Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38 results about "Urethral stents" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Polycaprolactone-collagen-fibroin nano three-dimensional porous stent and preparation method thereof
InactiveCN104874018AEasy to processEfficient manufacturing processFilament/thread formingConjugated cellulose/protein artificial filamentsUrethral stentsFresh Tissue
The invention relates to the field of the preparation of urethral stents, and discloses a polycaprolactone-collagen-fibroin nano three-dimensional porous stent and a preparation method thereof. The preparation method comprises the following steps of dissolving waste mulberry silk in a 0.1 percent of Na2CO3 aqueous solution, and repeatedly boiling for half an hour, so as to extract silk fibroin; dissolving the silk fibroin at a high temperature by using a ternary solution, dialyzing and filtering, and then putting at a room temperature for drying to form a regenerated silk fibroin membrane; dissolving the regenerated silk fibroin membrane, water-soluble collagen and polycaprolactone in hexafluoroisopropanol together, so as to form a mixed solution; carrying out electrostatic spinning on the mixed solution through a rotary receiving device, so as to obtain the nano three-dimensional porous stent, wherein a rotation speed is 300 to 500 revolutions per minute, and the diameter of a rotating shaft is 2 to 6 millimeters. The stent provided by the invention is convenient and efficient to process and manufacture, is convenient to apply, moreover, is convenient for adjusting various performances of a material, is stale in structure, and has certain mechanical strength to support the growth of a fresh tissue.
Owner:SHANGHAI TONGJI HOSPITAL
Urethral stent reducer
An apparatus for compressing a coiled stent having at least one protrusion, such as an enlarged coil disposed at the end of the stent, has a mandrel insertable into a lumen of the stent for holding the stent by friction and a coil compressor coupled to the mandrel. The mandrel is rotatable on an axis relative to the coil compressor and the coil compressor has a tab extending therefrom towards the mandrel. A stent is placed on the mandrel with the enlarged coil extending toward the coil compressor. The tab presses the enlarged coil inwardly toward the lumen of the stent when the mandrel is rotated relative to the coil compressor.
Owner:ADVANCED TECH & REGENERATIVE MEDICINE
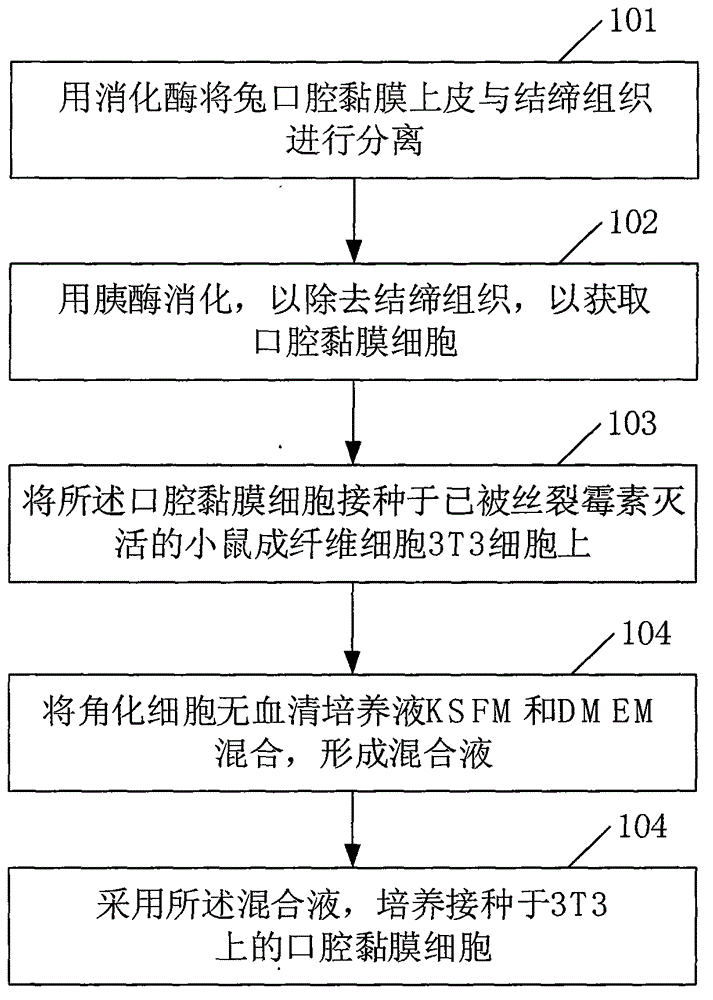
Method for culturing oral mucosa cells
InactiveCN104862269AHigh separation purityImprove proliferative abilityArtificial cell constructsVertebrate cellsUrethral stentsDigestive enzyme
The invention relates to the field of preparation of urethral stents and discloses a method for culturing oral mucosa cells. The method comprises the following steps: separating oral mucosal epithelium and connective tissues of a rat by using digestive enzymes, and digesting by using pancreatin so as to remove the connective tissues, thereby obtaining the oral mucosa cells; inoculating the oral mucosa cells to mouse fibroblast cells 3T3 which are inactivated by mitomycin, and mixing keratinocyte serum-free culture solution KSFM and DMEM to form the mixed solution; and culturing the oral mucosa cells inoculated to the cells 3T3 by adopting the mixed solution. According to the method disclosed by the invention, the separation purity of the oral mucosa cells is improved, and the adherence and proliferation capacities of seed cells can be improved.
Owner:SHANGHAI TONGJI HOSPITAL
Tissue-engineered urethral stent graft, and preparation method and application thereof
InactiveCN110960727ARepair damageDegradation rate matchingEpidermal cells/skin cellsArtificial cell constructsUrethral stentsUrethral injury
The invention discloses a tissue-engineered urethral stent graft, and a preparation method and application thereof, belonging to the technical field of tissue engineering. The tissue-engineered urethral stent graft is a macromolecular degradable nanofiber tube inoculated with epithelial cells and smooth muscle cells, wherein the epithelial cells are epithelial cells of epithelial progenitor cellswith monopotent differentiation potential, the degradation rate of the nanofiber tube can be matched with the regeneration rate of urethral repair, and urethral injury can be effectively repaired.
Owner:GUANGZHOU WOMEN AND CHILDRENS MEDICAL CENTER
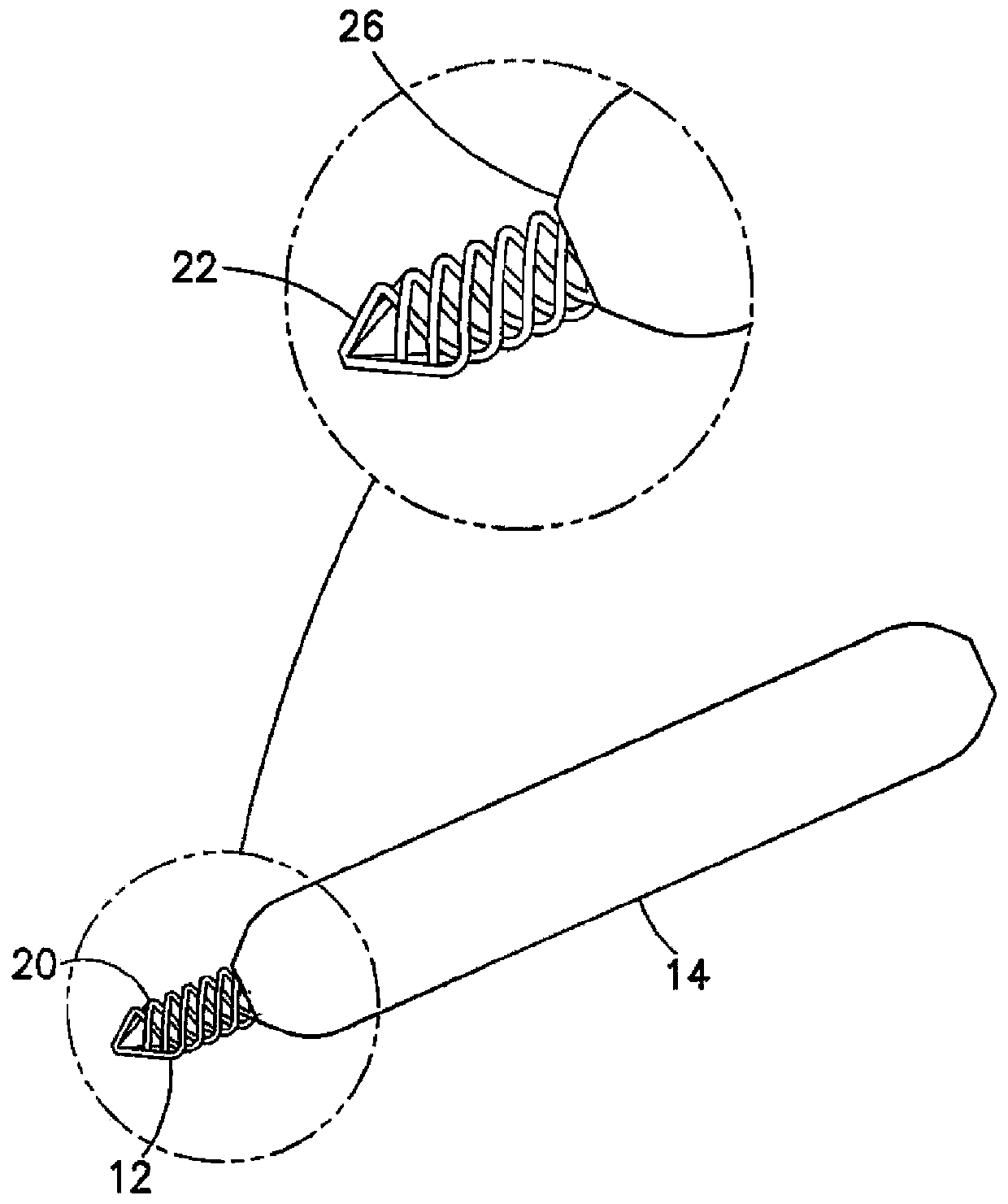
Spiral urethral stent and production method thereof
The invention aims to provide a spiral urethral stent and a production method thereof. The spiral urethral stent is made of polymer composites. The polymer composites are formed by compositing nondegradable high polymer materials and biodegradable high polymer materials. The nondegradable high polymer materials and the biodegradable high polymer materials are mixed uniformly according to a certain proportion, and are extruded to be high polymer material filaments through an extruding machine at a certain melting temperature. The production method of the spiral urethral stent comprises the following steps: winding the high polymer material filaments on a mold to be a spiral shape; and shaping for 0.5h to 48h at the temperature of 40 DEG C to 90 DEG C so as to form the spiral urethral stent. The stent can be partially degraded within a controlled range, and after urethral repair is finished, the partial degraded stent can be taken out from the urethral.
Owner:张自强
Urination control instrument for urethra
ActiveCN107280806AReduce stimulationImprove comfortAnti-incontinence devicesUrethral stentsEngineering
The invention discloses an urination control instrument for the urethra. The instrument comprises a urethral stent which is netlike tube, a connecting piece comprising a connecting ring and a urethral valve. The urethral valve is connected with the connecting ring to form a valve capable of controlling a longitudinal channel of the urethral stent to open and close. The urethral valve comprises at least one valve leaflet, and at least one of the valve leaflets can be opened in a swing manner. Magnet combinations attracting each other are embedded inside the edge of the swingable valve leaflet and the connecting piece movably connected with the same and / or inside the adjacent valve leaflet. The urethral stent is of a netlike structure and is in line contact with the urethra after being implanted to the urethra, stimulation to the urethra is reduced, occurrence of corresponding complications is reduced, and comfortableness of the implanted urethra is improved. The valve leaflets are controlled to swing by controlling the magnet combinations, further the valve leaflets are controlled to open and close, controllability in urination is realized, the valve orifice is large when the valve leaflets are opened to make urination smooth.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
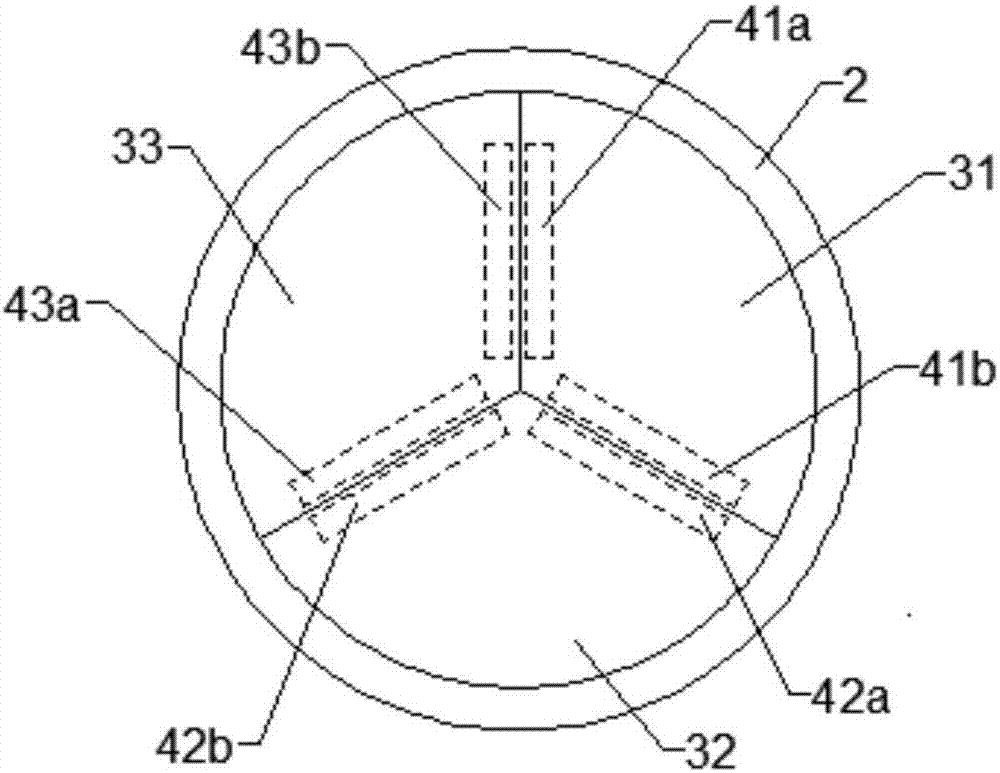
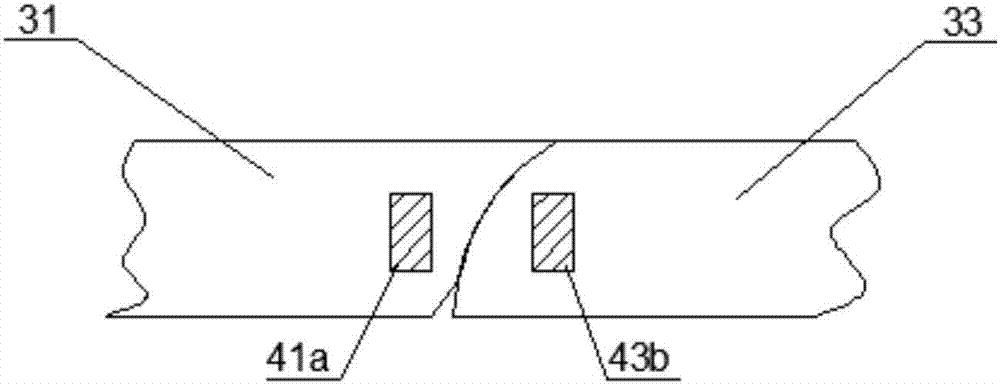
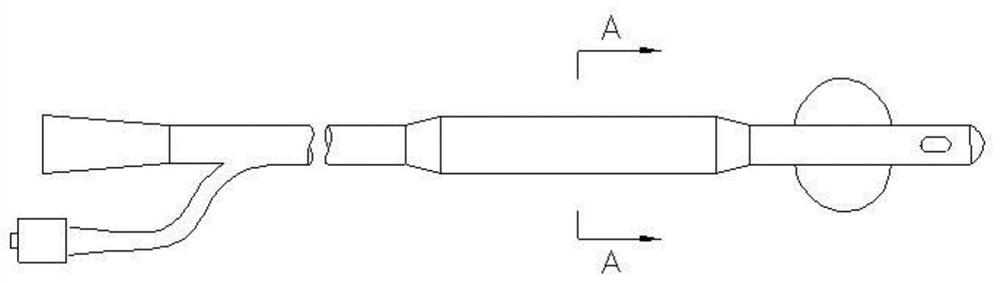
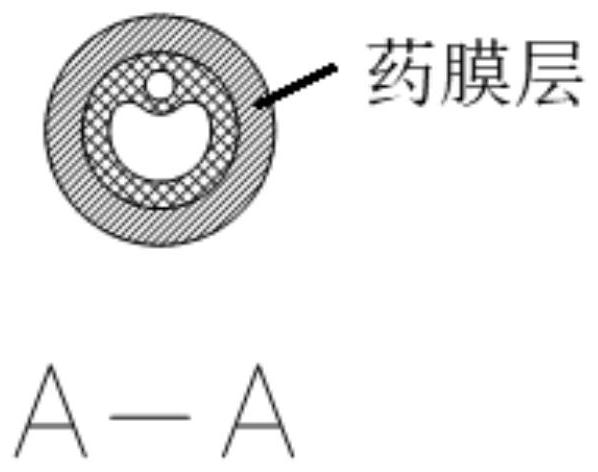
Urethral stent and preparation method and application thereof
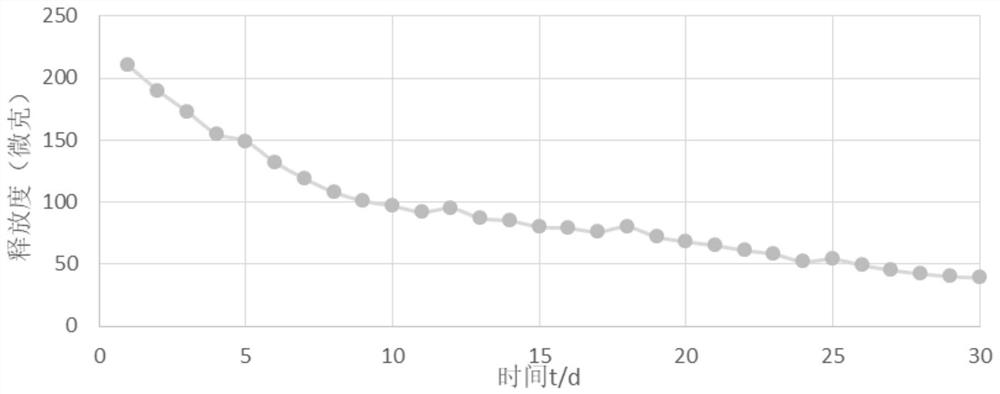
The invention provides a urethral stent and a preparation method and an application thereof. The urethral stent comprises a stent base body and a medicine film arranged on the outer surface of the stent base body, and the medicine film comprises controlled release factors and medicine; when the urethral stent is implanted into the urethra, the stent base body plays a role in supporting and draining the urethra, the medicine film is used for preventing or reducing restenosis of the urethra, and the urethral stent has the treatment effect of being low in dosage and high in efficiency, and has the long medicine release period and the good slow release capacity. Besides, the urethral stent can play a targeting role, the situation that the liver needs to be metabolized firstly during oral administration is effectively avoided, the dosage is about 10% of that of an oral administration or injection mode, and the side effect of the medicine on the whole body is greatly reduced.
Owner:YIPURUN SHANGHAI BIOTECH CO LTD
Biodegradable tissue engineering urethral stent and preparation method thereof
InactiveCN111450317AImprove toughnessStay flexibleStentsAdditive manufacturing apparatusUrethral stentsGlycolates
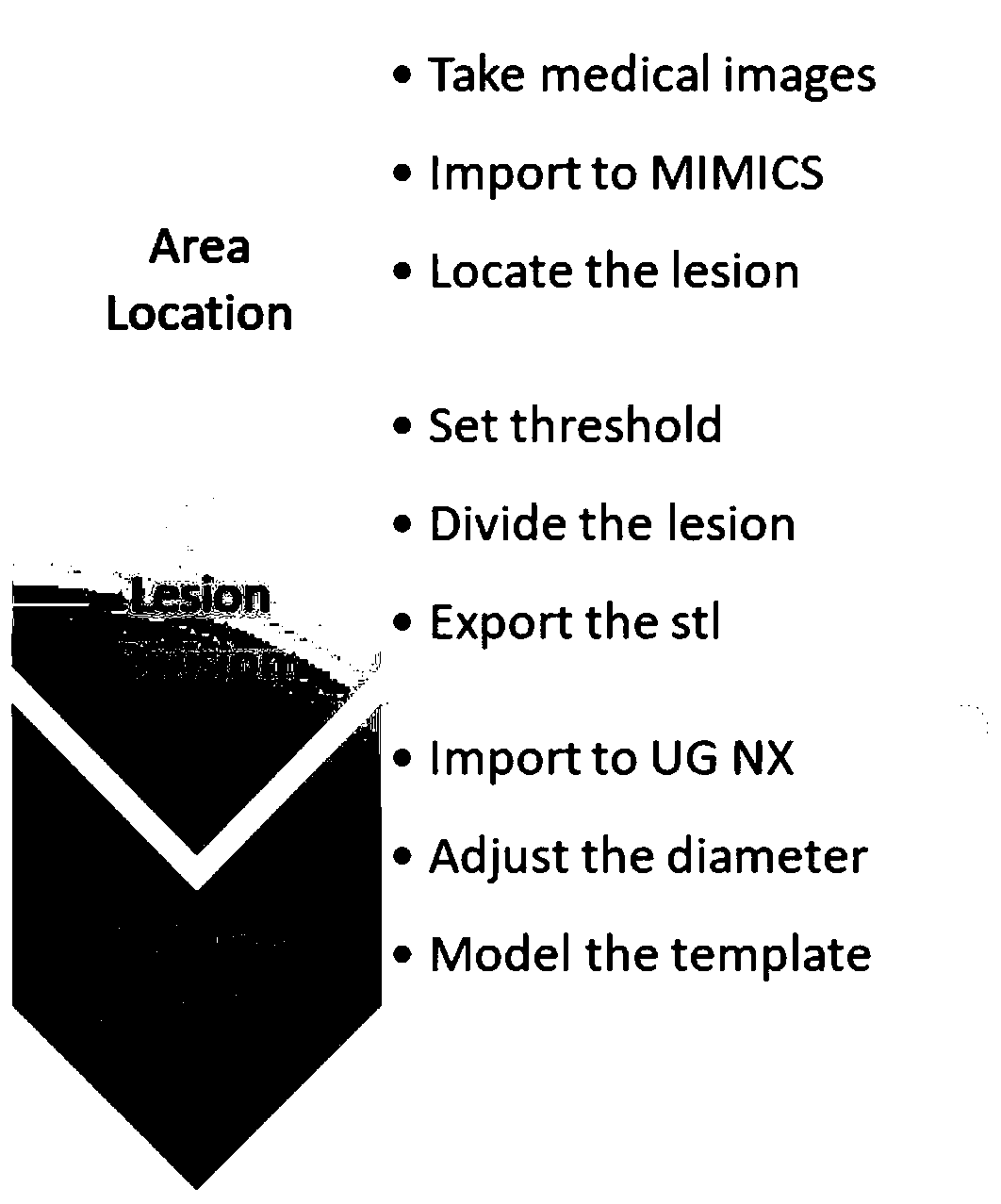
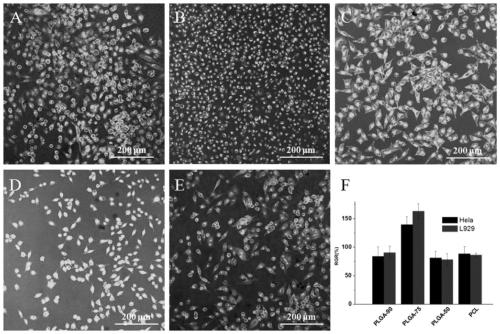
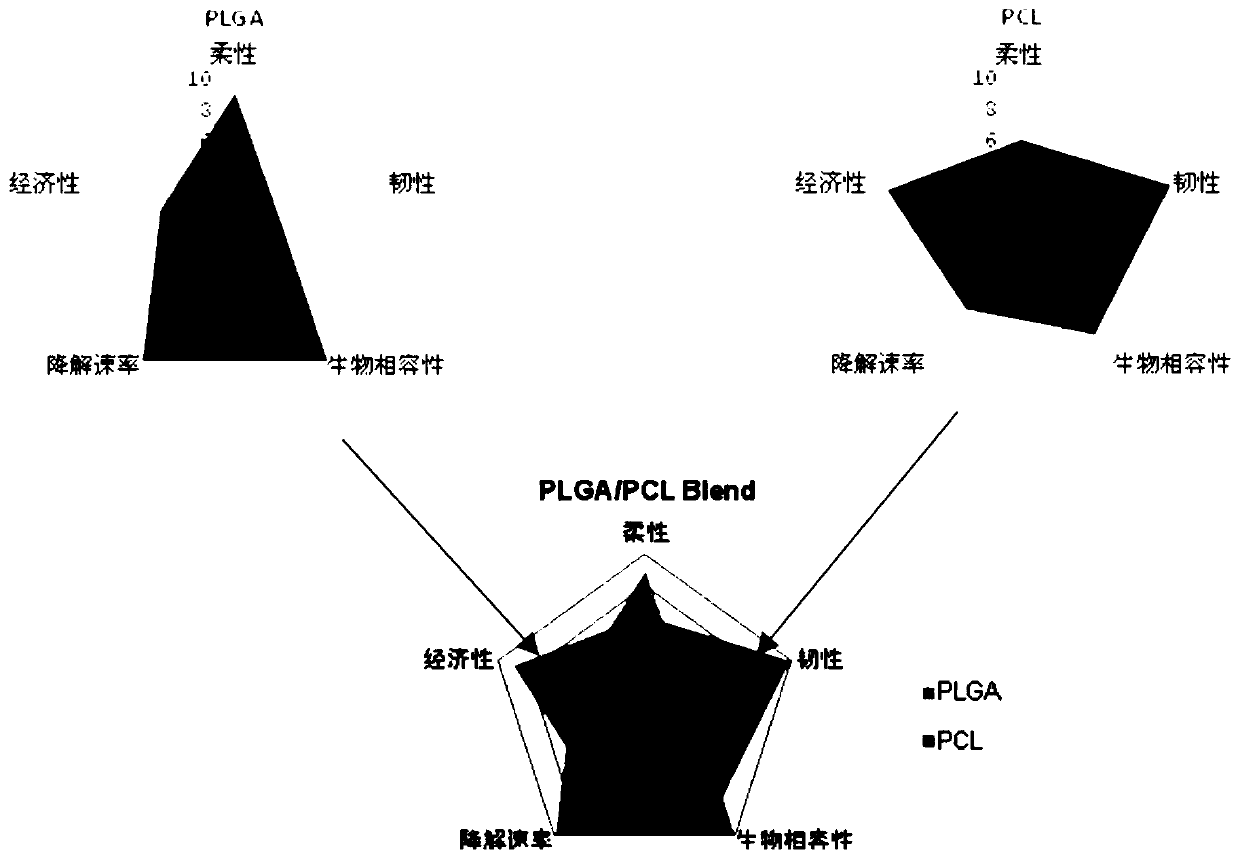
The invention discloses a biodegradable tissue engineering urethral stent and a preparation method thereof. The biodegradable tissue engineering urethral stent is made of a copolymer formed by polylactic acid-co-glycolate, polycaprolactone and a solubilizer according to the weight ratio of 70 to 30 to (2-10). The preparation method of the biodegradable tissue engineering urethral stent comprises the following steps of a) blending preparation of a modified material solution, b) medical image processing and modeling, c) 3D template printing and d) stent preparation. PLGA and PCL biodegradable polymers are subjected to solution co-mixing modification, and triethyl citrate is added as the solubilizer, and the blending ratio and the content of the solubilizer are adjusted, so that the toughnessof the modified material is relatively and greatly improved while the flexibility and the tensile strength are kept; and based on a 3D printing technology and in combination with medical image data and software processing, a soluble template can be printed for the focus of a patient, and the personalized tissue engineering urethral stent is prepared.
Owner:ZHEJIANG SINOU ENVIRONMENTAL PROTECTION EQUIP CO LTD
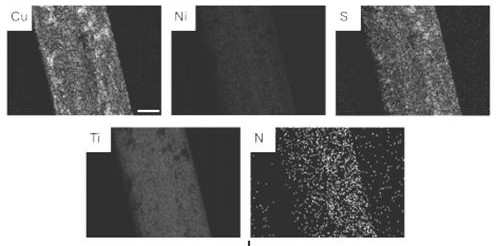
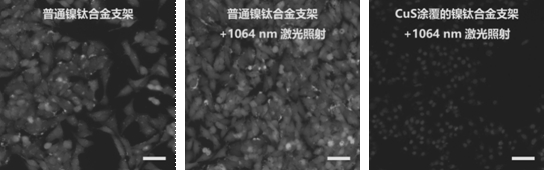
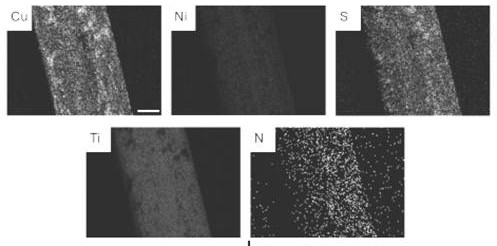
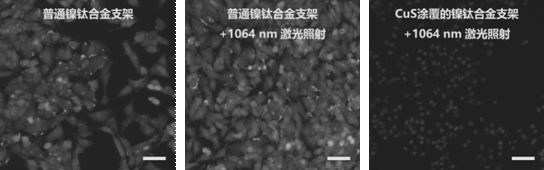
Memory alloy esophageal stent modified by nano copper sulfide coating and preparation method of memory alloy esophageal stent
ActiveCN113499483AStable in natureNot easy to decomposeStentsPharmaceutical delivery mechanismUrethral stentsNitinol stent
The invention belongs to the technical field of medical instruments, and relates to a memory alloy esophageal stent with a uniform nano copper sulfide film and an efficient photo-thermal physiotherapy function and a preparation method of the memory alloy esophageal stent. The stent is prepared by reducing dopamine under an alkaline condition to obtain a polydopamine-coated memory alloy stent, and then efficiently adsorbing copper ions through polydopamine under a heating condition to grow copper sulfide in situ. According to the method, a unique method for in-situ growth of nano CuS particles on the surface of the stent is adopted, the nano CuS material is safe and non-toxic, and the growth process is simple and rapid; photo-thermal conversion is efficient, and repeated use can be achieved; the method can be applied to nickel-titanium alloy stents of various shapes and other types, such as biliary tract stents, intestinal tract stents, urinary tract stents and tracheal stents.
Owner:FUZHOU UNIV
Stent replacement system suitable for urinary retention and urinary incontinence
ActiveCN111481327AExtended replacement cycleEliminate cumbersomenessStentsOperating means/releasing devices for valvesUrethral stentsStent replacement
The invention relates to a stent replacement system suitable for urinary retention and urinary incontinence. The stent replacement system comprises a stent system and a control system. The stent system comprises a cylindrical urethral stent with an anti-falling lug and a valve. The anti-falling lug is matched with the expansibility of the urethral stent, so that the stability of the device can beguaranteed. The valve can control the opening and closing of the urethra. The control system comprises a power supply module, a central control module, a pressure sensing module, a signal transmissionmodule and a power module and is used for realizing intelligent control. The power module comprises a transmission shaft and a spiral blade; opening and closing of the valve can be achieved in cooperation with a blocking rod on a fixed valve seat and a blocking piece on a movable valve plug. The spiral blade can form a vortex to assist urination. The system has the advantages that the urethra canbe expanded, urination can be assisted, operable intelligent control over opening and closing of the urethra is achieved, the stability is good, and the system can be placed for a long time so as toeffectively cope with urinary retention and urinary incontinence.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL
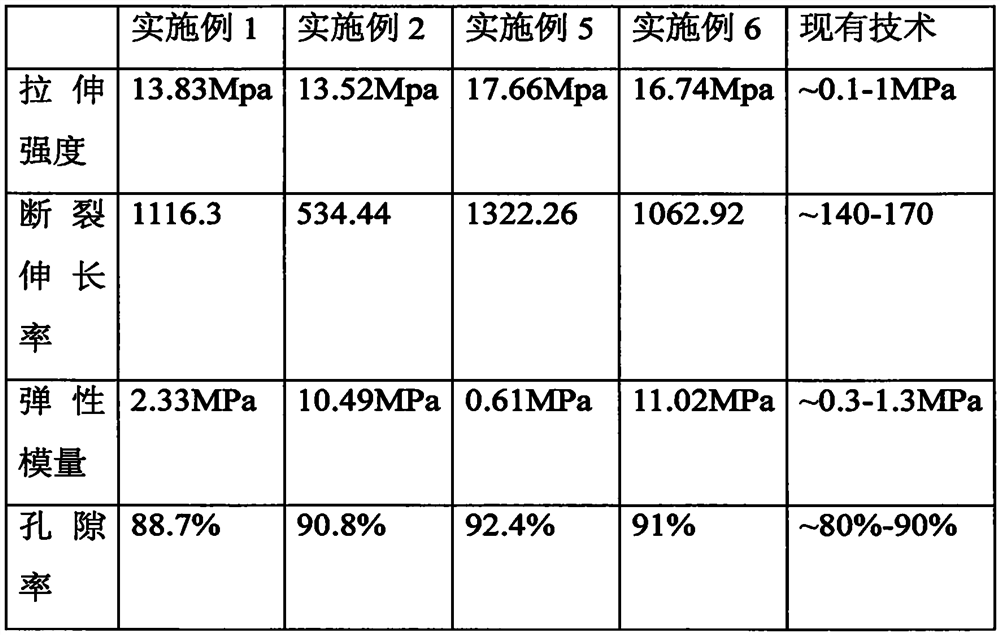
A biodegradable polymer porous urethral repair scaffold and its preparation method
InactiveCN103170007BGood biocompatibilitySimple preparation processStentsTubular organ implantsEpitheliumOrganic solvent
The invention discloses a biodegradable macromolecule urethra repairing support. The biodegradable macromolecule urethra repairing support is characterized by being a tissue engineering support with a tubular appearance shape and being prepared from macromolecular materials which can be completely biodegraded, the support is soft and has toughness, the microstructure of the support is a porous three-dimensional space, the average pore size is 20-200 microns, the support has good biocompatibility, and cells can be planted. The invention also discloses a preparation method of the biodegradable macromolecule urethra repairing support. The prepared urethra repairing support shows certain rigidity and necessary toughness, can endure strong tensile force, and is conducive to restoration of the shape of the support when being extruded and has good biocompatibility, and urethra mucous epithelium cells can be adhered and propagated on the support. According to the prepared urethra repairing support, residual of organic solvents and pore-foaming agents sare avoided, biological assessment is safer, a preparation process is simpler and more convenient, cost is lower, and the prepared urethra repairing support is more applicable to clinical application.
Owner:SHANGHAI NAT ENG RES CENT FORNANOTECH
Biodegradable articles and methods for treatment of pelvic floor disorders including extracellular matrix material
InactiveUS20160213815A1Promote tissue regenerationIncreased formationAnti-incontinence devicesSurgeryDiseaseUrethral stents
Biodegradable implants including an ECM material and methods for treating a pelvic floor condition are described. ECM particles can be present within or on the surface of a pelvic implant, such as a biodegradable mesh, a pouch, or a urethral stent. After implantation the implant can provide tissue support, degrade over a period of time, and deposit the ECM material in the implantation area for regeneration of tissue and long term benefit.
Owner:BOSTON SCI SCIMED INC
Absorbable tubular stent and preparation method and application thereof
InactiveCN105214144AHigh mechanical strengthGood grip rebound performanceSurgeryUrethral stentsEmbolization Therapy
The invention discloses an absorbable tubular stent. The absorbable tubular stent is characterized in that the tubular stent is made by blending of polylactic acid and acylated chitosan, and the wall of the tubular stent is provided with or without a porous structure or pattern structure. Due to addition of the acylated chitosan, local acidity generated by the polylactic acid in degradation can be regulated, and oligosaccharides and monosaccharide obtained by degradation of the acylated chitosan can be absorbed and used by organisms, so that high biocompatibility, degradability and absorbability are achieved; the tubular stent has excellent mechanical strength and press resilience. The absorbable tubular stent can be planted in vivo by surgeries to serve as an intravascular stent and is applicable to treatment of embolism or blood vessel lumina narrowing caused by vascular diseases; the absorbable tubular stent can also serve as a biliary stent to be applied to treatment of cholestasis and pancreaticobiliary obstruction caused by pancreatobiliary neoplasms; the absorbable tubular stent can further serve as a urethral stent to be applied to treatment of dysuria resulted from urethral compression caused by prostatic hyperplasia. The absorbable tubular stent can be degraded and absorbed in vivo finally along with lesion repair, in-vivo long-term retention is avoided, and the absorbable tubular stent has a promising market prospect.
Owner:OCEAN UNIV OF CHINA
Urological stent
An apparatus including a first layer, where the first layer includes a scaffold structure forming an inner lumen along a length of the scaffold structure, and where the first layer includes a bioresorbable material; and a second layer on the first layer, where the second layer includes a bioresorbable material, wherein the second layer surrounds a majority of the first layer, and where the secondlayer is configured to hydroscopicly swell.
Owner:OLYMPUS CORP
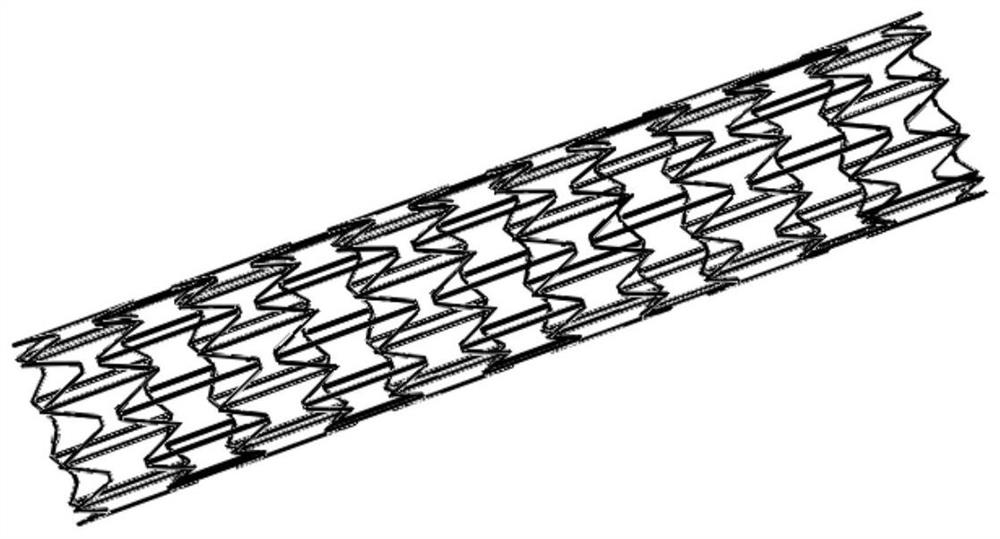
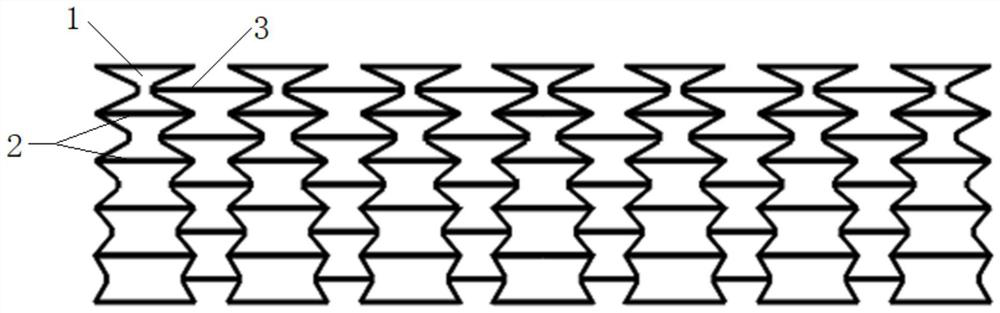
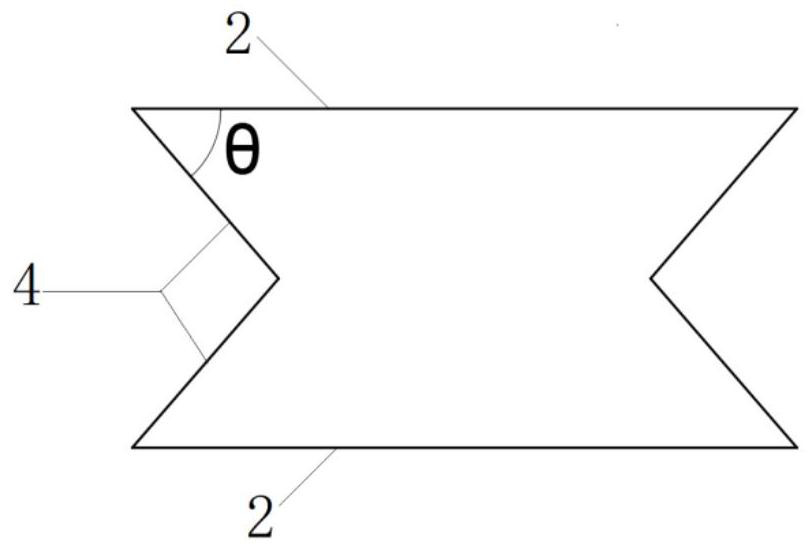
Stent suitable for urethral bent part
ActiveCN112107401AReduce problems such as restenosisEasy implantationStentsAdditive manufacturing apparatusUrethral stentsHuman body
The invention provides a stent suitable for a urethral bent part. A body of the urethral stent is of a tubular structure made of a degradable or non-degradable material; the tubular structure is in astraight tube state before being implanted; at least one part of the tubular structure is bent after being implanted into a human body; the tube wall of the tubular structure is formed by arranging and combining concave hexagonal structures; and, in the concave hexagon at the bent position after being implanted into the human body, the included angle theta formed by the straight edges and the inclined edges of the concave hexagons sequentially arranged from the bent outer side to the bent inner side is gradually increased. After the urethral stent in the invention is implanted into a urethralstenosis part, both the inner diameter and the length of the stent can be increased by means of balloon dilatation and the like; furthermore, the stent can be bent at the same time after being implanted so as to adapt to physiological bending of a urethral membrane part, a ball part and a pubis anterior bending part; and thus, the problems of intimal hyperplasia, stent restenosis and the like caused by urethral straightening due to overlarge rigidity of the stent are solved.
Owner:BEIHANG UNIV
Urethral stent with long-acting antibacterial and anti-stenosis functions and preparation method thereof
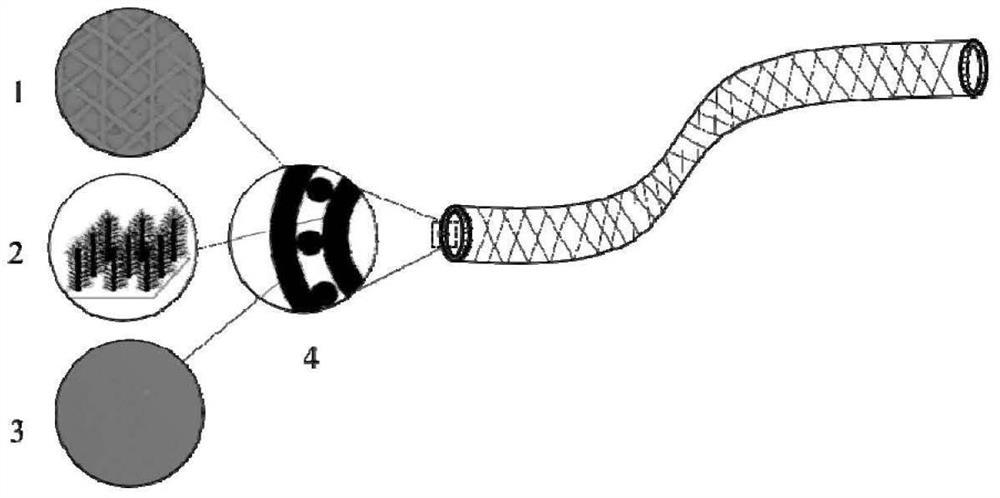
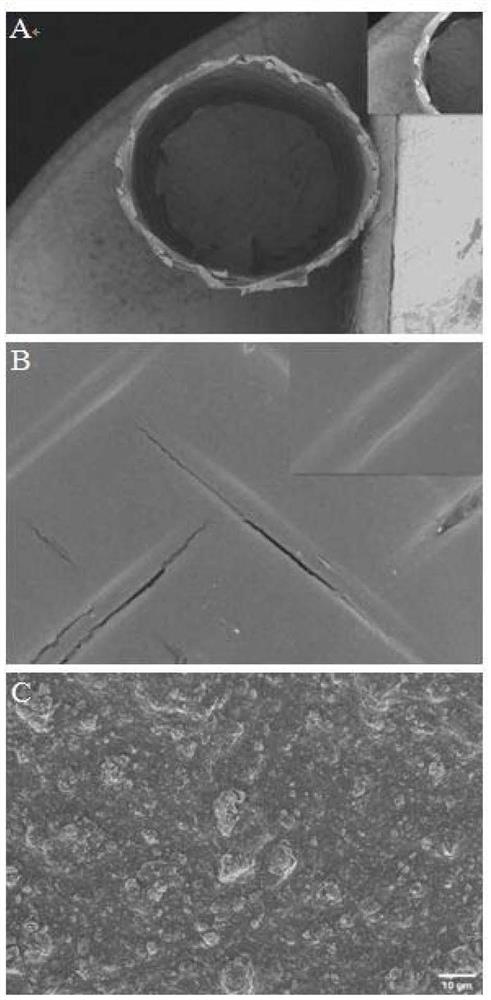
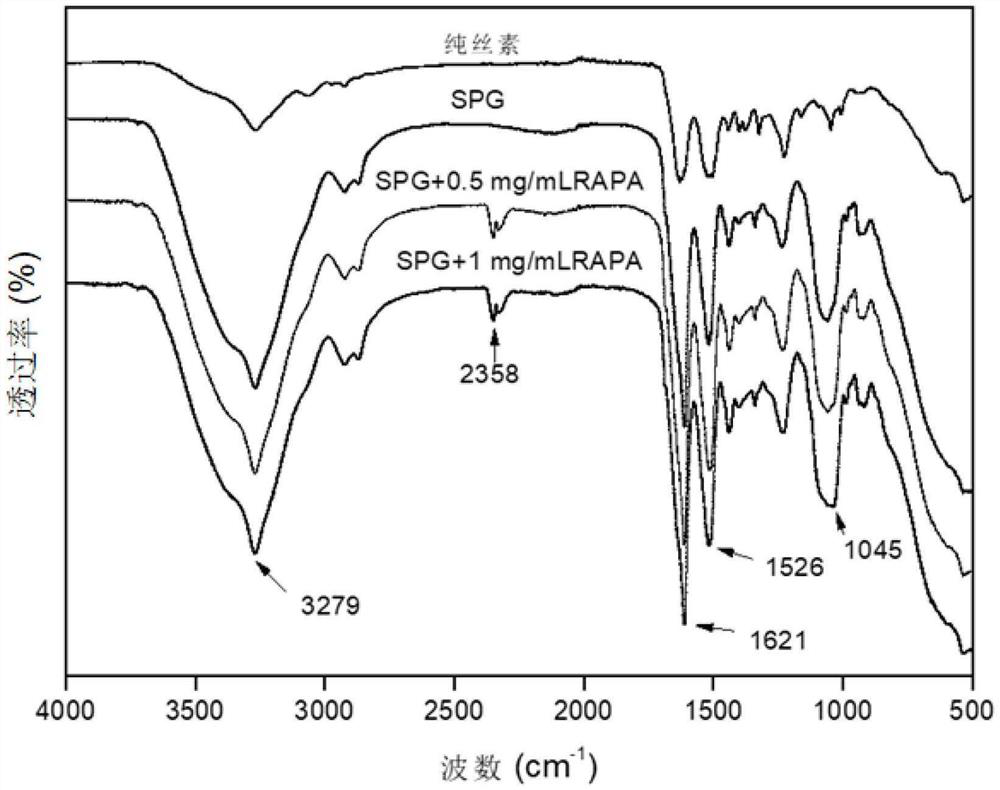
ActiveCN113577401AImprove mechanical propertiesImprove stress resistanceSurgeryMedical devicesUrethral stentsEngineering
The invention relates to a urethral stent with long-acting antibacterial and anti-stenosis functions and a preparation method thereof, the urethral stent is of a tubular structure and is composed of an inner layer, a middle layer and an outer layer; the middle layer is a fabric tube; the outer layer is a silk fibroin film carrying an anti-stenosis drug; the inner layer is an antibacterial and antifouling coating and is composed of a chitosan-nano silicon dioxide array, villus, micro villus and an antibacterial agent; and the preparation method comprises the following steps: preparing the yarns into fabric tubes by adopting a spinning forming method, preparing the antibacterial and anti-fouling coatings on the inner surfaces of the fabric tubes, and preparing the silk fibroin films carrying the anti-stenosis medicines on the outer surfaces of the fabric tubes, so as to prepare the urethral stent with the long-acting antibacterial and anti-stenosis functions. The method disclosed by the invention is simple, and the prepared urethral stent is smooth in surface and good in biocompatibility, not only has excellent performances such as radial compression force, circumferential expansionary force, bending torsion resistance and elastic resilience, but also has long-acting antibacterial and anti-stenosis functions, and can prevent excessive deposition of urethral collagen tissues, so that the purpose of treating urethral stenosis is achieved.
Owner:NANTONG TEXTILE & SILK IND TECH RES INST +1
A method for constructing segmental individualized three-dimensional scaffold material for human urethra
ActiveCN105031725BHas a physiological curvatureAdditive manufacturing apparatusProsthesisHuman anatomyPenis
The invention discloses a method for constructing a segmental individualized human urethral three-dimensional scaffold material, which comprises using three-dimensional ultrasound technology to obtain the overall imaging information of the patient's thin-layer penis; using a manual outline method to screen out the overall imaging information to be constructed Image area: Use 3D technology to perform 3D reconstruction and processing of the image area to be constructed, import it into a 3D printer for printing, and prepare a urethral model, and then construct a material-fillable urethral model after the urethral model is reversed by a silica gel inversion process. Silicone model: the filling material is placed in the silicone model for reorganization, and a three-dimensional scaffold material of human urethra with individual characteristics is constructed. The solid model constructed by the technical solution of the present invention fits the physiological and anatomical position structure of the normal human urethra, conforms to the individual characteristics of the patient, and enables the constructed material to have the advantage of being adjustable in macroscopic and microscopic multi-scales.
Owner:SHANGHAI SIXTH PEOPLES HOSPITAL +1
A kind of tissue engineering urethral stent and preparation process thereof
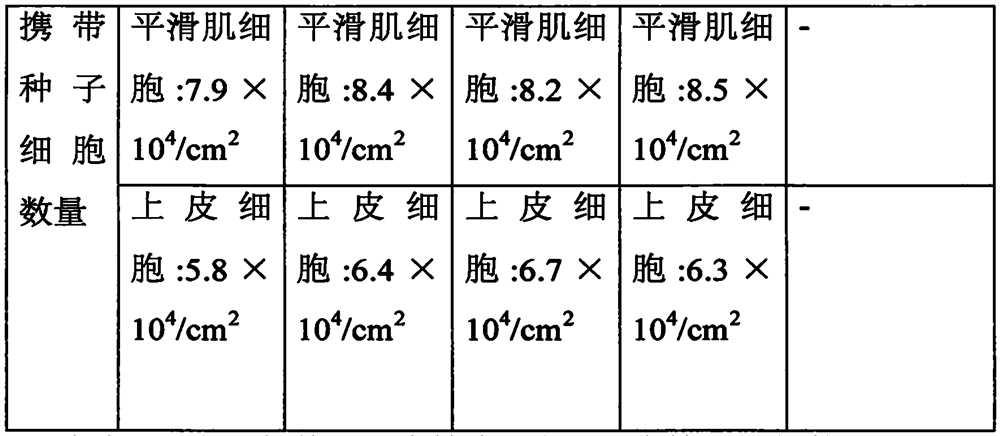
The invention discloses a tissue engineering urethral stent and a preparation process thereof, and relates to the technical field of polymer materials. Specifically, the preparation process can produce tissue engineering urethral stents suitable for different individuals, and the preparation process is beneficial to large-scale batches Production. Secondly, the tissue engineering urethral scaffold prepared by this preparation process has good biocompatibility, biodegradability and biomechanical properties, and its microporous structure can ensure the adhesion and proliferation of seed cells, so that the tissue engineering urethral scaffold can carry enough A large number of seed cells are suitable for different forms of urethral injury, especially long-term urethral injury repair.
Owner:GUANGZHOU WOMEN AND CHILDRENS MEDICAL CENTER
Preparation method of 3D printing hydrogel urethral stent
PendingCN114681675AReduced swelling energyDegradation has no noticeable effectProsthesisUrethral stents3d print
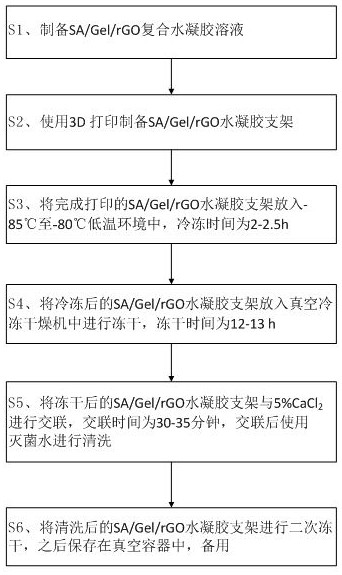
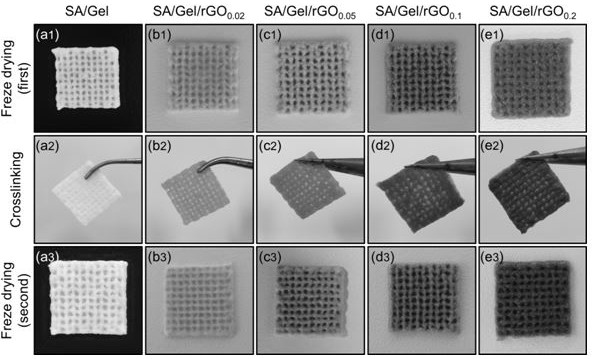
The invention discloses a preparation method of a 3D printing hydrogel urethral stent. The preparation method comprises the following steps: S1, preparing an SA / Gel / rGO composite hydrogel solution; s2, preparing an SA / Gel / rGO hydrogel scaffold by using 3D printing; s3, putting the printed SA / Gel / rGO hydrogel stent into a low-temperature environment of-85 DEG C to-80 DEG C, and freezing for 2-2.5 hours; s4, putting the frozen SA / Gel / rGO hydrogel stent into a vacuum freeze-drying machine for freeze-drying, wherein the freeze-drying time is 12 to 13 hours; s5, the freeze-dried SA / Gel / rGO hydrogel scaffold and 5% CaCl2 are subjected to crosslinking, the crosslinking time is 30-35 minutes, and after crosslinking, the scaffold is washed with sterilized water; and S6, carrying out secondary freeze-drying on the cleaned SA / Gel / rGO hydrogel scaffold, and then storing in a vacuum container for later use. The SA / Gel / rGO nano-composite hydrogel is prepared by introducing an rGO solution into an SA / Gel mixed solution and is printed, so that the swelling property, the pore size and the tensile property of the hydrogel can be well improved.
Owner:张楷乐
Preparation method of urethral stent and urethral stent
The invention provides a preparation method of a urethral stent and the urethral stent. The preparation method comprises the following steps: preparing a PLLA membrane with good toughness and good mechanical strength from lecithin and PLLA, putting a first PLLA stent into an alkaline solution to modify the first PLLA stent so as to activate carboxyl on a surface of the first PLLA stent to obtain a second PLLA stent, firstly reacting the second PLLA stent with EDC to generate an intermediate product, then reacting with NHS to form a group easy to bind to amino to obtain a third PLLA stent, covalently grafingchitosan on a surface of the third PLLA stent to obtain a fourth PLLA stent, and then binding individual molecules of chitosan into a network structure through a surface cross-linking agent to obtain the urethral stent. Due to a fact that chitosan molecules are only distributed on a surface of the third PLLA stent, the prepared urethral stent is not compact and crisp, has good mechanical properties, and can prevent disintegration and fragmentation from blocking the urethra.
Owner:BEIJING INSTITUTE OF GRAPHIC COMMUNICATION
Close-cell structured stent, a preparation method and use thereof
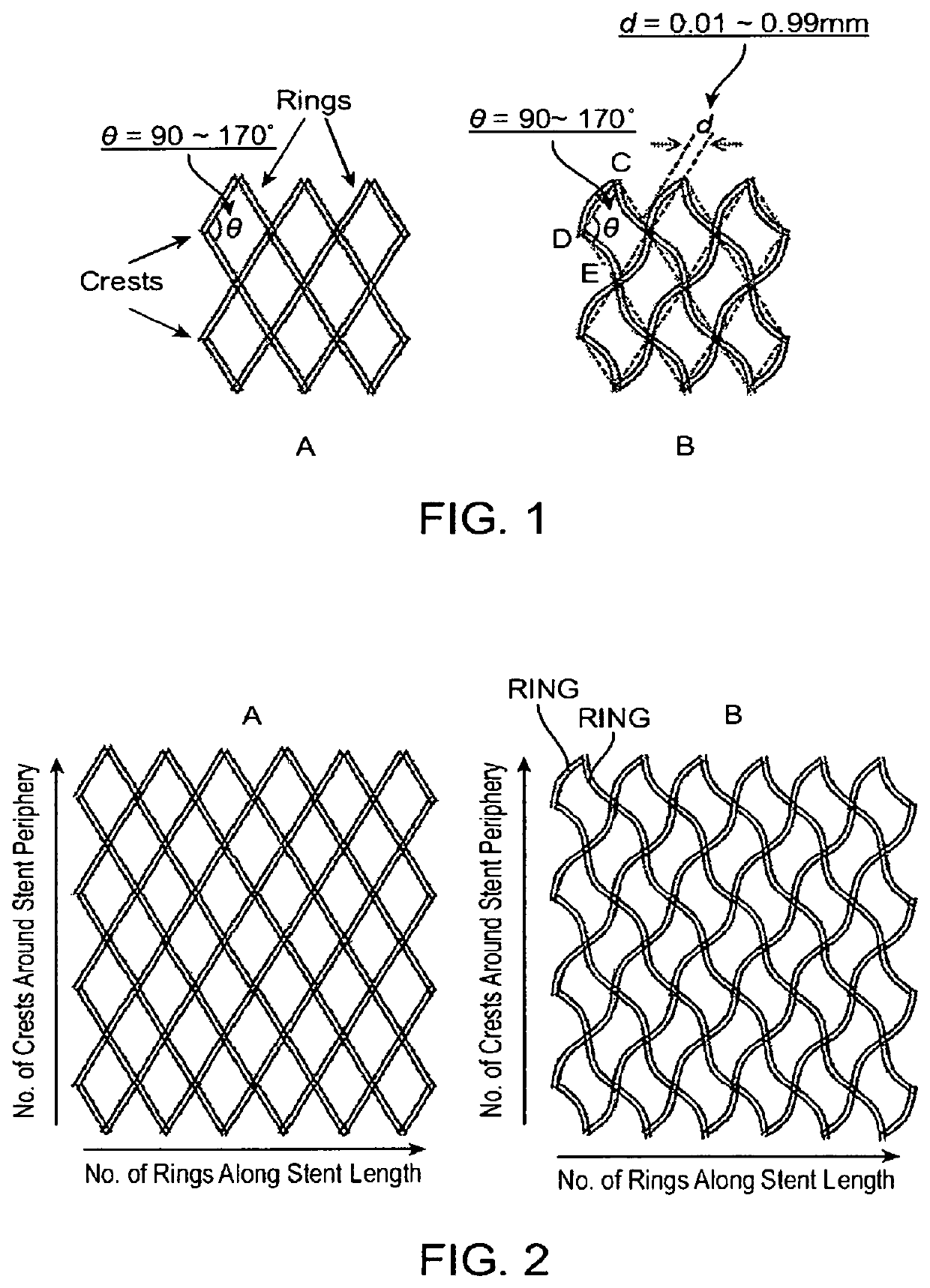
ActiveUS10603194B2Increase flexibilityHigh strengthUrinary bladderStentsUrethral stentsStent grafting
Disclosed herein is a close-cell structured stent which is composed of curved struts that intersect at a crossing angle of 90 to 170 degrees in the longitudinal direction. Methods for making the stent, use of the stent as a vascular stent, an esophagus stent, an intestine stent, a bile conduct stent, or a urinary tract stent, and use of the stent in the manufacture of stent graft for the treatment of abdominal aortic aneurysms are also provided. The stent has both excellent longitudinal flexibility and radial strength.
Owner:BEIJING ADVANCED MEDICAL TECH
A urethral stent for non-invasive surgery and its preparation method
The present invention provides a urethral stent for non-invasive surgery. The urethral stent includes: a main structure made of polyglyceryl dodecanoate (PGD), the main structure is in the shape of a long and thin strip at normal temperature, After being implanted in the human body, it transforms into a helical structure; the degradable alloy wire is coiled on the outer surface of the main structure. The main structure of the stent in the present invention is made of polylaurin, and the main structure is in the shape of a slender strip at room temperature, which is convenient to be placed in the urethra during surgery. The polylaurin has a memory effect at body temperature, After it is implanted into the human body, it can deform and become a helical structure, which can expand the urethra, thereby significantly reducing the difficulty of the operation and improving the comfort of the stent after implantation. In the present invention, the degradable alloy wire is coiled around the main structure, which can deform together with the PGD material while providing support for the stent structure, so as to expand the urethra.
Owner:BEIHANG UNIV
A structure and function bionic urethral stent and preparation method thereof
ActiveCN113750297BGood urethral defect repair effectSimple processSurgeryPharmaceutical delivery mechanismUrethral stentsCross linker
The invention provides a bionic urethral scaffold with structure and function and a preparation method thereof; the preparation method is as follows: (1) mix the oxidized bacterial cellulose nanofiber dispersion and the acellular matrix solution and pour it into the mold I until it is completely filled and frozen Dry to obtain an uncrosslinked porous scaffold; (2) put the uncrosslinked porous scaffold into a crosslinking agent solution, rinse with water after crosslinking, and then freeze-dry to obtain a crosslinked porous scaffold; (3) oxidize the bacterial fiber The mixed liquid II composed of plain nanofibers, modified or unmodified natural polymer materials, gel-forming aids and water is poured into the silica gel mold II until it is completely filled, and the cross-linked porous scaffold is fixed on the mold II, so that Contact with the mixed solution II and let it stand for a period of time to obtain a structural and functional bionic urethral scaffold; the prepared scaffold is composed of a hydrogel layer of the bionic urethral mucosa and a porous layer of the bionic urethral spongy body, and can be rapidly epithelialized and Vascularized, has a good repair effect on urethral defects.
Owner:DONGHUA UNIV
High-strength urethral stent easy to recycle
PendingCN113288504ASmooth positioningPrevent abrupt size changesStentsUrethraeUrethral stentsEngineering
The invention discloses a high-strength urethral stent easy to recycle, and the stent comprises a stent body; the stent body comprises a guiding section, a transition section and an anchoring section which are sequentially arranged from the near end to the far end; the outer diameter of the anchoring section is greater than that of the guiding section, one end of the transition section is connected with the guiding section, and the other end of the transition section is connected with the anchoring section; the outer diameter of the transition section is gradually increased from one end to the other end, the outer diameter of the anchoring section is gradually decreased from the near end to the far end, and the stent body is of an integrally-formed structure. When the stent changes from the guide section to the anchoring section through the transition section, the outer diameter of the stent is gradually increased, so that the anchoring section can be limited at a bladder neck opening after entering the bladder neck due to the size difference; due to the fact that the far end of the anchoring section of the support is in an inward-contracting shape, the sheath tube is arranged at the far end of the anchoring section of the support, the support can be smoothly contracted into the sheath tube, and guiding and recycling of the support are achieved.
Owner:上海惠凯医疗科技有限公司
A memory alloy esophageal stent modified by nano-copper sulfide coating and its preparation method
ActiveCN113499483BStable in natureNot easy to decomposeStentsPharmaceutical delivery mechanismUrethral stentsNitinol stent
The invention belongs to the technical field of medical devices, and relates to a memory alloy esophageal stent with a uniform nano-copper sulfide film with high-efficiency photothermal physiotherapy function and a preparation method thereof. The stent reduces dopamine under alkaline conditions to obtain a polydopamine-coated memory alloy The scaffold is prepared by the method of in-situ growth of copper sulfide by polydopamine efficiently adsorbing copper ions under heating conditions. The method of the present invention adopts a unique method of in-situ growing nano-CuS particles on the surface of the support. The nano-CuS material is safe and non-toxic, and the growth process is simple and fast; the light-to-heat conversion is efficient and can be used repeatedly; the present invention can be used in various shapes and Other types of nickel-titanium alloy stents, such as biliary stents, intestinal stents, urinary tract stents, and tracheal stents.
Owner:FUZHOU UNIV
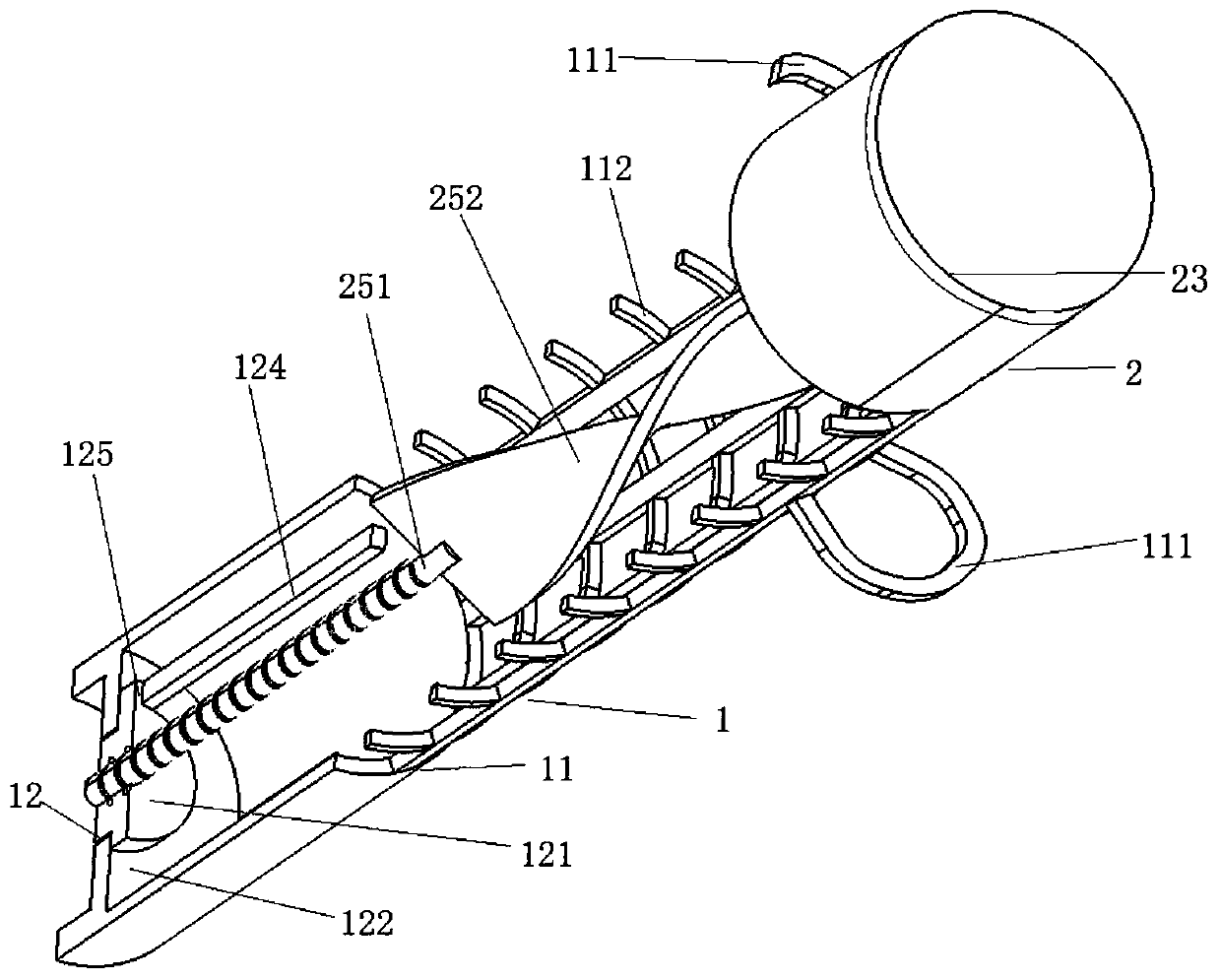
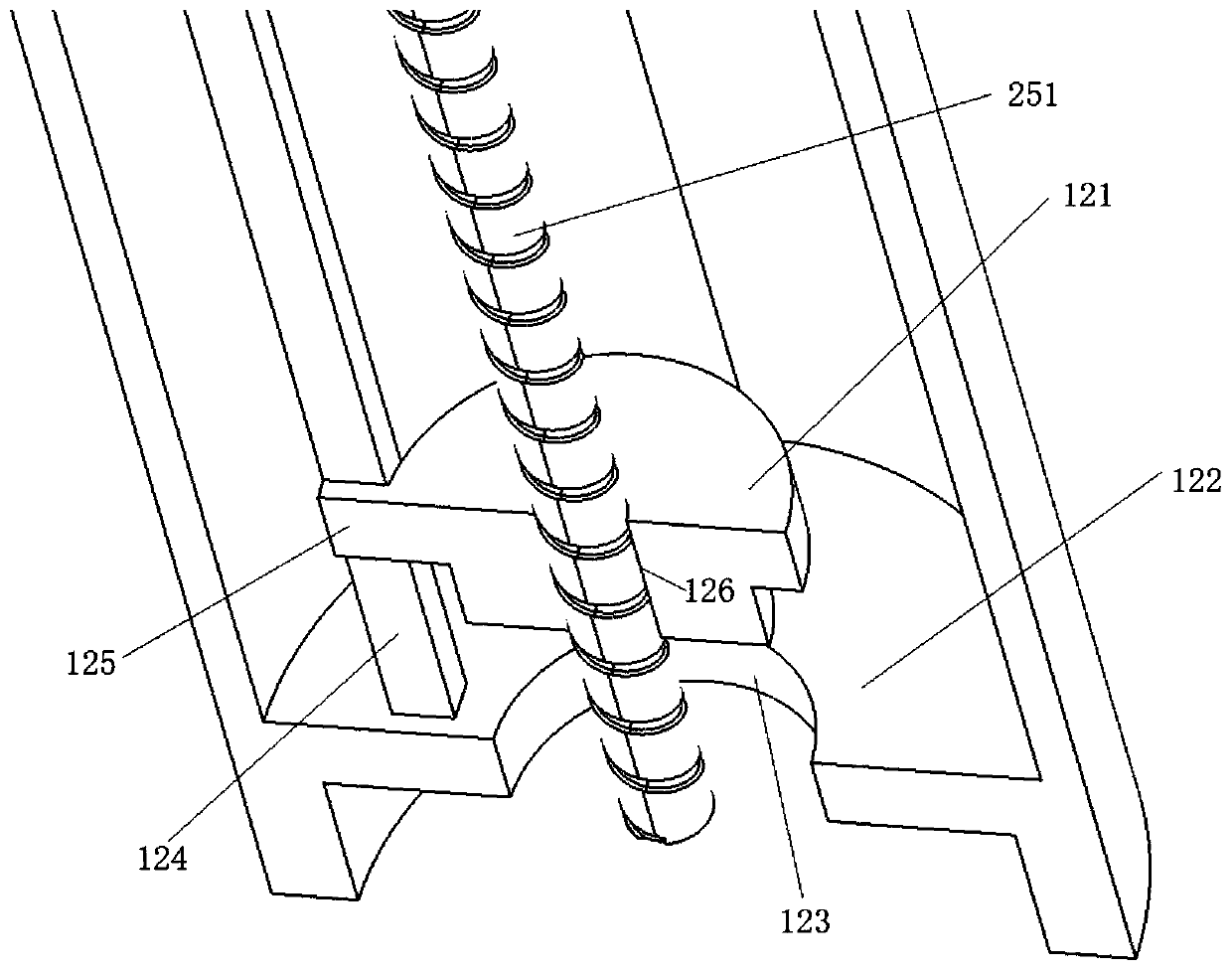
Self-anchored prostate hanging-type urethral stent, visible release catheter and use method
PendingCN112891032ATreat stenosisTreating Difficulty UrinatingStentsUrethral stentsAbsorbable suture
The invention relates to the technical field of medical instruments, and provides a self-anchored prostate hanging-type urethral stent, a visible release catheter and a use method. The self-anchored stent comprises a supporting line; barbs are arranged on the supporting line; the tail of the supporting line is provided with a gasket; the gasket is used for distracting prostate tissue to expand the urethra; the supporting line is a non-absorbable suture line; a first barb is made of metal and used for being interlocked with a puncture needle; and the barbs play a role of a self-anchored fixing stent. By means of the technical scheme, the defects of the prior art in treatment of prostatic hyperplasia: the cost of generally adopted surgical treatment is high, bleeding and tissue losses can be caused, and the male sexual function is affected, are solved. While existing urethral stent interventional therapy has the problems that the treatment effect is poor, recurrent calculous obstruction is easy to occur, the stent is easy to displace and fail, and difficult to take out after failure and the like.
Owner:张保
Structure and function bionic urethral stent and preparation method thereof
ActiveCN113750297AGood urethral defect repair effectSimple processSurgeryPharmaceutical delivery mechanismUrethral stentsFreeze-drying
The invention provides a structure and function bionic urethral stent and a preparation method thereof. The preparation method comprises the following steps of: (1) uniformly mixing an oxidized bacterial cellulose nanofiber dispersion liquid and a decellularized matrix solution, pouring the mixture into a mold I until the mold I is completely filled, and performing freeze drying to obtain an uncrosslinked porous stent; (2) putting the uncrosslinked porous stent into a cross-linking agent solution, performing cross-linking, rinsing the cross-linked porous stent with water, and performing freeze drying to obtain a cross-linked porous stent; and (3) pouring a mixed solution II consisting of oxidized bacterial cellulose nanofibers, a modified or unmodified natural polymer material, a gelling aid and water into a silica gel mold II until the silica gel mold II is completely filled, fixing the cross-linked porous stent on the mold II, enabling the cross-linked porous stent to be in contact with the mixed solution II, and standing for a period of time to obtain the structure and function bionic urethral stent. The prepared stent consists of a hydrogel layer of a bionic urethral mucosa and a porous layer of a bionic urethral cavernous body, can be rapidly epithelized and vascularized after being implanted into a body, and has a good urethral defect repair effect.
Owner:DONGHUA UNIV
Preparation method of degradable metal molybdenum and alloy for ureteral and urethral stents
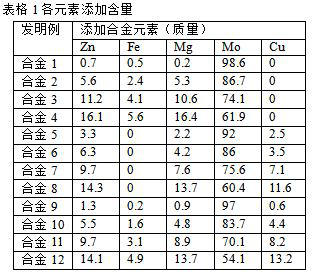
The invention discloses a preparation method of degradable metal molybdenum and alloy for ureteral and urethral stents. The alloy comprises the following components in percentage by mass: 0.2-17 wt% of Zn, 0-6 wt% of Fe, 0.2-17 wt% of Mg and 0-15 wt% of Cu, and the rest metal is Mo. The elements such as Mo, Zn, Fe, Mg, Cu and the like are uniformly mixed, and vacuum arc melting is performed to obtain the alloy. The molybdenum-based alloy has good mechanical properties and is easy to process, and the properties such as strength, toughness and plasticity meet the requirements of human body implant materials for biliary tracts and the like. The surface of the stent is coated with anti-stenosis, anti-urethral infection, antibacterial and other polymer drugs. The five elements of Mo, Zn, Fe, Mg and Cu are the elements necessary for a human body, magnesium is an activating agent and an auxiliary factor of various enzymes, zinc ions participate in in-vivo balance and immune reaction, iron is a main substance for maintaining life and is an important component of hemoglobin of the human body, a degradation product of copper has a certain antibacterial effect, and molybdenum can prevent anemia and promote development. The molybdenum-based alloy ureteral and urethral stent has good biological performance, and the degradation process and the product are safe and effective to a matrix.
Owner:SHANDONG RIENTECH MEDICAL TECH
Viscous penis construction template and urethral stent tube for new penis surgery
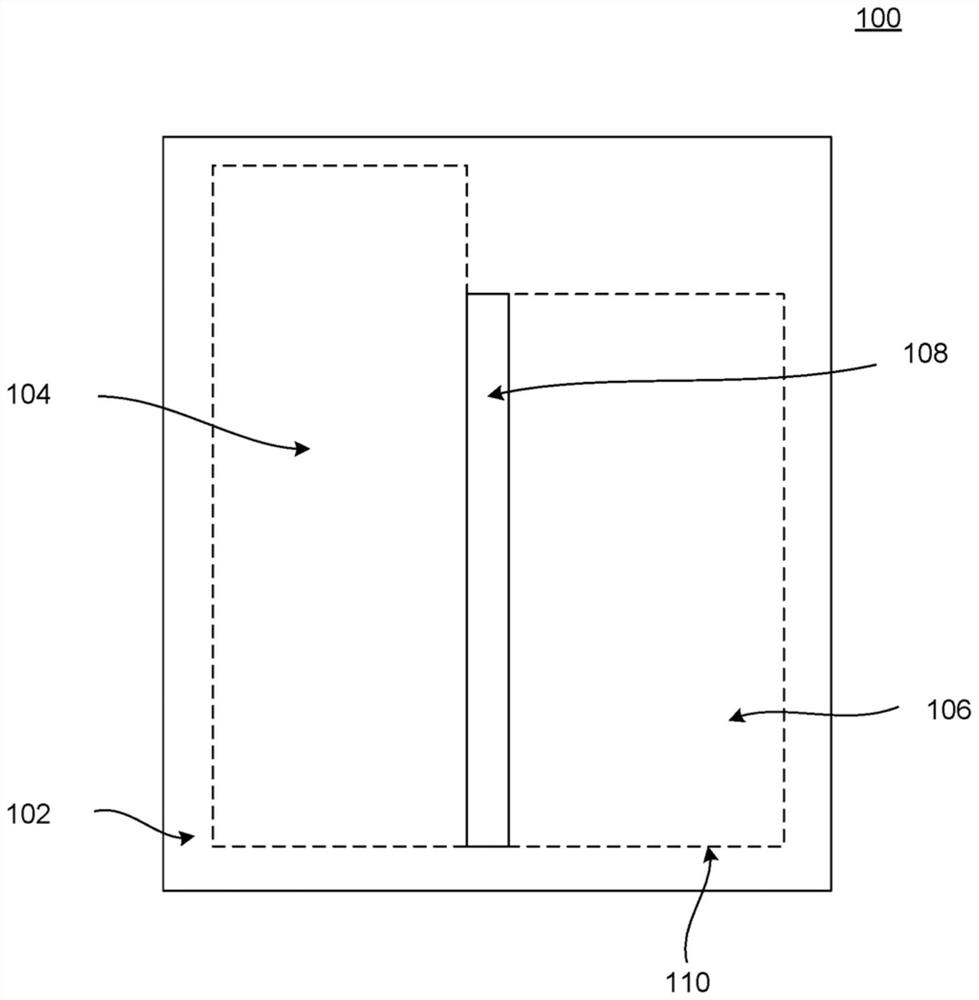
A penis build template (100) may include an adhesive film (102) configured for adhesion to donor skin. The penis build template includes a first section (106) defined on the adhesive film to identify an outer penis flap of the extracted donor skin when the adhesive film adheres to the donor skin, and a second section (108) defined on the adhesive film to identify an epithelium-removed flap of the extracted donor skin when the adhesive film adheres to the donor skin. An implantable device for urethral construction includes a tubular stent (500) configured to be coupled to a natural urethra of a patient, the tubular stent being inoculated with urethral cells of the patient from which a urethral segment grows on the tubular stent while the tubular stent is disposed within the patient.
Owner:BOSTON SCI SCIMED INC
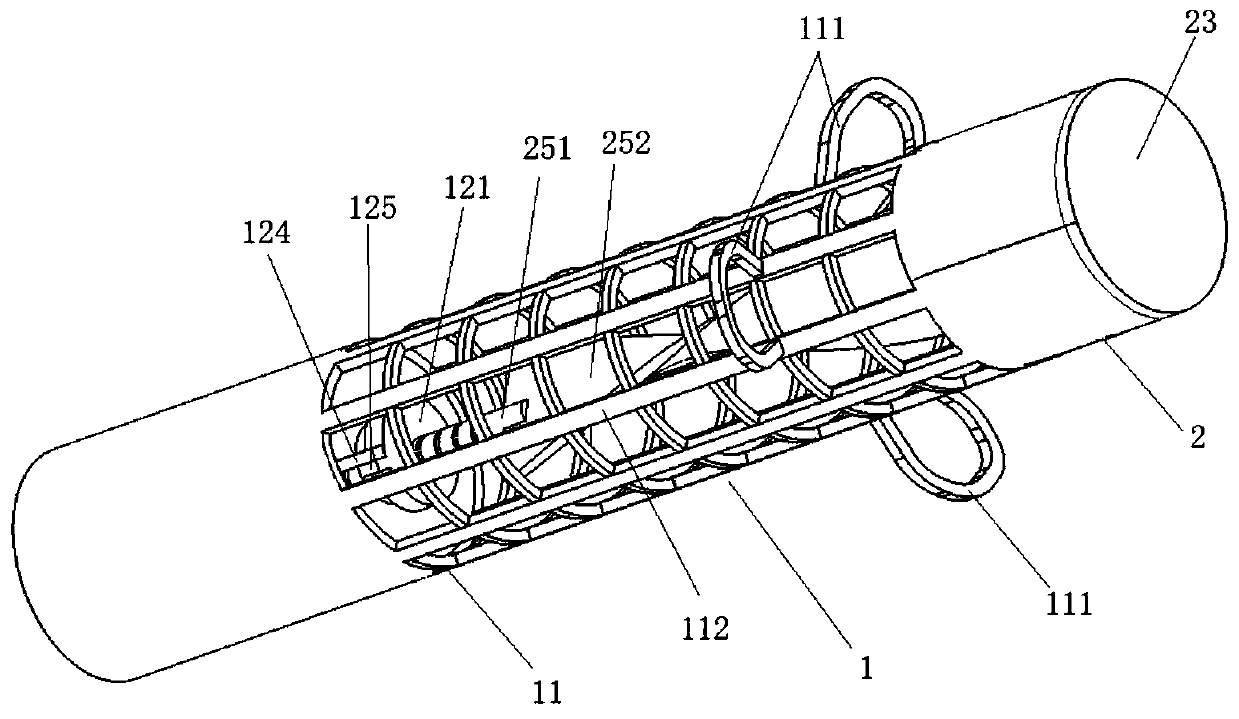
Anti-infection absorbable nursing urethral stent suitable for post-urethral-fissure operation
The invention discloses an anti-infection absorbable nursing urethral stent suitable for post-urethral-fissure operation. The anti-infection absorbable nursing urethral stent comprises a tube body, a first balloon, a second balloon, a degradable stent ring, an inner partition tube, a first anti-infection liquid medicine sleeve, a degradable partition body A, a degradable partition body B, a second anti-infection liquid medicine sleeve, a degradable partition body C and a degradable partition body D. Firstly, through the cooperation effect of the first balloon and the second balloon, the bladder can be supported, the urethra can be supported, and the problem of urethral stricture is effectively prevented; secondly, through the degradable stent ring, the problem that in a traditional technology, when the tube body is disassembled, due to the fact that the degradable stent ring is not made of degradable materials, pain is brought to a patient when the degradable stent ring and the tube body are withdrawn together is solved; and finally, through an antibacterial and anti-inflammation structure, dissolution is subjected to an arrangement sequence, thus antibacterial and anti-inflammation liquid medicine can conveniently permeate into the urethra in batches, disinfection in batches is achieved, and meanwhile, the antibacterial and anti-inflammation time is prolonged, and the problem that external germs invade the urethra of the patient in the tube placement process is prevented.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com