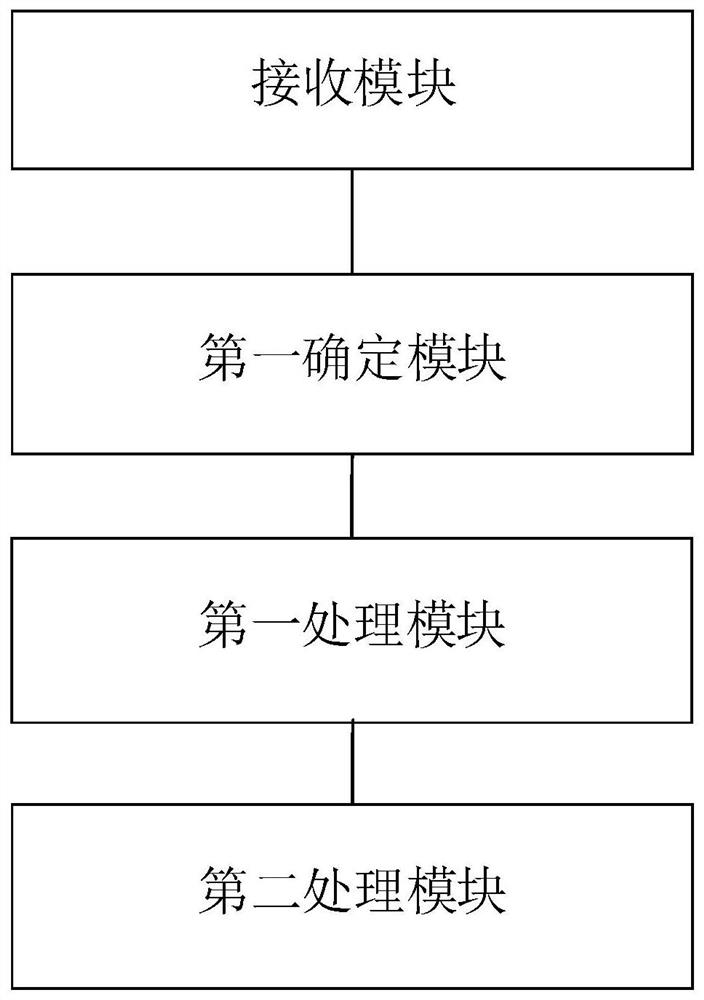
PendingCN111932167ARealize automatic management of the whole processAvoid Biosecurity RisksLogisticsSpecial data processing applicationsLogistics managementSoftware system
The invention relates to a system and a method for carrying out whole-process management on a sample. The system comprises a receiving module used for receiving sample information of an input sample and storing the sample information; a first determining module used for determining whether a logistics starting request for the sample from a consigner is received or not; a first processing module used for carrying out logistics on the sample when the logistics starting request of the sample is received, and recording the logistics progress of the sample in real time; and a second processing module used for updating the logistics progress of the sample and displaying the logistics progress under the interface of the sample logistics management applet. By means of the technical scheme, the logistics situation of the sample can be recorded and tracked through a software system method, the logistics situation of the sample can be recorded, checked and managed in real time, and full-process automatic management of the sample is achieved.
Owner:戴纳智慧医疗科技有限公司
Preparation method of antigen protein for detecting rabies virus antibody, and kit
PendingCN109828109ASolve the problem that it cannot accurately reflect the true level of effective protective antibodies in the bodyAvoid Biosecurity RisksMaterial analysisViral antibodyNeutralizing antibody
The invention relates to the technical field of in-vitro diagnostic reagents, in particular to a preparation method of antigen protein for detecting a rabies virus antibody and a kit. The method comprises the steps: employing a recombinant baculovirus containing a rabies virus G protein expression cassette for infecting insect cells to obtain infected insect cells; culturing and propagating the infected insect cells; extracting antigen protein of the rabies virus antibody from insect cells; and preparing the kit through the antigen protein. The problem that rabies virus whole-virus particles are used as coating antigens in a traditional method due to a fact that the antibody detection result contains a neutralizing antibody and a non-neutralizing antibody is solved. The method can be usedfor large-scale production and detection of other infectious disease positive serum, has no cross reaction, has the advantages of strong specificity, high sensitivity, good repeatability and wide linear range, avoids biological safety risks, and has very strong creativity.
Owner:WUHAN LIFE TECH
Rapid nucleic acid sequencing method based on fluorescence PCR
InactiveCN105695564AEasy to storeAvoid Biosecurity RisksMicrobiological testing/measurementMicrobiologyNucleic acid sequencing
The invention relates to a rapid nucleic acid sequencing method based on fluorescence PCR, is successfully applied in sequencing of bacterial genes, and can be used for identification of pathogenic bacteria. An FTA card nucleic acid is used for saving pathogenic bacteria nucleic acid, a real-time fluorescence PCR technology is used for amplifying a target fragment, and machine loading is performed for double deoxidization sequencing. A developed fluorescence PCR rapid sequencing reagent kit includes the following reagents: 1) one piece of FTA card; 2) one tube of PCR reaction liquid internally filled with an SYBR GREEN fluorescent PCR reaction solution; 3) one tube of an upstream primer and one tube of a downstream primer which are internally filled with target sequence amplification and sequencing primers; 4) a set of double deoxidization sequencing reagents; 5) a set of sequencing product purification reagents including one tube of a XTerminator solution and one tube of an SAM solution; and 6) one tube of deionized water internally filled with DNA enzyme-free sterile deionized water. Because the FTA card is used for saving the nucleic acid, after the card is dried naturally, the card can be stored for a long time at room temperature, is easy to save, has no need for nucleic acid extraction during PCR amplification, and avoids biological security risks of a bacterial suspension treating operation process during common bacteria gene sequencing.
Owner:汕头国际旅行卫生保健中心
Method for promoting earlier blooming of chrysanthemums by utilizing grafting mediated RNAi (Ribonucleic Acid interfere) technology
InactiveCN107258336AImprove applicabilityAvoid Biosecurity RisksPlant tissue cultureHorticulture methodsRootstockNormal growth
The invention belongs to the technical field of plant cultivation, relates to a regulation and control method for a plant blooming stage and more specifically relates to a method for promoting earlier blooming of chrysanthemums by utilizing a grafting mediated RNAi (Ribonucleic Acid interfere) technology. The method comprises the following steps: carrying out rootstock preparation, carrying out scion selection, carrying out grafting operation and the like; and a rootstock is a transgenic chrysanthemum material with a methylation gene which is silenced by adopting the RNAi technology. According to the method provided by the invention, the transgenic chrysanthemum material is only used as the rootstock and is not used for blooming, so that bio-safety risks of genetic drift can be avoided relatively well; and furthermore, a grafting technology is applied so that the earlier blooming of different chrysanthemum varieties can be realized through the material of only one transgenic chrysanthemum variety, transgenic operation of each variety is avoided and the applicability is relatively good. According to the method for promoting the earlier blooming of the chrysanthemums, provided by the invention, the earlier blooming aim is realized relatively well on the basis of ensuring the normal growth of plants and stabilizing the quality of the chrysanthemums, so that the method has relatively good application values on cultivation of earlier blooming series of good varieties.
Owner:HENAN UNIVERSITY
Sheep pox inactivated vaccine and preparation method thereof
InactiveCN109200282ASolve source difficultiesSolve difficult puzzlesViral antigen ingredientsPharmaceutical delivery mechanismIntramuscular injectionAdjuvant
The invention relates to a sheep pox inactivated vaccine and preparation method thereof. The invention adopts AV41 strain of goat pox virus to inoculate BHK-21 cell, harvesting culture, concentrate, purifying, inactivating BEI, adding suitable adjuvant, mixing and emulsifying. Used to prevent goat pox and sheep pox. The suspension culture BHK-21 cell produce an inactivated sheep pox vaccine, compared with the live vaccine prepared from sheep testicular cells, The present invention not only solves the problem that sheep testicles are difficult to obtain in the production process of live sheep pox vaccine, but also avoids the biological safety risk of primary generation cells carrying exogenous virus, and the prepared live sheep pox vaccine has no potential safety hazards such as lamb death,ewe abortion and the like. In addition, the vaccine can be injected subcutaneously or intramuscularly through the neck to solve the problem of intradermal immunization of live sheep pox vaccine, which is difficult to operate in clinical use.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Method for increasing pork yield by CRISPR/Cas9
InactiveCN109082439AIncrease meat productionPromotes myogenic differentiation potentialStable introduction of DNANucleic acid vectorGene targetsLean meat
The invention provides a method for increasing pork yield by CRISPR / Cas9. The method includes:adopting CRISPR / Cas9 technique for constructing a targeting vector containing swine IGF2 gene targeting sgRNA, transfecting swine cells to obtain cells containing editing type IGF2 genes, and performing somatic cell nuclear transfer and embryo transfer, wherein a nucleotide sequence of the sgRNA for constructing the targeting sector is as shown in SEQ ID NO.1. The method has advantages that an IGF2 gene expression inhibiting effect of ZBED6 can be relieved, IGF2 expression is promoted, and the lean meat rate and the meat yield of pigs are increased.
Owner:SUN YAT SEN UNIV
Broad-spectrum neutralizing antibody for resisting novel coronavirus and application of broad-spectrum neutralizing antibody
ActiveCN114560930AAvoid Biosecurity RisksEfficient and broad-spectrum neutralizing activityImmunoglobulins against virusesAntiviralsAntigenMolecular biology
The invention relates to a broad-spectrum neutralizing antibody for resisting novel coronavirus and application of the broad-spectrum neutralizing antibody. The invention provides an anti-novel coronavirus broad-spectrum neutralizing antibody or an antigen binding fragment thereof, the broad-spectrum neutralizing antibody or the antigen binding fragment thereof has a heavy chain variable region containing VHCDR1, VHCDR2 and VHCDR3 and a light chain variable region containing VLCDR1, VLCDR2 and VLCDR3, the VHCDR1, the VHCDR2 and the VHCDR3 respectively comprise amino acid sequences shown as SEQ ID No.1, SEQ ID No.2 and SEQ ID No.3, and the VLCDR1, the VLCDR2 and the VLCDR3 respectively comprise amino acid sequences shown as SEQ ID No.4, SEQ ID No.5 and SEQ ID No.6. The finally screened antibody has efficient and broad-spectrum neutralizing activity, the IC50 of the antibody to a wild type of the novel coronavirus and 13 mutant strains is less than 0.05 g / ml, and the IC50 of the antibody to WT, Beta, Delta and Omicro BA.1 live viruses is less than 0.1 g / ml.
Owner:TSINGHUA UNIV +1
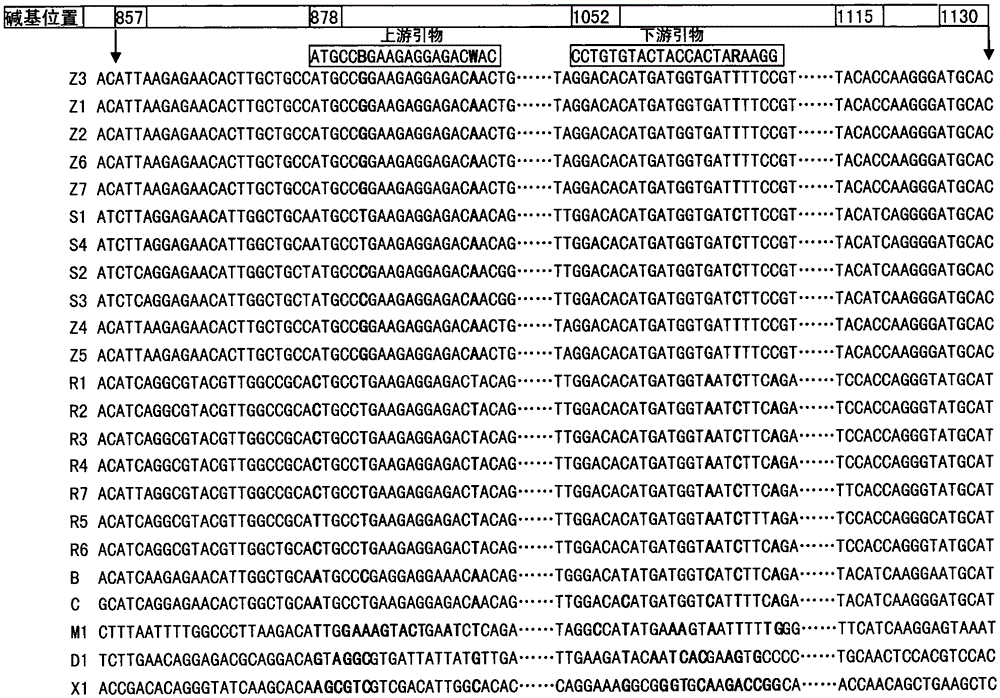
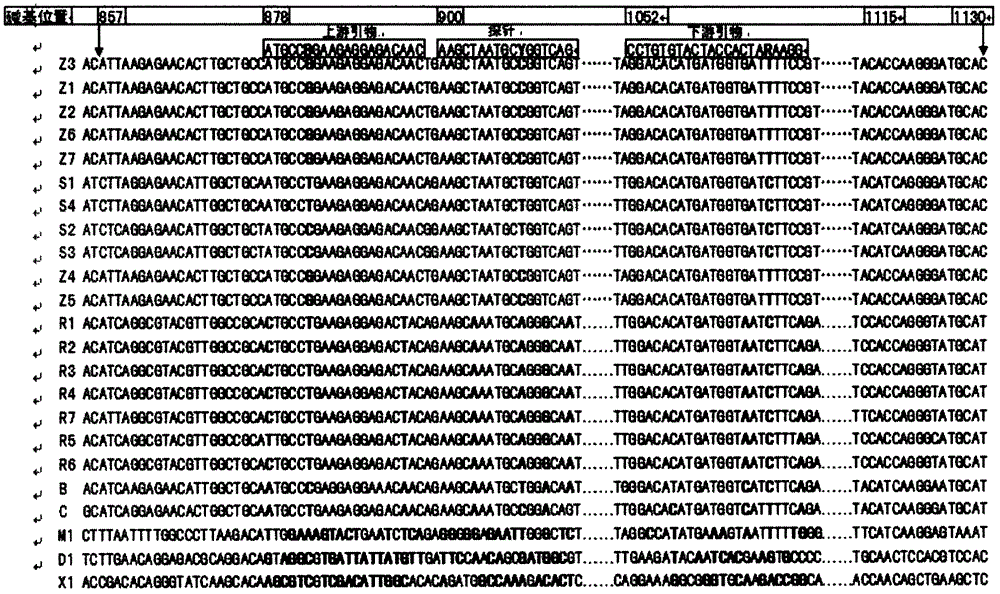
A fluorescent quantitative PCR detection method for detecting Ebola virus and its primers and kit
InactiveCN103045755BNo extension requiredIncreased sensitivityMicrobiological testing/measurementMicroorganism based processesPositive controlEbola virus
The invention discloses a fluorescent quantitative PCR (polymerase chain reaction) method, primer and kit for detecting the EBOV (Ebola virus). The general method can be used for detecting that the sample to be detected is positive as long as the sample contains one or more of the five types of subtype EBOVs which are Z, S, B, C and R at the same time. The method overcomes the defects of the conventional PCR method for detecting by adopting the advantages of high-efficiency nucleic acid amplification of the PCR technology and the sensitivity of the fluorescence-dye SYBR Green I and the computer-aided fluorescent technology for detecting and improves the detection sensitivity, specificity and operation convenience greatly. In addition, the positive control adopted by the method is a section of RNA molecules transcribed in vitro of a NP gene, and the method is safer than the method for detecting by taking the inactivated virus solution as the positive control. The RNA molecules transcribed in vitro can be prepared in quantity, and the sources of the positive control are stable and reliable.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
A vector and recombinant cells for goat blg knockout based on talen-mediated gene targeting
ActiveCN104726495BKnockout precisionKnockout achievedFermentationVector-based foreign material introductionExonGene targeting
The invention discloses a vector and a recombinant cell for knocking out goat BLG based on TALEN-mediated gene targeting. According to the goat β-lactoglobulin gene, the present invention designs and constructs a TALEN eukaryotic expression vector targeting its first exon and a BLG gene knockout vector containing a shorter homology arm, and uses the TALEN expression vector as a template in vitro Transcribed mRNAs were co-transfected into goat fetal fibroblasts, and β-lactoglobulin gene knockout cells were obtained after drug screening and identification. The present invention uses TALEN technology to greatly improve gene targeting efficiency, and at the same time, the use of shorter homology arms can more conveniently and efficiently screen target cells, and the use of mRNAs avoids random integration of TALEN expression vectors in the genome.
Owner:NORTHWEST A & F UNIV +1
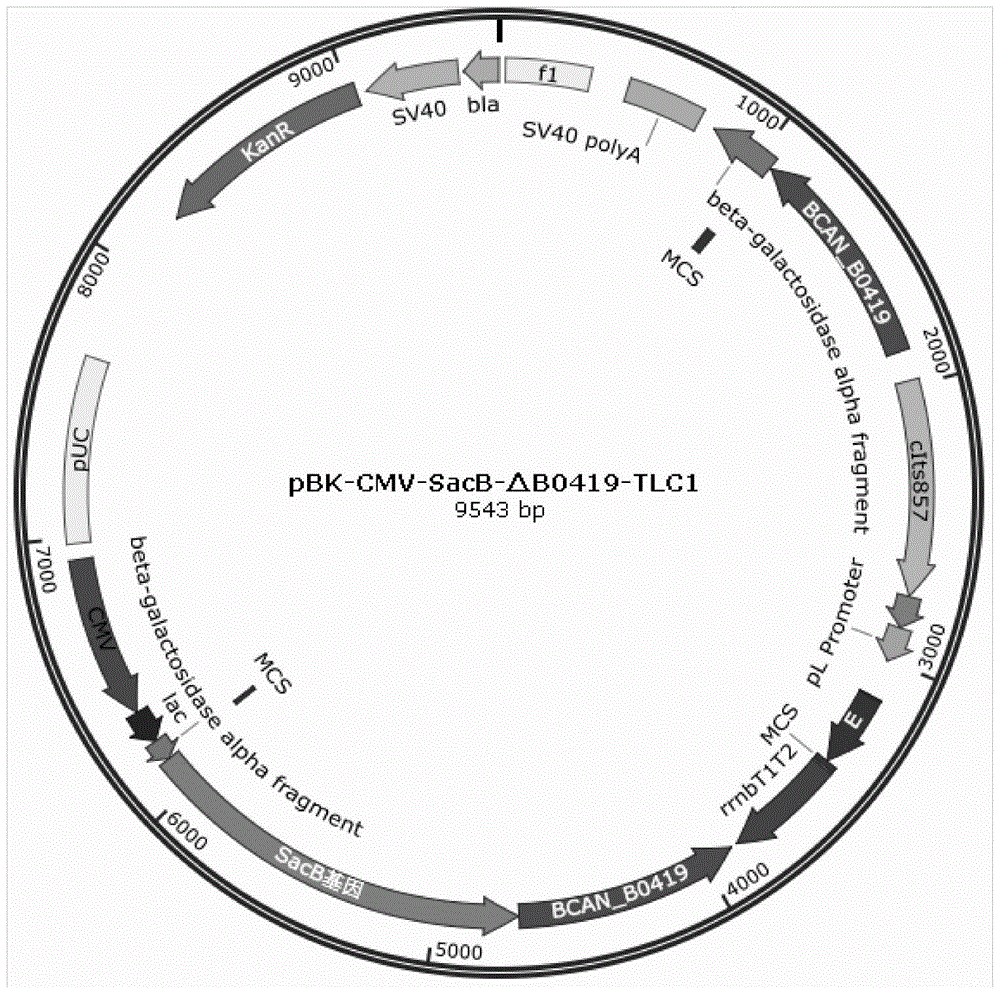
Preparation method of brucella capsid vaccine strain
InactiveCN103952428BWith temperature control cracking functionGood genetic stabilityBacteriaMicroorganism based processesTemperature controlBrucella Vaccine
A preparation method for Brucella shell vaccine strain is disclosed, relates to the field of molecular biology and microbiology, and helps to solve the problem that conventional bacteria shell preparation method is high in cost and not suitable for large-scale production. The method comprises: respectively cloning PCR products of homogenous arms at the upstream and at the downstream of a specific gene of Brucella strain genome to a pMD18-T Simple vector to construct recombinant plasmids, performing double digestion on the recombinant plasmids, successively connecting with plasmid pBK-CMV-SacB subjected to digestion processing for constructing a suicide plasmid, performing PCR amplification on a temperature-control lysis part of a temperature-control expression plasmid pBV220-E, performing digestion processing on the amplification product, inserting into the suicide plasmid; and transforming the temperature-control lysis type suicide plasmid into competent Brucella, and performing positive and negative screening by utilizing kanamycins resistance and fructose sucrase gene, so as to obtain the Brucella shell vaccine strain. The Brucella shell vaccine strain has good heredity stability, security, effectiveness and immunogenicity.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
A Fluorescence Quantitative PCR Detection Method of Xijiang Virus Sybr Green I
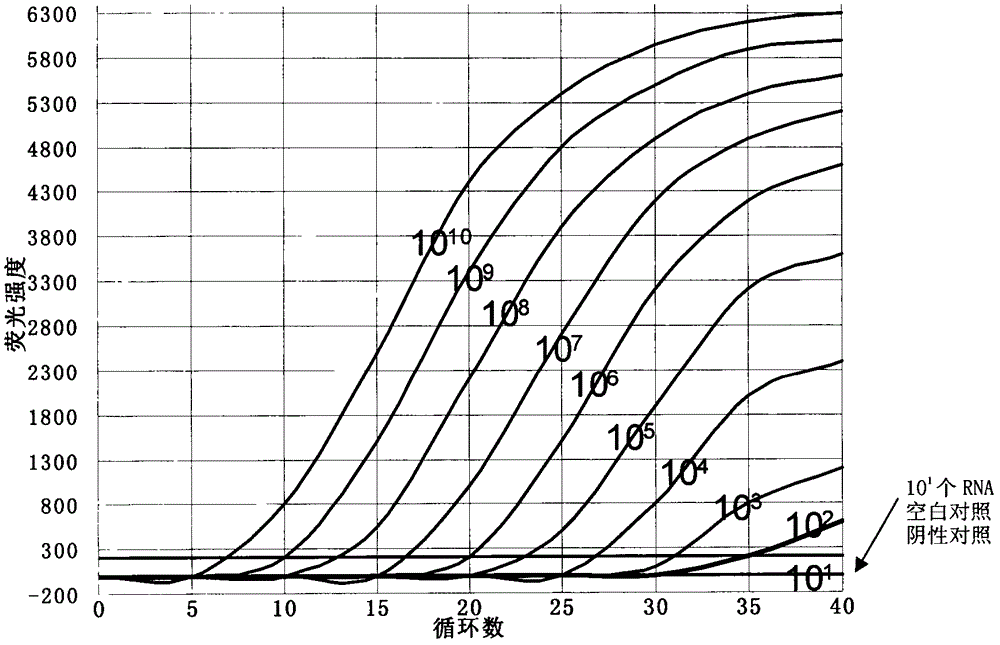
ActiveCN105219888BEasy to operateFinish quicklyMicrobiological testing/measurementSingle sampleVirus
The invention relate to a method for detecting XRV through an SYBR Green I fluorogenic quantitative PCR. The method aims at solving the problem that in the prior art, a method for detecting the XRV through a fluorogenic quantitative PCR is not available. The method comprises the steps that firstly, a primer is designed; secondly, a standard curve is structured; thirdly, negative and positive judgment is carried out. The detection sensitivity of the method can reach 100 copied virus RNA molecules, a single sample can be detected within two hours, operation is easy, and high flux sample detection can be carried out.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
A fully automatic extractor for paramagnetic particles
The invention relates to the nucleic acid extraction field, in particular to a full-automatic extractor for paramagnetic particles. The full-automatic extractor comprises a heat sealing area, a negative pressure reflux area and a nucleic acid extraction reaction area disposed between the heat sealing area and the negative pressure reflux area, the heat sealing area includes a powerful air intake fan and an air inlet cavity disposed below the powerful air intake fan, and the negative pressure reflux area includes a powerful air blower and an air outlet cavity disposed above the powerful air blower. The embodiment of the invention can provide product support for realization of reliable full-automatic high flux nucleic acid extraction in clinical practice and scientific research. And the full-automatic nucleic acid extraction does not suffer from between-sample cross contamination and amplification product contamination. The full-automatic extractor provided by the invention realizes fewconsumables and reagents used in the workflow and low cost. The full-automatic extractor provided by the invention can realize continuous sample introduction and continuous extraction of single sample, has fast extraction speed, no need to wait for batch samples, and high efficiency.
Owner:深圳市优提基因科技有限公司
A kind of Bacillus coagulans and its microbial preparation, preparation method and application
ActiveCN107400637BAvoid secondary pollutionAvoid the risk of secondary pollutionGas treatmentBacteriaBiotechnologyFeces
The invention discloses a strain of bacillus coagulans (Bacillus coagulans) Daoduo 4 and a screening method thereof, and also discloses a microbial preparation containing the strain, a preparation method and an application of the microbial preparation. The microbial preparation of the present invention has simple cultivation, stable structure, non-toxic, harmless and no side effects, can effectively inhibit the reproduction and growth of spoilage bacteria, pathogenic bacteria and other harmful microorganisms, has strong stress resistance to the environment, has high temperature resistance, acid resistance, and Choline and other advantages can inhibit the growth of harmful bacteria in garbage, feces and sewage under complex conditions, eliminate odors, reduce pollution, and improve the environment. On the other hand, it can effectively remove volatile organic compounds (VOC), and it can be used in newly renovated houses and other places, which can significantly reduce the harm caused by decoration pollution to human health.
Owner:上海道多生物科技有限公司
Livestock breeding waste treatment and fermentation system
InactiveCN113526994AAddress Biosecurity RisksHigh degree of automationBio-organic fraction processingClimate change adaptationPlunger pumpAgricultural engineering
The invention discloses a livestock breeding waste treatment and fermentation system which comprises a manure collection pool used for collecting waste generated in the livestock breeding process; a first treatment module which comprises a crusher and a first plunger pump, wherein the crusher is used for carrying out crushing treatment on waste, the crusher is connected with the sealed plunger pump, and the waste treated by the crusher enters the plunger pump; an auger conveyor, wherein the input end of the auger conveyor is communicated with the manure collection pool, and the output end of the auger conveyor is connected with a stock bin of the crusher in a sealed mode, so that the waste can be conveyed into the crusher from the interior of the manure collection pool; and a fermentation tank which is used for realizing fermentation treatment of the wastes. By means of the pipeline transportation mode, the whole transportation pipeline is sealed, so that the biological safety risk of open-air transportation is avoided; the whole system is controlled through a PLC, so that the automation degree is higher; and compared with a traditional transportation mode, labor use is greatly reduced.
Owner:青岛凯昇环保设备制造有限公司
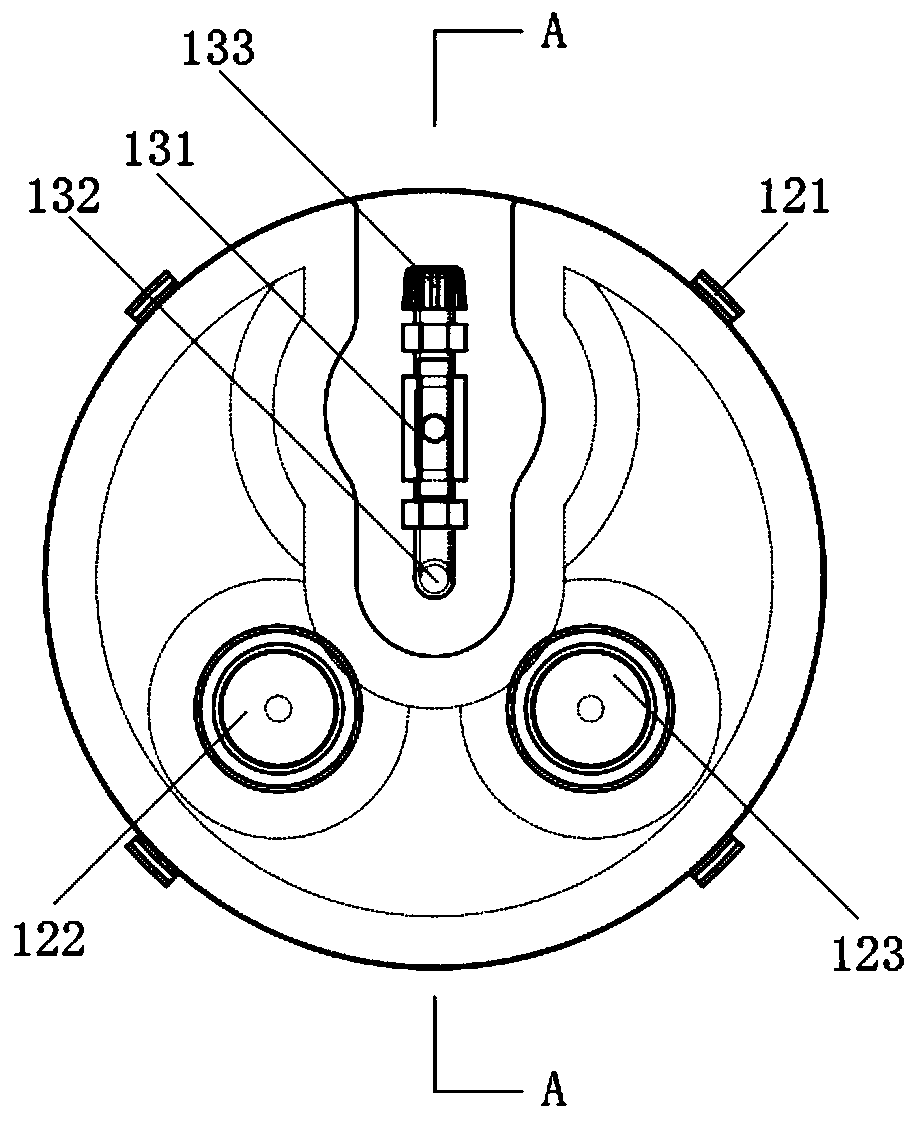
Biological safety type full-automatic capping and sample information management device
A biological safety type full-automatic capping and sample information management device comprises a closed working box, an operation panel is fixed to the left side of the working box, a working cabin is arranged on one side of the operation panel, an aerosol harmless treatment cabin is arranged on one side of the working cabin, a negative pressure cover is fixed to the top of the working box, and an exhaust fan is fixed to the negative pressure cover. The exhaust fan is connected with the aerosol harmless treatment cabin through an air supply pipe; and the working box is provided with a data processing cabin and a power supply cabin, a PCB is fixed in the data processing cabin, a storage battery is fixed in the power supply cabin, the operation panel is fixed on the outer sides of the data processing cabin and the power supply cabin, the operation panel is connected with the PCB, and the PCB is connected with the storage battery. The device not only can cover the test tubes in batches, but also can scan information bar codes on the test tubes and sort and insert the test tubes according to the detection sample information on the test tubes according to the sampling time, so that the test tubes with overdue detection samples can be conveniently taken out; and the biological safety of the device is better.
Owner:FIRST AFFILIATED HOSPITAL OF KUNMING MEDICAL UNIV
Blood sample separation method and blood sample separation device
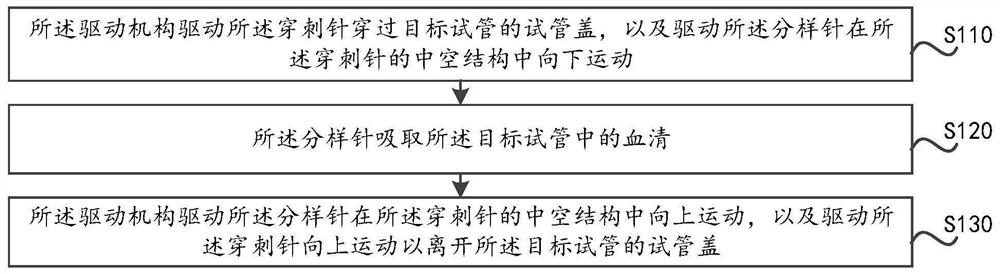
PendingCN113759142AAvoid Biosecurity RisksReduce workloadMaterial analysisNeedle punctureHematological test
The invention provides a blood sample separation method and a blood sample separation device. The blood sample separation device comprises a sample separation needle, a puncture needle and a driving mechanism connected with the sample separation needle and the puncture needle, the puncture needle is provided with a hollow structure, and the sample separation needle is arranged in the hollow structure in a penetrating mode. The method comprises the steps that the driving mechanism drives the puncture needle to penetrate through a test tube cover of a target test tube and drives the sample separation needle to move downwards in the hollow structure of the puncture needle; serum in the target test tube is taken by the sample separation needle; the driving mechanism drives the sample separation needle to move upwards in the hollow structure of the puncture needle and drives the puncture needle to move upwards to leave the test tube cover of the target test tube. The puncture needle penetrates through the test tube cover of the test tube, and then the sample separation needle moves downwards in the hollow structure of the puncture needle to take serum in the test tube, so that a blood sample can be taken without taking off the test tube cover, the test tube cover covers the tube body of the test tube after the blood sample is taken, a cover or a film does not need to be added, biological safety risks can be effectively avoided, the workload is reduced, and the cost is saved.
Owner:SHENZHEN MINDRAY BIO MEDICAL ELECTRONICS CO LTD
A kind of vero cell serum-free culture medium and application thereof
ActiveCN111733126BClear chemical compositionLow costCulture processArtificial cell constructsBiotechnologyAluminium chloride
The invention relates to a Vero cell serum-free culture medium and an application thereof. The Vero cell serum-free culture medium comprises an amino acid component, a vitamin component, an inorganicsalt component, trace element components, carbohydrate and other molecular compound components, wherein the trace element components include the following components: zinc sulfate heptahydrate, coppersulfate pentahydrate, ferric nitrate nonahydrate, ferrous sulfate heptahydrate, sodium selenite, nickel chloride, stannous chloride, silver nitrate, cobalt chloride and aluminum chloride. The serum-free culture medium provided by the invention has the advantages of definite chemical components and low cost, avoids the biological safety risk caused by the use of serum when the serum is not needed,and provides convenience for the large-scale production of biological products, especially vaccines. Vero cells grow well in the culture medium, and the serum is not used, so that the stability of the production process of the biological products and the stability of the quality of finished products are improved, and particularly, the research, development and production of novel coronavirus (SARS-CoV-2) vaccines are accelerated.
Owner:苏州依科赛生物科技股份有限公司
Real-time fluorescence quantitative RT-PCR method and its kit for one-step detection of z/s subtype Ebola virus
InactiveCN103045754BStrong specificityHigh sensitivityMicrobiological testing/measurementFluorescence/phosphorescenceEbola virusFluorescence
The invention discloses a one-step process real-time fluorescent quantitative RT-PCR (Reverse Transcription-Polymerase Chain Reaction) method and kit as well as a primer and a probe for detecting Z / S subtype ebola viruses (EBOV). The one-step process real-time fluorescent quantitative RT-PCR method is a general detection method; and the PCR detection process can be used for detecting Z and S subtype EBOVs after being carried out once. A sample is positive as long as any one of the Z and S subtype EBOVs or both the Z and S subtype EBOVs exist in the sample to be detected. The one-step process MGB (Minor Groove Binder) probe fluorescent quantitative RT-PCR technology provided by the invention combines the advantages of efficient amplification of nucleic acids in a PCR technology and sensitivity of a MGB probe and a computer-assisted fluorescence detection technology, overcomes the shortcomings of conventional PCR detection and greatly increases detection sensitivity, specificity and convenience of operation.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Reagent lid and sample reagent loading device
ActiveCN110360795BAvoid Biosecurity RisksImprove accuracyLighting and heating apparatusDomestic refrigeratorsCold airAqueous droplet
Owner:CHENGDU SHEN MINDRAY MEDICAL ELECTRONICS TECH RES INST +1
Biodegradable Metal Vascular Stents
InactiveCN107158479BGrain refinementImprove mechanical propertiesSurgeryCoatingsMetalMaterials science
Owner:青岛市即墨区人民医院
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com