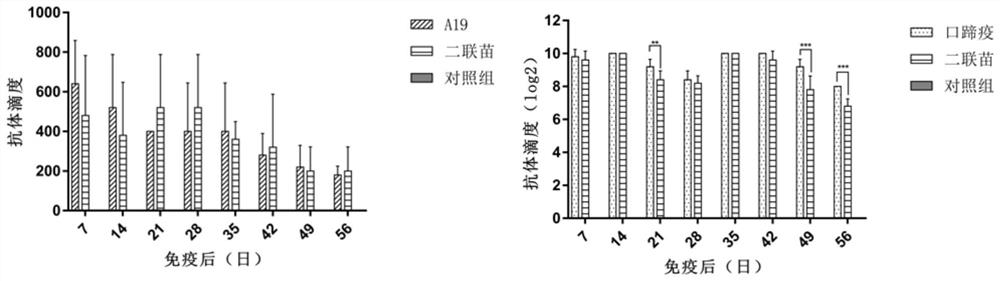
Brucellosis and foot-and-mouth disease bivalent vaccine and preparation method and application thereof
A technology of brucellosis and dual vaccine, which is applied in the field of brucellosis and foot-and-mouth disease dual vaccine and its preparation, can solve problems such as loss of appetite, decreased milk production, infection of humans and animals, etc., and achieves Reduced side effects, convenient operation, and the effect of resisting attacks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Preparation and cultivation of embodiment 1 Brucella ghost bacterial strain
[0063] 1. Preparation of Brucella ghost strains
[0064] 1.1 Construction of the Brucella ghost strain is constructed by transforming the plasmid containing the phage cleavage protein E gene, Staphylococcus aureus nuclease A gene (SNA), and temperature control elements into the A19 strain by electroporation.
[0065] 1.2 Amplification and cloning of phage E gene
[0066] 1.2.1 E gene amplification
[0067] Using the pUC57-E plasmid as a template, use the E gene amplification primers E-F, E-R for amplification. The reaction conditions are: 94°C pre-denaturation for 5 minutes, 94°C denaturation for 30 seconds, 56°C annealing for 30 seconds, and 72°C extension for 30 seconds. 30 cycles, 72°C extension for 10 minutes. A 50 μl reaction system is used, and the specific components are as follows:
[0068] E gene amplification PCR reaction system table
[0069]
[0070]
[0071] 1.2.2 E gen...
Embodiment 2
[0098] Embodiment 2 Preparation of foot-and-mouth disease virus-like particles
[0099] 1 Recombinant plasmid construction
[0100] 1.1 VP1, VP2, VP4 gene amplification
[0101] Using the O-type foot-and-mouth disease virus genome as a template, use primers VP1-F / VP1-R (enzyme cutting sites EcoRI, NdeI), VP2-F / VP2-R (enzyme cutting sites NdeI, SacI) containing restriction sites , VP4-F / VP4-R (enzyme cutting sites SacⅠ, BamHI) were respectively amplified to obtain VP1, VP2, VP4 gene sequences.
[0102] 1.2 VP1, VP2, VP4 gene cloning
[0103] Recover the VP1, VP2, and VP4 genes with the agarose gel recovery kit, connect the gene fragments to the pMD18-T Simple vector overnight at 4°C, and transform them into DH5α competent cells. Take 100 μl of the competent cell and vector mixture and spread it on Take the plate containing Amp (100 μg / ml) on TSA, and incubate at 36-38°C for 24 hours.
[0104] Connection System Information
[0105]
[0106] 1.3 Identification of positive...
Embodiment 3
[0115] Embodiment 3 Preparation of brucellosis and foot-and-mouth disease dual vaccine
[0116] Concentrate the FMD virus-like particles 10 times with a hollow fiber column, mix them with Brucella bacterial shadows at a mass ratio of 1:2.5, mix them at 115 rpm at 4-6°C for 26 minutes, and the resulting mixture is cloth Secondary immunization vaccines for rucellosis and foot-and-mouth disease, in which the content of Brucella ghost antigens is not less than 28 μg per serving, and the content of foot-and-mouth disease virus-like particles is not less than 11.2 μg per serving.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com