Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
54 results about "Metacresol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
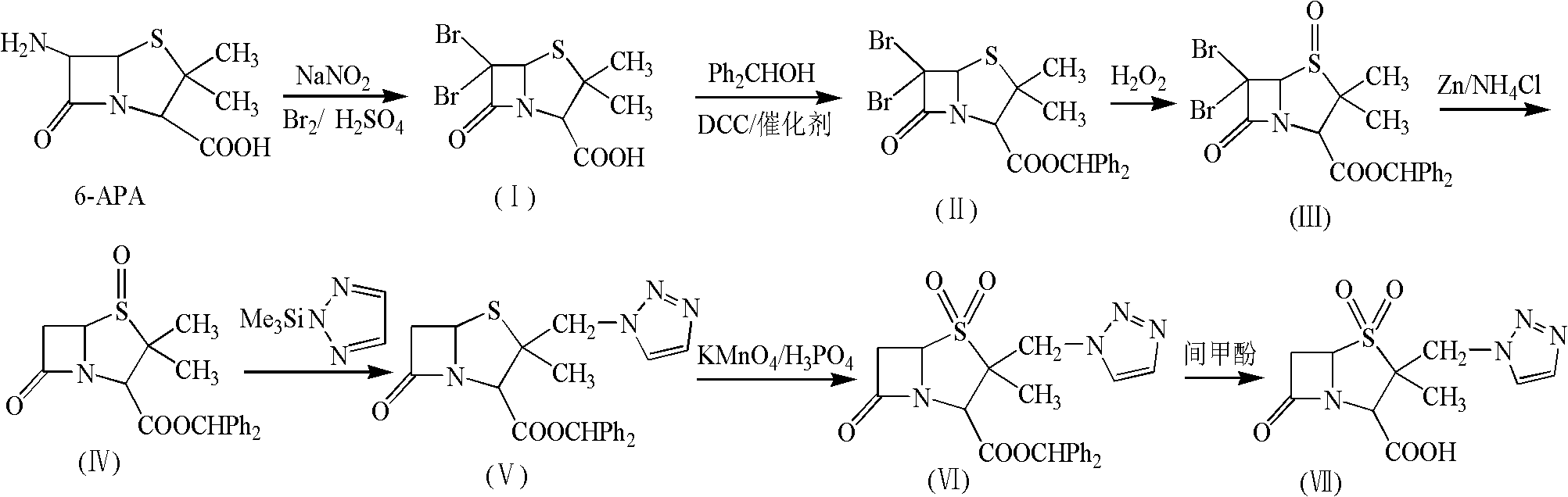
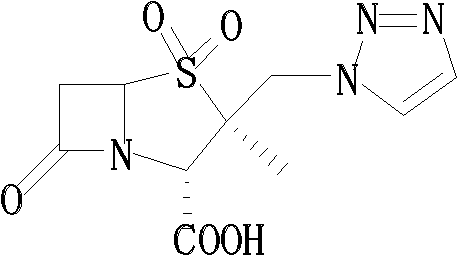
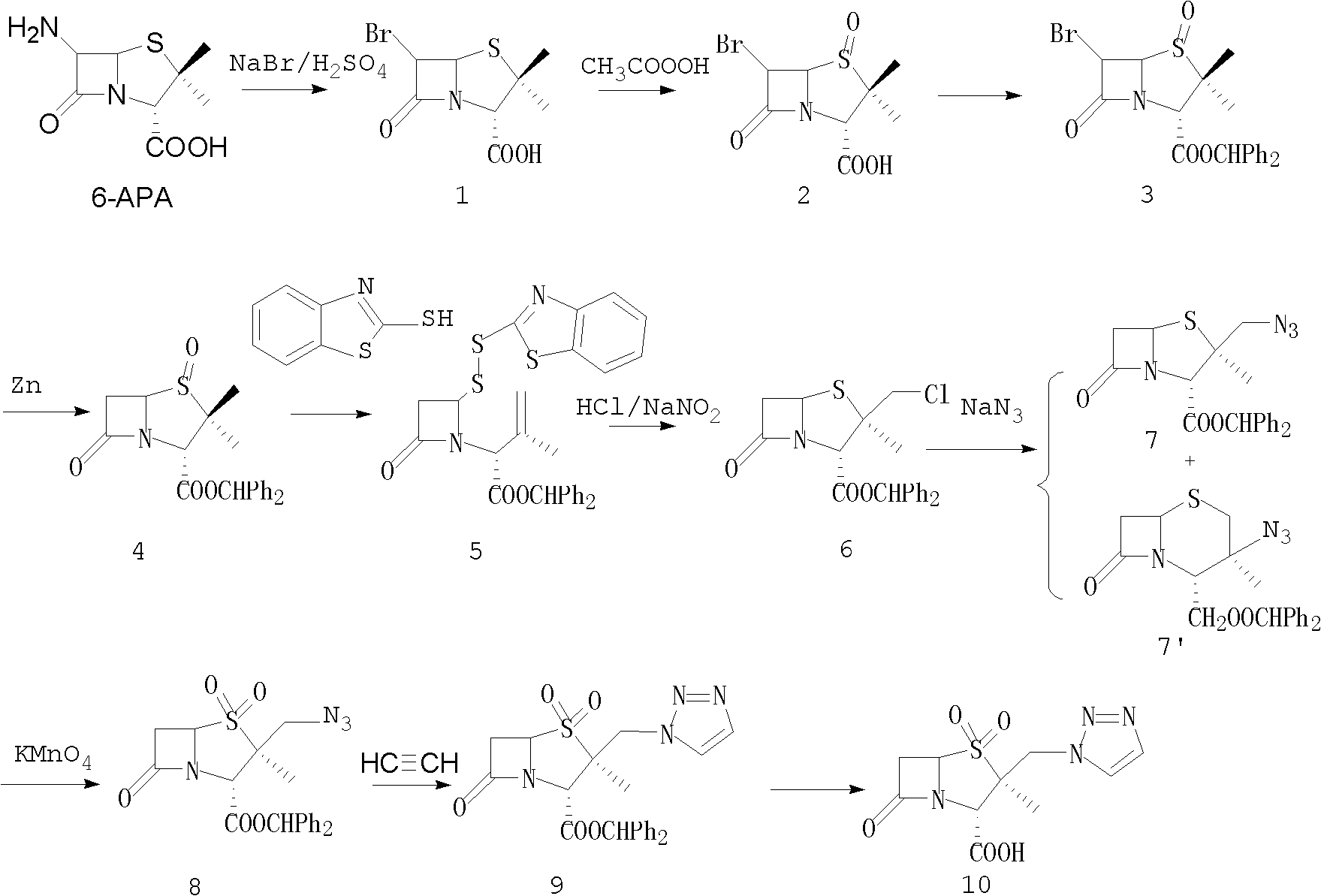
Tazobactam synthesis method
ActiveCN102020663AReduce usageWill not polluteOrganic chemistryChemical recyclingMetacresolSynthesis methods
The invention relates to a tazobactam synthesis method which comprises the steps of: with 6-APA(Amino Penicillanic Acid) as raw material, preparing a key intermediate 6,6-dihydro penam sulphoxide acid diphenylcarbinol ester through successive reactions of esterification, oxidation, reduetive debromination and the like without separation; then, reacting with 2-triphenyl silicon-1,2,3-triazole; introducing a triazole ring; and finally obtaining the final product of tazobactam through potassium permanganate oxidation and metacresol deprotection. The tazobactam synthesis method is mainly characterized in that a phase transfer catalyst is introduced in the first step, therefore, the reaction rate and the product purity are improved; since an environment-friendly hydrogen peroxide-cobalt acetate catalytic oxidation system is adopted in the third step, the characteristics of good reaction selectivity, high yield, catalyst recyclability and the like are achieved; a method for synthesizing 2 alpha-methyl-2 beta-(1,2,3- triazole-1- radical) methyl penam-3 alpha-carboxylic acid diphenylcarbinol ester by using 2-triphenyl silicon-1,2,3-triazole is adopted in the fifth step, and the tazobactamsynthesis method is simple and convenient to operate, is safe and reliable, shortens the reaction route and improves the total yield. Compared with the traditional process, the tazobactam synthesis method greatly reduces the production cost and the environment pollution and has greater implementation value and economic benefits.
Owner:YIYUAN XINQUAN CHEM
Pharmaceutical composition applicable to body tissue
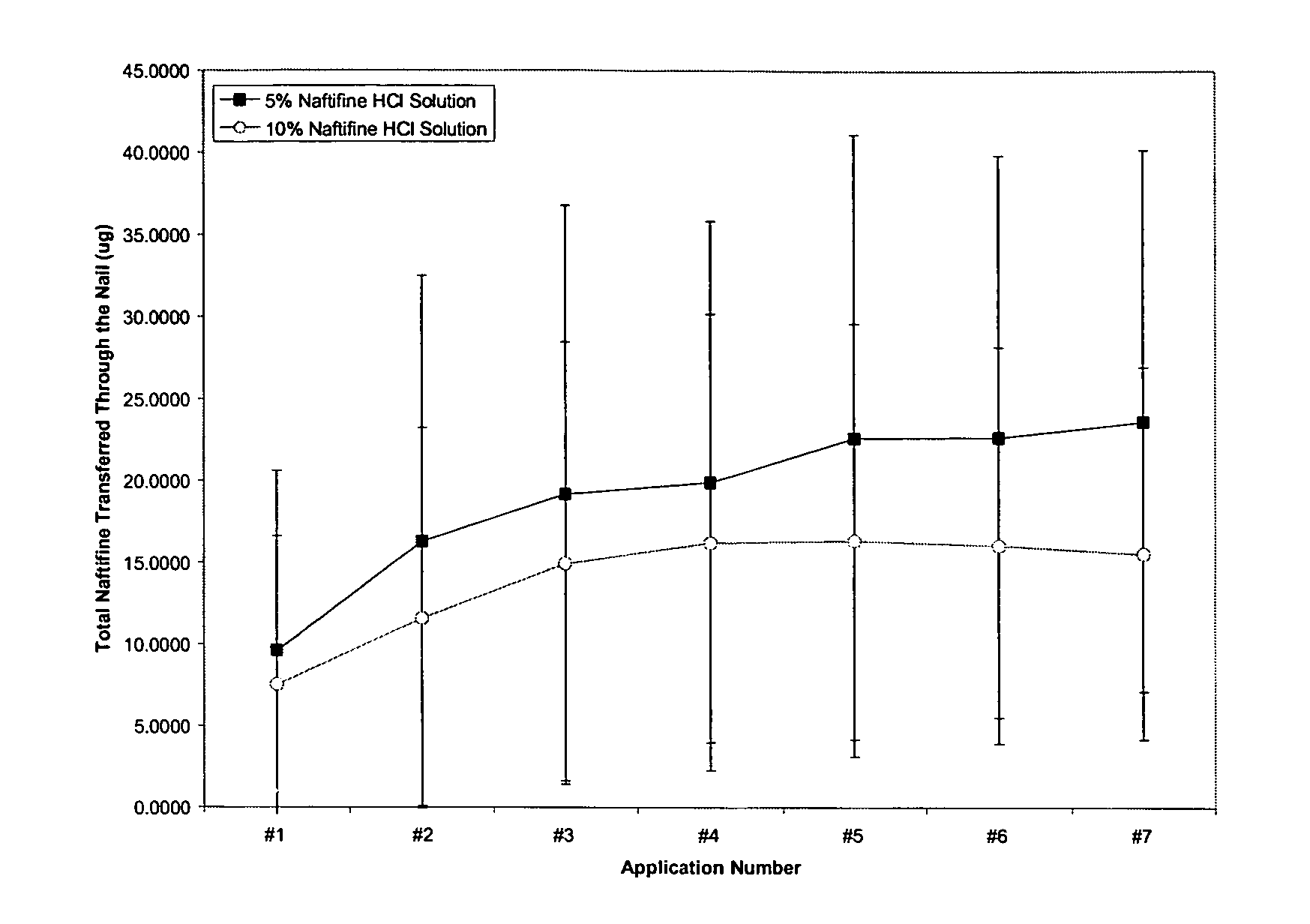
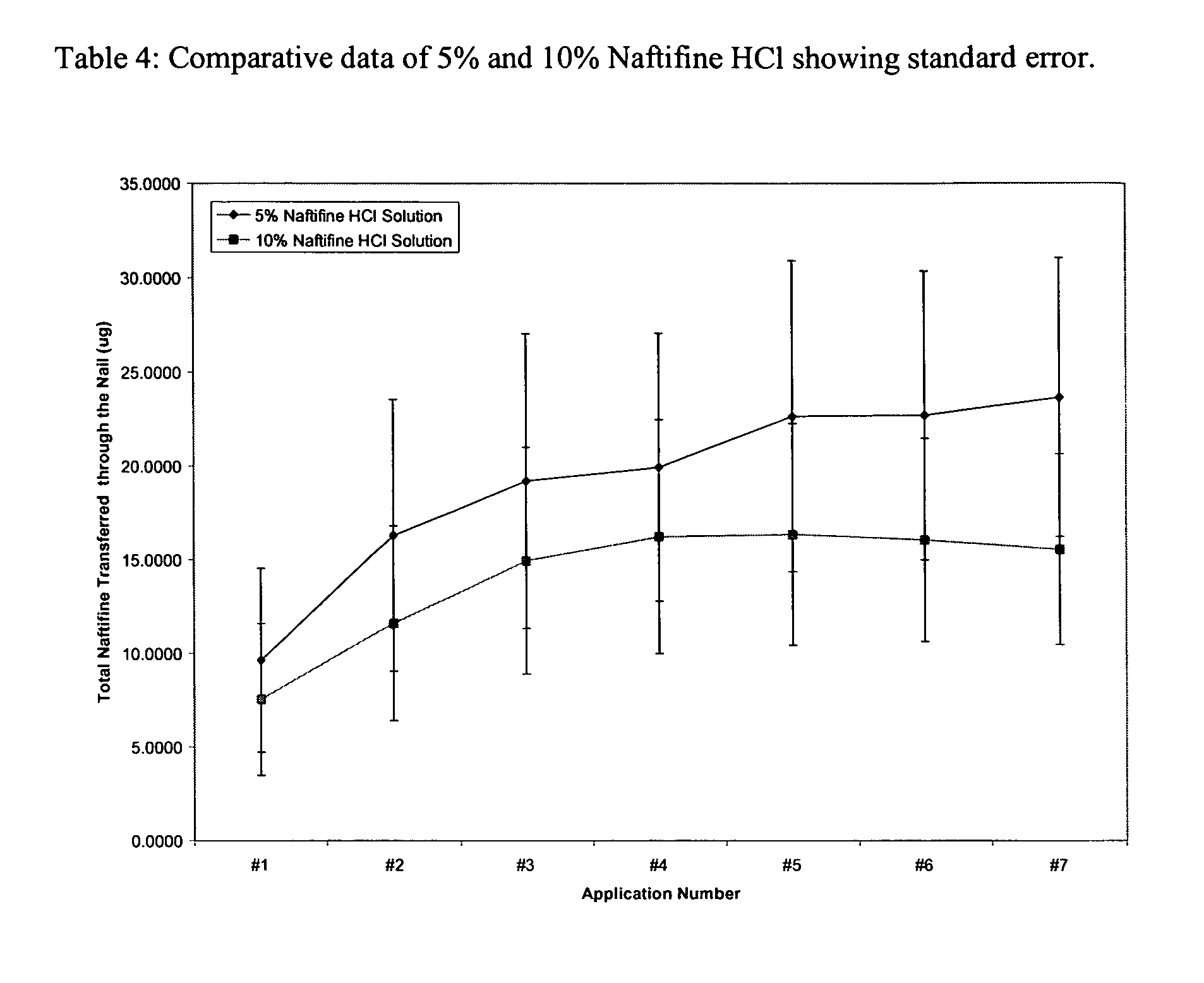
The present invention provides a non-water soluble, film-forming composition which adheres to body tissue and forms a pharmaceutical carrier to provide localized delivery of an antifungal agent to a treatment site. The composition will typically include: (a) an alkyl cellulose; (b) a hydroxyalkyl cellulose; (c) a pharmaceutically acceptable polar protic solvent; (d) an antifungal agent selected from the group of naftifine, ciclopirox, terbinafine, pharmaceutically acceptable salts thereof, and combinations thereof; (e) an glycol ether; (f) an antipruritic agent selected from the group of camphor, menthol, butamben picrate, metacresol, benzyl alcohol, camphorated metacresol, juniper tar, phenol, phenolate sodium, resorcinol, camphorated metacresol, carbolic acid, and combinations,; and (g) a solubility enhancing agent, a surfactant, a wetting agent, or a combination thereof. The present invention also provides for the use of the composition composition of the present invention, in treating a fungal infection (e.g., nail fungus) in a mammal afflicted with such an infection.
Owner:QLT USA INC
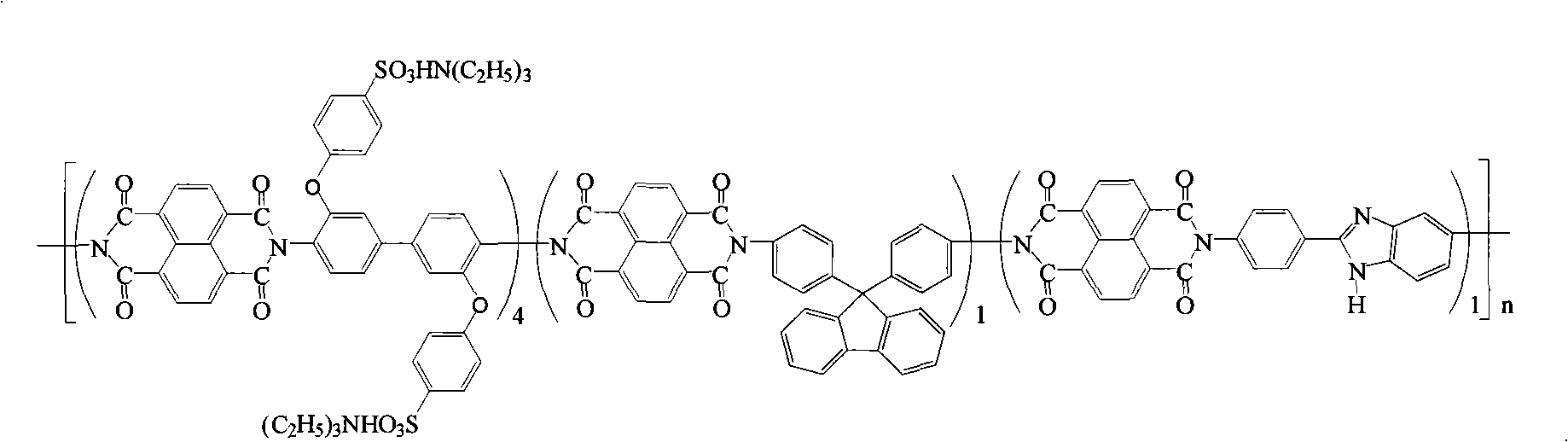
Preparation of glyoxalinyl-containing sulphonation polyimides covalence-ionomer membrane
InactiveCN101407592AExcellent anti-free radical oxidation performanceHigh proton conductivityCell component detailsFuel cell detailsBenzoic acidCross-link
The invention relates to a preparation method of a covalent-ion cross-linked film of sulfonated polyimide containing imidazolyl. The preparation method comprises the following steps: 1, 4, 5, 8-naphthoic tetracarboxylic dianhydride, sulfonated diamine, common non-sulfonated diamine and diamine containing imidazolyl are added into a metacresol medium, the sulfonated polyimide containing imidazolyl is obtained by copolycondensation under the presence of triethylamine and benzoic acid and the protection of nitrogen; a solution casting method and proton exchange are adopted to prepare non-covalent cross-linked sulfonated polyimide film containing imidazolyl which is then processed by methane-sulfonic acid / phosphorus pentoxide solution or phosphoric acid solution or polyphosphoric acid containing phosphorus petoxide so as to prepare the covalent-ion cross-linked film of sulfonated polyimide containing imidazolyl. The covalent-ion cross-linked film obtained by the preparation method has anti-free radical oxidation susceptibility which is far better than the common sulfonated polyimide or non-covalent cross-linked film without containing imidazolyl and has potential application prospect in the fields of fuel cells and the like.
Owner:SHANGHAI JIAO TONG UNIV
Tazobactam synthesis method
ActiveCN102643292ASteps to increase monoxidationBlocking affinityOrganic chemistryMetacresolSynthesis methods
The invention discloses a tazobactam synthesis method, which belongs to the technical field of medicines, and includes the steps: firstly, enabling 6,6-dihydropenam sulfoxide acid diphenylmethyl ester serving as raw materials to undergo thermal cracking and chloromethylation reaction to obtain 2beta-chloromethyl penicillanic acid diphenylmethyl ester; secondly, adding oxidizing agent to oxidize the 2beta-chloromethyl penicillanic acid diphenylmethyl ester-1beta-oxide, enabling the oxidized 2beta-chloromethyl penicillanic acid diphenylmethyl ester-1beta-oxide to react with sodium azide to generate 2beta-hydrazoic methyl penicillanic acid diphenylmethyl ester-1beta- oxide, and then generating 2beta-hydrazoic methyl penicillanic acid diphenylmethyl ester-1,1- dioxide by means of oxidization under the action of potassium permanganate and acetic acid; and finally, preparing the tazobactam by means of deprotection under the action of acetylene cyclization and metacresol. Compared with a past 6-APA (aminopenicillanic acid) route, the tazobactam synthesis method has the advantages that the step of sulfur atom single oxidization is added, so that possibility of ring expansion due to affinity of lone pair electrons on a sulfur atom is blocked, and transformation of five-membered ring products to six-membered ring by-products during hydrazoic reaction can be effectively controlled.
Owner:山东安信制药有限公司 +1
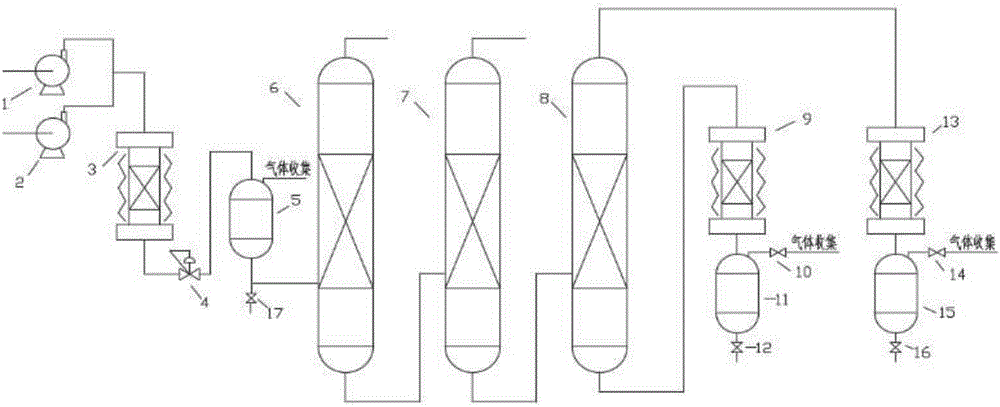
Technology for complexing, crystallizing, separating and purifying metacresol
InactiveCN102167658AReduce usageHigh yieldOrganic chemistryOrganic compound preparationWater bathsChemical industry
The invention provides a technology for complexing, crystallizing, separating and purifying metacresol, belonging to the field of separation and purification in a chemical industry. The technology comprises the steps of dissolving urea in mixed phenol: adding the mixed phenol into a reactor, wherein the mixed phenol comprises 10.907wt% of o-cresol, 34.276wt% of p-cresol, 53.176wt% of m-cresol and 1.641% of dimethyl phenol; adding the urea into the reactor, wherein the mole ratio of the urea to the m-cresol is 1.0-1.8; heating and rising the temperature to be 85-105DEG C in a water bath or an oil bath for 40-80min, so that the urea is completely dissolved in the mixed phenol; cooling, adding normal hexane under the temperature of 60DEG C, and crystallizing for 1-2h under the temperature from -10DEG C to -20DEG C; transporting crystallized serous fluid into a filter to separate solid from liquid; recovering filter liquor in a rectifying way, adding water into crystalline solid, and hydrolyzing during heating at 40DEG C to obtain water phase and organic phase; and recovering the water phase, so that the organic phase is the m-cresol. In the technology, methylbenzene is replaced by normal hexane to be taken as a dissolvent for complexing and crystallizing reaction, so that the technology is higher in the yield of the cresol, the usage amount of the dissolvent can be reduced by one third, and the dissolvent can be covered by less energy consumption.
Owner:BEIJING UNIV OF CHEM TECH
Method for producing 2-tertiary butyl-p-cresol and 6-tertiary butyl-m-cresol
InactiveCN101353293AReduce manufacturing costReduce processing costsOrganic chemistryOrganic compound preparationCresolMetacresol
The invention relates to a method for producing 2-tertiary butyl-paracresol and 6-tertiary butyl-metacresol from mixed cresol, which is characterized in that the mixed cresol is taken as raw material; firstly, the mixed cresol is rectified and separated to obtain refined mixed cresol; then the refined mixed cresol is alkylated to obtain the mixture of 2-tertiary butyl-paracresol and 6-tertiary butyl-metacresol; and the mixture of 2-tertiary butyl-paracresol and 6-tertiary butyl-metacresol is further rectified; as the difference between the boiling point of 2-tertiary butyl-paracresol and the boiling point of 6-tertiary butyl-metacresol is 6 DEG C, the rectification method can be used for separating 2-tertiary butyl-paracresol from 6-tertiary butyl-metacresol to obtain a 2-tertiary butyl-paracresol product and a 6-tertiary butyl-metacresol product with high purity. The method greatly reduces the production cost.
Owner:天津天大天海化工新技术有限公司
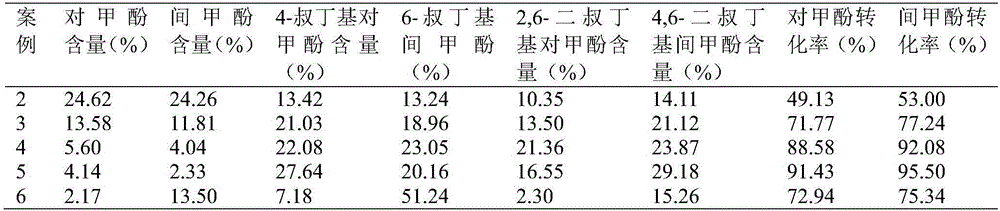
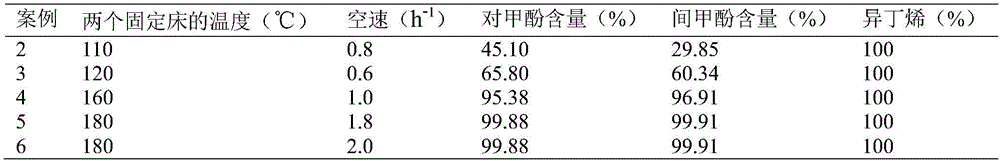
Method for separating m-cresol and p-cresol mixture by liquid-phase alkylation method
InactiveCN106810422AImprove conversion rateIncrease profitOrganic chemistryOrganic compound preparationCresolMetacresol
The invention discloses a method for separating an m-cresol and p-cresol mixture by a liquid-phase alkylation method. According to the method, alkylation reaction between isobutene mixed gas and the m-cresol and p-cresol mixture are performed through a fixed-bed reactor under the liquid phase state, the reacted mixture is separated to obtain metacresol and paracresol, the isobutene mixed gas serves as an alkylating agent, the m-cresol and p-cresol mixture serves as a raw material, the alkylation reaction is performed for the alkylating agent and the m-cresol and p-cresol mixture, and the treated mixture is separated to obtain pure metacresol and pure paracresol. The method overcomes the shortcomings of low utilization rate and serious environmental pollution of alkylating agents in gas-phase alkylation reaction in the prior art and solves the problems that production cost is high as high-purity isobutene serves as the alkylating agent in the gas-phase alkylation reaction.
Owner:HEBEI UNIV OF TECH +1
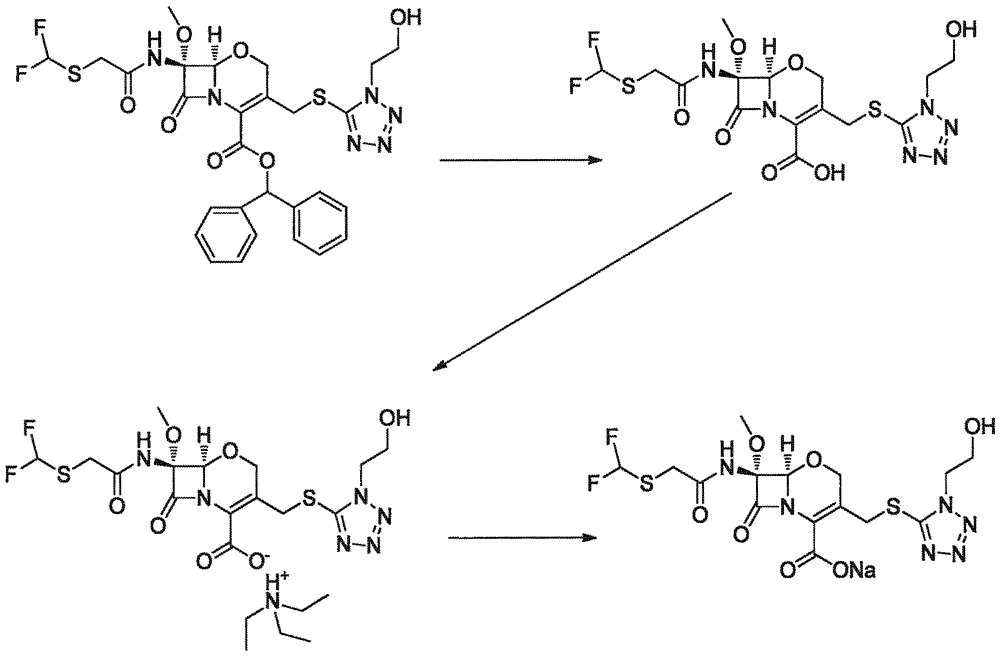
Synthesis method of flomoxef sodium
The invention provides a synthesis method of flomoxef sodium. The synthesis method comprises steps as follows: a, (6R,7R)-3-chloro-7-(2-((difluoromethyl) sulfenyl) acetamido)-7-methoxy-8-oxo-5-oxa-1-azabicyalo[4.2.0] octan-2-ene-2-(benzhydryl) carboxylate ester and metacresol are added to a reaction bottle and subjected to a reaction at the temperature of 65-70 DEG C; after the reaction ends, the mixture is cooled, a solvent is added for follow-up treatment, and a flomoxef acid solution is obtained; b, triethylamine is added to the flomoxef acid solution, solids are separated out, filtration and drying are performed, and flomoxef triethylamine salt is obtained; c, the flomoxef triethylamine salt is added to the reaction bottle, an organic solvent is added for dissolution, the temperature is reduced to subzero 25 DEG C to subzero 20 DEG C, sodium salt and an organic solvent are continuously dropwise added, then, crystal growing, filtration, washing and drying are performed after adding, and flomoxef sodium is obtained.
Owner:LIVZON PHARM GRP INC
Organic luminescent material 4, 6-diphenyl-1, 9-anthralin compounds, synthesis method and application thereof
The invention provides a 4, 6-diphenyl-1, 9-anthralin compound luminescent material, a preparation method and application thereof. In the invention, 4, 6-dibenzoyl-1, 3-m-phenylenediamine and p-alkyl hypnone, p-acetyl biphenyl, p-acetyl diphenyl ether, 2-acetamidofluorene, 3-acetyl carbazole and mono-acetyl compounds substituted by alkyl thereof are used as raw materials, polyphosphoric acid is adopted as a catalyst, metacresol is employed as solvent, and a Friedlander condensation reaction is performed to obtain a series of 4, 6-diphenyl-1, 9-anthralin compounds which can be used as a luminous layer or an electron transport layer of an organic light emitting diode.
Owner:NANJING UNIV OF TECH
Polyphenol mixture recycling purification technology
ActiveCN108558609AReduce pollutionRealize sequential separation and purificationOrganic chemistryOrganic compound preparationMetacresolPhase splitting
The invention provides a polyphenol mixture recycling purification technology which comprises the steps of alkalization and phase splitting: heating a polyphenol mixture to react with a sodium carbonate solution to general phenol sodium salt, conveying reaction liquid into an alkalization and phase splitting tower for continuous reaction to generate the phenol sodium salt and phase splitting and obtaining a water phase rich in the phenol sodium salt and an organic light component, acidification, water washing and phenol extraction: conveying the water phase into an acidification tower for acidification, allowing an organic phase on the upper layer in the acidification tower to overflow into a water washing tower for washing treatment, ethanol crystallization mixing and adding the organic phase after the water washing and ethanol into a crystallizer for crystallization to form 2,3-dimethyl phenol, and rectification and separation: continuously conveying primary mother liquid into a primary rectification tower for rectification till a metacresol finished product with a content greater than 98% is generated continuously in the primary rectification tower, and conveying kettle liquid with a 3,5-dimethyl phenol content greater than 80% from the primary rectifying tower into a secondary rectification tower for continuous rectification and purification to form a product with the 3,5-dimethyl phenol content greater than 98%.
Owner:JIANGSU HUANXIN NEW MATERIAL CO LTD
Catalyst for synthesizing 2,3,6-trimethylphenol and preparation method thereof
InactiveCN102974354AExtended service lifeReduce usageOrganic chemistryOrganic compound preparationMetacresolAlkali metal oxide
The invention discloses a method for preparing 2,3,6-trimethylphenol, and the 2,3,6-trimethylphenol is an important intermediate of vitamin E. The method comprises the following steps of: enabling a mixture of metacresol, methanol and water to pass a fixed bed reactor containing an o-position methylation catalyst in a liquid space velocity of 0.4-10 h<-1>, and preparing the 2,3,6-trimethylphenol by carrying out a gas solid phase catalytic reaction under a temperature of 280-450 DEG C and a pressure of 0-10 MPa, wherein the o-position methylation catalyst consists of iron oxide, silicon dioxide and alkali metal oxide, and in the oxides, the metal ion molar ratio of iron, silicon, aluminum and alkali metal is equal to 100:(0.1-10):(0.2-5):(0-1). According to a catalyst for synthesizing the 2,3,6-trimethylphenol and a preparation method thereof, the conversion rate of the metacresol for compounding the 2,3,6-trimethylphenol is 100%, the yield of the 2,3,6-trimethylphenol is above 99.9%, and the service life of the catalyst is longer than 3000 hours.
Owner:SOUTHEAST UNIV
Polymerizable organic strong acid / sulfuric acid composite curing agent and foamable phenolic resin combination thereof
InactiveCN103059338AHighlight substantial advantagesHighlight significant progressChemical LinkageHydrogen
The invention discloses a polymerizable organic strong acid / sulfuric acid composite curing agent and a foamable phenolic resin combination thereof. The combination comprises 100 parts of liquid A-stage phenolic resin, 7-9 parts of foaming agents, 2-6 parts of foam stabilizer, and 6-10 parts of polymerizable organic strong acid / sulfuric acid composite curing agents. The polymerizable organic strong acid / sulfuric acid composite curing agents are obtained through phenol or metacresol sulfonated in slightly excessive concentrated sulfuric acid, postprocessing processes of separation and the like are not needed, preparation processes are simple, and cost in low. The polymerizable organic strong acid / sulfuric acid composite curing agent can be chemically bonded with phenolic resin and directly brought in structure of foams, the polymerizable organic strong acid / sulfuric acid composite curing agent is not easy to permeate, small in corrosivity of base materials due to the fact that the potential of hydrogen (PH) value of the obtained foams reaches up to 5.8, rapid in foaming speed, and free from foam collapse and foam contraction.
Owner:北京聚源达科技有限公司
Preparation method for thymol
ActiveCN104744219AReduce the temperatureLow reaction temperatureOrganic chemistryOrganic compound preparationAlkyl transferMetacresol
The invention discloses a preparation method for thymol. Metacresol and isopropanol are used as reaction raw materials. The preparation method comprises the following preparation steps of stirring the metacresol and the isopropanol to be uniform; adding a catalyst in a mixture of the metacresol and the isopropanol and stirring to be uniform again to obtain a reaction mass; reacting the reaction mass at the reaction temperature of 100-200DEG C under the radiation of microwave with the power of 200 to 1,000W for 0.1 to 60 minutes to obtain the thymol as a target product, wherein the amount ratio of the metacresol to the isopropanol is 1:(0.01-10), and the dose of the catalyst is 1 to 30 percent of the mass sum of the metacresol and the isopropanol. According to the preparation method for the thymol, disclosed by the invention, the reaction temperature is 100 to 200DEG C and is greatly lower than the preparation temperature of the thymol by using an existing friedel-crafts alkylation reaction; the reaction time is shorter, and is only 0.1 to 60 minutes.
Owner:JIANGSU UNIV OF TECH
Method for measuring content of seven harmful phenol in mainstream flue gas by high efficiency liquid chromatography
ActiveCN101587105AThe result is accurateSimple processComponent separationFluorescence/phosphorescenceLiquid Chromatography-FluorescenceMetacresol
The invention provides a method for measuring the content of seven harmful phenol in the mainstream flue gas by high efficiency liquid chromatography, comprising the steps of mainstream flue gas collection, flue gas sample preparation and high efficiency liquid chromatography fluorescence detection. Firstly, the fluorescence detection spectrum data output by a high efficiency liquid chromatographyfluorescence detector of different mixed concentration sample is processed with a partial least square method, and a mathematical model is built, and then the fluorescence detection spectrum data ofthe metacresol and the paracresol in high efficiency liquid chromatography fluorescence detection data of the flue gas sample is input into the mathematical model to obtain the concentration of the metacresol and the paracresol. The method can be used for simultaneously analyzing the content of seven harmful phenol compounds in the mainstream flue gas and realizes the measurement of the metacresolcompound and the paracresol compound with a chemometrics method without adopting any other instrument, equipment or reagent, has accurate mausruing result, simple measure process and rapid calculation speed.
Owner:CHINA TOBACCO GUIZHOU IND +1
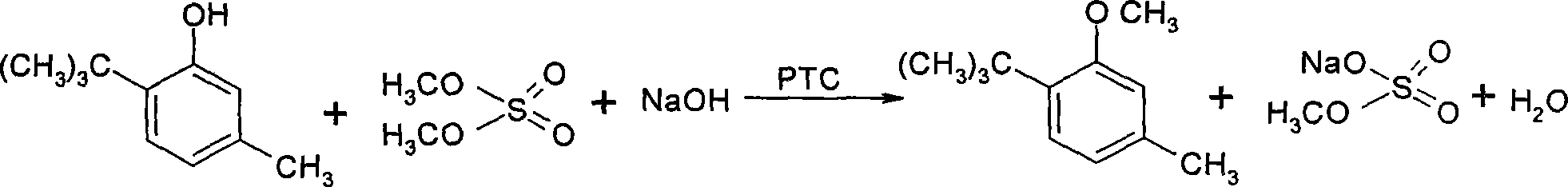
Method for preparing 3-methoxyl-4-t-butyltoluene
InactiveCN101234955AReduce pollutionSimple processEther preparation by organic exchangeMetacresolToluene
The invention discloses a method for preparing 3-methoxyl-4-tert-butyl toluene. Under the existence of quaternary ammonium salt type phase transfer catalysts, 6-tert-butyl metacresol reacts with dimethyl sulphate and water solution of hydroxides or carbonates of alkali metals at 20-50 DEG C, and 3-methoxyl-4-tert-butyl toluene can be produced with high yield. Post treatment of the reaction mixture is very simple and 3-methoxyl-4-tert-butyl toluene with a content of higher than 99.2 percent can be obtained after only two water washing operations. The method has the advantages of simple process, easy product quality control, low cost, low environment pollution and safe production and is suitable for industrialized application.
Owner:孔繁昇
Synthesis method for 2-methoxy-4-methylbenzylamine
InactiveCN102311352AReduce manufacturing costWide variety of sourcesOrganic compound preparationAmino-hyroxy compound preparationMetacresolSynthesis methods
The present invention discloses a synthesis method for 2-methoxy-4-methylbenzylamine. The method comprises the following three steps that: 1, metacresol and formaldehyde are mixed in a solvent A, the resulting solution is subjected to a carbonylation reaction under an alkaline condition through a catalytic action of a Lewis acid catalyst to generate 2-hydroxy-4-methyl-benzaldehyde; 2, the 2-hydroxy-4-methyl-benzaldehyde and a methylating regent are subjected to heating reflux in a solvent B to carry out a methylation reaction to generate 2-methoxy-4-methyl-benzaldehyde; 3, the 2-methoxy-4-methyl-benzaldehyde and ammonium formate are subjected to a reductive amination reaction under a heating condition to generate the 2-methoxy-4-methylbenzylamine. According to the synthesis method provided by the present invention, the used raw materials are mass industrial products, such that the production cost is low; in the third step of the reductive amination reaction, a hazardous reducing agentof lithium aluminum hydride is prevented from using; the reaction conditions are mild, the operation is safe and convenient; the yield of the final product is high, the generated three waste are less, and the method is applicable for the industrial production.
Owner:SHANGHAI INST OF TECH +1
Method for preparing metacresol by directly hydrolyzing meta-aminotoluene
InactiveCN101591223AReduce manufacturing costReduce processingOrganic chemistryOrganic compound preparationOrganic solventMetacresol
The invention discloses a method for preparing metacresol by directly hydrolyzing meta-aminotoluene. The method comprises the following steps: acid, meta-aminotoluene and water are made into a solution which is put into a reactor; nitrogen is introduced into the reactor; heating and stirring are carried out, so that the hydrolysis reaction is carried out fully; when the reaction is finished, the temperature is cooled to room temperature, the material is extracted by an organic solvent to obtain the metacresol crude product. The hydrolysis of the invention is carried out by one step or multiple steps of continuous reactions, the method is simple and practicable with low cost, the product of primary hydrolysis is removed, a plurality of hydrolyses of the mixture can improve the yield; after metacresol is extracted, the residual mixed liquor is added with reactant which are heated to hydrolysis temperature, and multiple times of hydrolysis reactions are carried out, which is capable of effectively reducing waste water; the reaction processes are shortened, the treatment of waste water is greatly reduced, the conversion rate of the reaction is up to 100%, and the yield is up to above 80%; the invention is a new method for synthesizing metacresol and can remarkably reduce the production cost of metacresol.
Owner:NANJING UNIV OF SCI & TECH
Carbon/carbon composite material preform and preparation method thereof
The invention relates to a carbon / carbon composite material preform. The carbon / carbon composite material preform comprises 40-70wt% of short carbon fibers, and the balance of a carbon nano-adhesive. The invention provides a preparation method of the preform. The method comprises the following steps: dispersing the short carbon fibers in a polyoxyethylene and hydroxypropyl methyl cellulose ether dispersed aqueous solution; standing the obtained solution, and removing a supernatant; heating and drying the obtained material to obtain a carbon fiber preform; carrying out vacuum impregnation to make a n-propanol solution of phenol-metacresol-furfural-acetyl chloride be impregnated into the carbon fiber preform, heating the obtained carbon fiber preform to carry out in situ polymerization in order to form a phenolic aldehyde-based nano-adhesive, and drying the phenolic aldehyde-based nano-adhesive to volatilize n-propanol in order to obtain a phenolic resin nano-adhesive reinforced carbon fiber preform; and carbonizing the phenolic resin nano-adhesive reinforced carbon fiber preform to obtain the carbon nano-adhesive reinforced short carbon fiber preform. The preparation method has the advantages of no need of expensive long carbon fibers, no need of a complex knitting technology, convenient operation, short period, low cost, excellent performances and easy industrial production.
Owner:SHANGHAI INST OF TECH
DOT6 boric acid ester type braking fluid
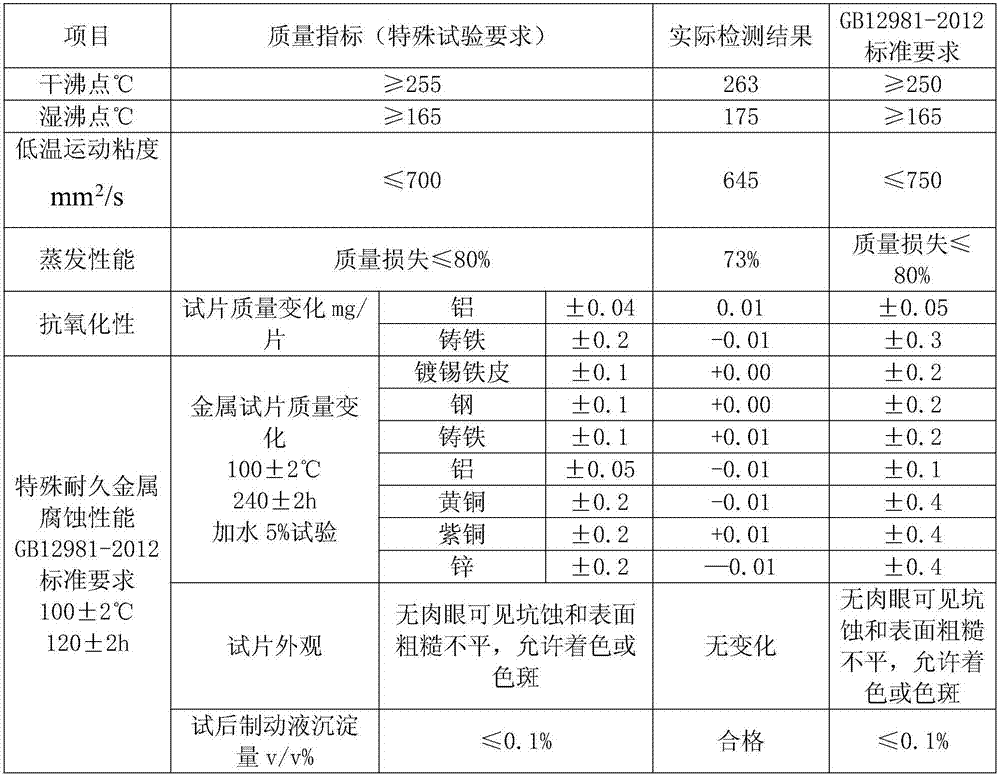
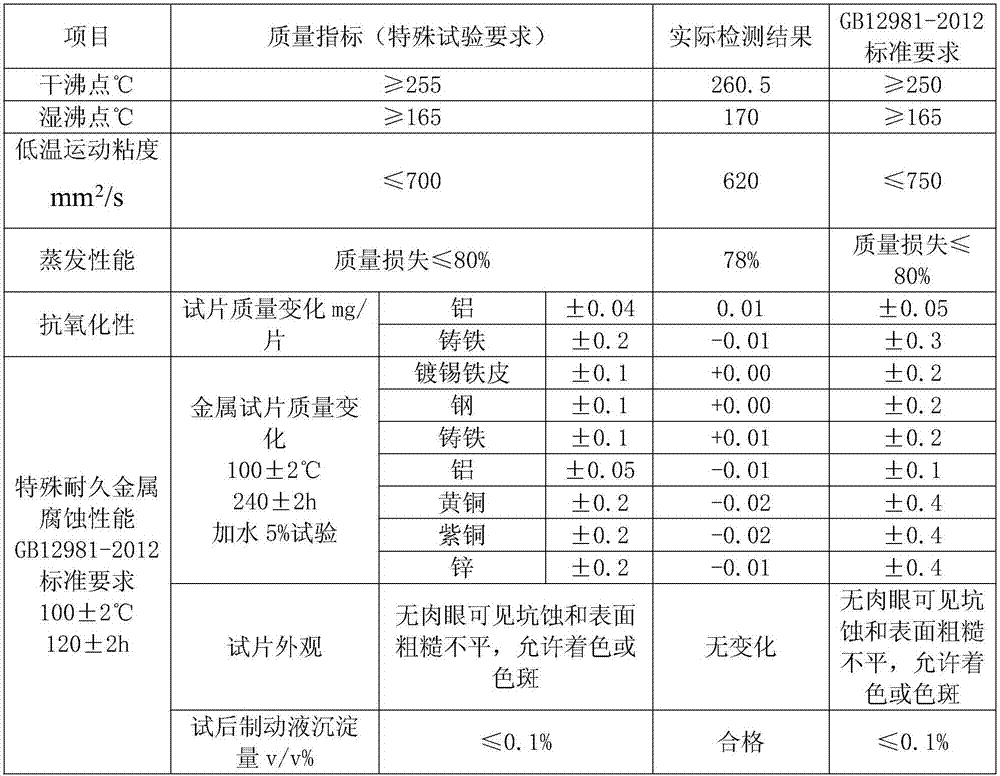
ActiveCN107573998AMeet the corrosion test requirementsHigh wet and dry boiling pointLubricant compositionMetacresolAntioxidant
The invention discloses DOT6 boric acid ester type braking fluid, which is prepared from the following ingredients in percentage by mass: 3 to 16 percent of polyethylene glycol ether, 82 to 95 percentof tris[2-[2-(2-methoxyethoxy)ethoxy]ethyl]orthoborate, 0.05 to 0.15 percent of 6-methyl-2-mercaptobenzothiazole, 0.05 to 0.15 percent of methylbenzotriazole, 0.5 to 1.0 percent of N-methylmonoethanolamine, 0.05 to 0.1 percent of antioxidants 1010, 0.04 to 0.2 percent of acrylic acid 2-ethylhexyl acrylate, 0.01 to 0.05 percent of 4,4'-butylidene bis-(6-tertiary butyl metacresol), 0.01 to 0.05 percent of paraben, 0.01 to 0.05 percent of dodecencylsuccinic acid (T746) and 0.5 to 1.0 percent of alkalinity regulators. The tris[2-[2-(2-methoxyethoxy)ethoxy]ethyl]orthoborate obtained through optimizing the esterification process can effectively solve the problem of high dry and wet boiling point requirements of DOT6; meanwhile, the excellent low-temperature driving performance is realized; thebraking is more sensitive.
Owner:张家港迪克汽车化学品有限公司
Tazobactam synthesis method
ActiveCN102643292BSteps to increase monoxidationBlocking affinityOrganic chemistryMetacresolSynthesis methods
The invention discloses a tazobactam synthesis method, which belongs to the technical field of medicines, and includes the steps: firstly, enabling 6,6-dihydropenam sulfoxide acid diphenylmethyl ester serving as raw materials to undergo thermal cracking and chloromethylation reaction to obtain 2beta-chloromethyl penicillanic acid diphenylmethyl ester; secondly, adding oxidizing agent to oxidize the 2beta-chloromethyl penicillanic acid diphenylmethyl ester-1beta-oxide, enabling the oxidized 2beta-chloromethyl penicillanic acid diphenylmethyl ester-1beta-oxide to react with sodium azide to generate 2beta-hydrazoic methyl penicillanic acid diphenylmethyl ester-1beta- oxide, and then generating 2beta-hydrazoic methyl penicillanic acid diphenylmethyl ester-1,1- dioxide by means of oxidization under the action of potassium permanganate and acetic acid; and finally, preparing the tazobactam by means of deprotection under the action of acetylene cyclization and metacresol. Compared with a past 6-APA (aminopenicillanic acid) route, the tazobactam synthesis method has the advantages that the step of sulfur atom single oxidization is added, so that possibility of ring expansion due to affinity of lone pair electrons on a sulfur atom is blocked, and transformation of five-membered ring products to six-membered ring by-products during hydrazoic reaction can be effectively controlled.
Owner:山东安信制药有限公司 +1
Preparation method of single-component high-ortho-position-content thermosetting phenolic resin used for extrusion
The invention discloses a preparation method of a single-component high-ortho-position-content thermosetting phenolic resin used for extrusion, and belongs to the field of phenolic resin preparation method. The preparation method comprises following steps: complete pyrolysis of paraformaldehyde is carried out so as to obtain a formaldehyde solution; a certain amount of acetic acid is added, phenol and zinc oxide are added to control pH value of an obtained reaction liquid to be 5 to 7, and reaction is carried out for 120 to 180min at 80 to 95 DEG C; other aldehyde raw materials and an alkali catalyst are added for reaction; when the Abbe refractive index of an obtained mixed reaction liquid ranges from 1.460 to 1.470, temperature is reduced to 50 to 70 DEG C quickly, a mixed phenol composed of resorcinol, metacresol, and 3,5-xylenol is added for reaction; vacuum dehydration is carried out, an auxiliary material is delivered into a reaction still, and discharging is carried out after viscosity adjusting so as to obtain a finished product. The single-component high-ortho-position-content thermosetting phenolic resin used for extrusion is friendly to the environment, is low in curing temperature, high in curing speed, long in storage duration, high in product strength, stable in performance, and excellent in comprehensive properties; and no bubble is generated in curing.
Owner:洛阳双瑞橡塑科技有限公司
Stable insulin glargine injection and preparation method thereof
ActiveCN104688677AImprove stabilityGuarantee safe and effectivePeptide/protein ingredientsPharmaceutical delivery mechanismMetacresolInsulin injection
The invention discloses a stable insulin glargine injection and a preparation method thereof. The stable insulin glargine injection comprises the following components: 100IU / mL of insulin glargine, 10-100 mu g / mL of zinc, 1.5-3.5 mg / mL of metacresol, 0.7-1.8 mg / mL of phenol, 15-20 mg / mL of glycerin, and hydrochloric acid and / or sodium hydroxide and injection water, the pH value is 3.8-4.2. By means of modifying the raw material components and the preparation process of the insulin glargine injection, the stability of the insulin glargine injection is increased and the quality of the preparation is improved, and the insulin glargine injection does not have allergic reactions and the safety is good.
Owner:TONGHUA DONGBAO PHARMA
Catalyst for synthesizing 2,3,6-trimethylphenol and preparation method thereof
InactiveCN102974354BExtended service lifeReduce usageOrganic chemistryOrganic compound preparationMetacresolAlkali metal oxide
The invention discloses a method for preparing 2,3,6-trimethylphenol, and the 2,3,6-trimethylphenol is an important intermediate of vitamin E. The method comprises the following steps of: enabling a mixture of metacresol, methanol and water to pass a fixed bed reactor containing an o-position methylation catalyst in a liquid space velocity of 0.4-10 h<-1>, and preparing the 2,3,6-trimethylphenol by carrying out a gas solid phase catalytic reaction under a temperature of 280-450 DEG C and a pressure of 0-10 MPa, wherein the o-position methylation catalyst consists of iron oxide, silicon dioxide and alkali metal oxide, and in the oxides, the metal ion molar ratio of iron, silicon, aluminum and alkali metal is equal to 100:(0.1-10):(0.2-5):(0-1). According to a catalyst for synthesizing the 2,3,6-trimethylphenol and a preparation method thereof, the conversion rate of the metacresol for compounding the 2,3,6-trimethylphenol is 100%, the yield of the 2,3,6-trimethylphenol is above 99.9%, and the service life of the catalyst is longer than 3000 hours.
Owner:SOUTHEAST UNIV
Preparation method of thermosensitive sensitizer
The invention relates to a preparation method of thermosensitive sensitizer. The preparation method is characterized in that methyl substituted phenol (metacresol, o-cresol or p-cresol) and dichloroethane have condensation reaction at the temperature of 70 to 80 DEG C, then the thermosensitive sensitizer 1,2-dimethyl phenoxyl ethane crude product with the purity of 95 to 98 percent (the main impurity is the single substituted phenoxyl ethane) is obtained in the steps of layering, continuous reaction, extraction and layering, and then the crude product is rectified to obtain the high-purity thermosensitive sensitizer. The method is simple to operate, environmentally friendly, high in yield, moderate in reaction condition and low in production cost; the extracted impurity can be used as the raw material to be continued in the reaction.
Owner:山东瑞康精化有限公司
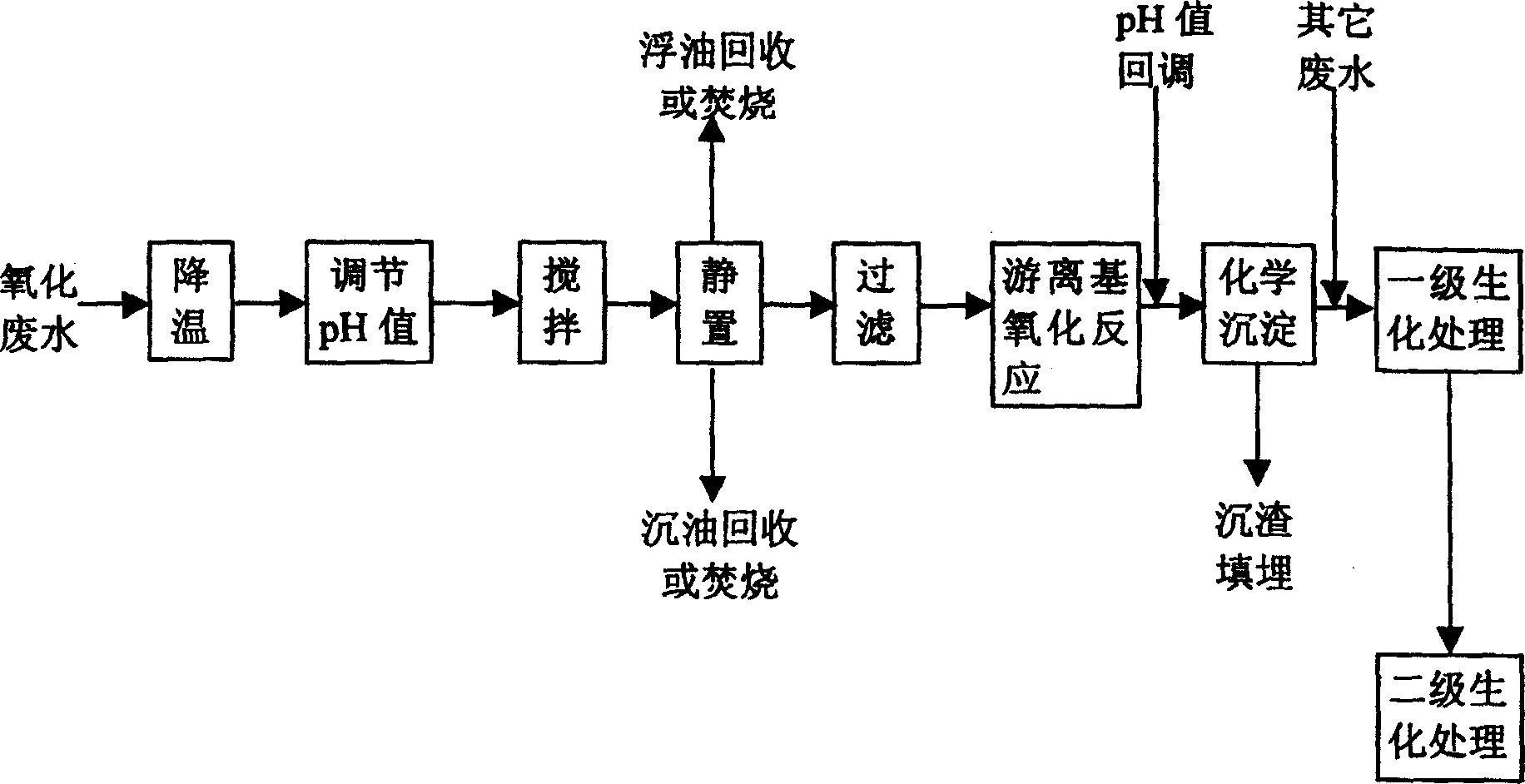
Pre-treating method of metacresol production effluent
InactiveCN1169734CEasy to handleImprove the efficiency of subsequent biochemical treatmentMultistage water/sewage treatmentNon-miscible liquid separationPretreatment methodMetacresol
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for preparing thymol through isopropyl m-cresols
InactiveCN105061153AHigh purityHigh selectivityOrganic chemistryOrganic compound preparationMolecular sieveMetacresol
The invention relates to a method for preparing thymol through isopropyl m-cresols, which adopts a molecular sieve as a catalyst, adds raw material metacresol and isopropyl reagent isopropyl alcohols into a reaction kettle according to certain proportion, generates the thymol and isomers thereof through a catalytic reaction in a given temperature, and can achieve above 98% selective yield of the thymol. The method for preparing the thymol through the isopropyl m-cresols has the advantages of high catalysis efficiency, wide source of raw materials, excellent product selective yield and easy operation of industrial production. The method for preparing the thymol through the isopropyl m-cresols has great guidance and application value in production preparation of the isopropyl m-cresols.
Owner:侯颖
Tazobactam synthesis method
ActiveCN102020663BHigh yieldReduce usageOrganic chemistryChemical recyclingMetacresolSynthesis methods
The invention relates to a tazobactam synthesis method which comprises the steps of: with 6-APA(Amino Penicillanic Acid) as raw material, preparing a key intermediate 6,6-dihydro penam sulphoxide acid diphenylcarbinol ester through successive reactions of esterification, oxidation, reduetive debromination and the like without separation; then, reacting with 2-triphenyl silicon-1,2,3-triazole; introducing a triazole ring; and finally obtaining the final product of tazobactam through potassium permanganate oxidation and metacresol deprotection. The tazobactam synthesis method is mainly characterized in that a phase transfer catalyst is introduced in the first step, therefore, the reaction rate and the product purity are improved; since an environment-friendly hydrogen peroxide-cobalt acetate catalytic oxidation system is adopted in the third step, the characteristics of good reaction selectivity, high yield, catalyst recyclability and the like are achieved; a method for synthesizing 2 alpha-methyl-2 beta-(1,2,3- triazole-1- radical) methyl penam-3 alpha-carboxylic acid diphenylcarbinol ester by using 2-triphenyl silicon-1,2,3-triazole is adopted in the fifth step, and the tazobactamsynthesis method is simple and convenient to operate, is safe and reliable, shortens the reaction route and improves the total yield. Compared with the traditional process, the tazobactam synthesis method greatly reduces the production cost and the environment pollution and has greater implementation value and economic benefits.
Owner:YIYUAN XINQUAN CHEM
Pharmaceutical raw material hexafluoroacetone synthesis method
InactiveCN108238870AReduce intermediate linksShort reaction timeOrganic compound preparationCarbonyl compound preparationMolybdateMetacresol
The invention relates to a pharmaceutical raw material hexafluoroacetone synthesis method, which mainly comprises: adding 3 mol 2,3-dihydroxy-hexafluoroisopentane and 4-6 mol N-methylpropionamide solution to a reaction container, increasing the temperature of the solution to 70-80 DEG C, maintaining for 60-80 min, adding 2-3 mol bismuth molybdate, continuously carrying out the reaction for 50-70 min, reducing the temperature to 40-50 DEG C, carrying out pressure reducing distillation, collecting the distillate at a temperature of 80-89 DEG C, washing with a metacresol solution, washing with am-chloroaniline solution, and re-crystallizing with a methoxytoluene solution to obtain the crystal hexafluoroacetone.
Owner:CHENGDU DONG DIAN AI ER TECH
Treating method of metacresol production effluent
The present invention relates to the technology of organic chemical effluent treatment. The treating method can reduce the consumption of treating agent, and the CODcr eliminating rate of the effluent in the pre-treatment may reach 60% being favorable to subsequent biochemical treatment. The treating process includes the pre-treatment of oxide effluent from metacresol production, mixing of the effluent after the pre-treatment and other two kinds of waste water exhausted from metacresol production, and the subsequent oxidation treatment. The said process has stable treatment effect, low treatment cost and simple and practical operation.
Owner:CHINA PETROLEUM & CHEM CORP +1
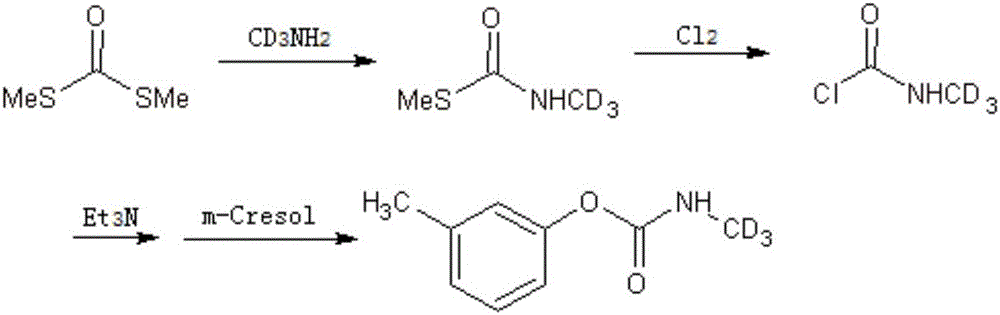
A kind of synthetic method of isotope-labeled metilcarb-d3
ActiveCN104610098BEasy to separateSimple processCarbamic acid derivatives preparationOrganic compound preparationIsotopic labelingMetacresol
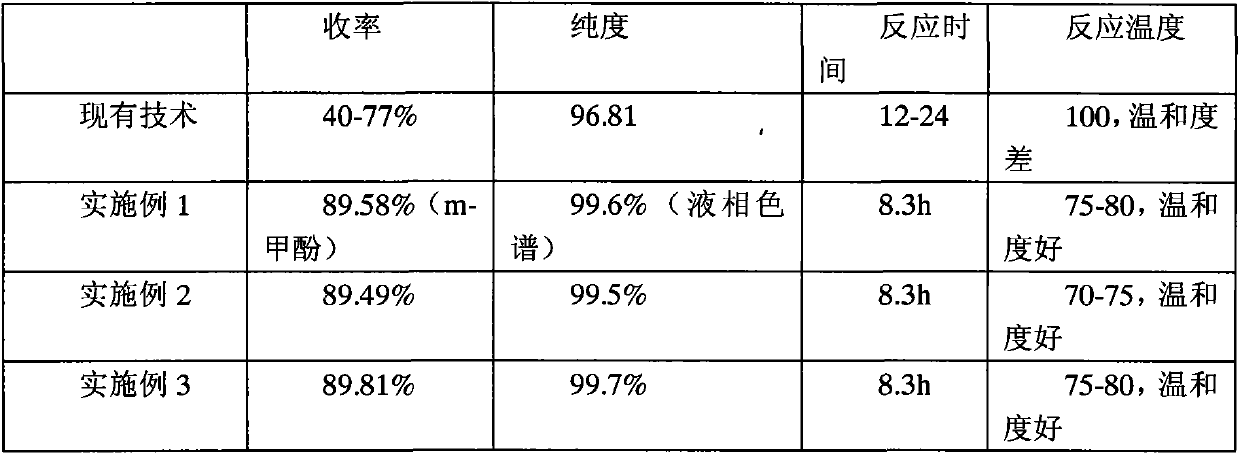
The present invention relates to a synthetic method for isotopically labeled tsumacide-D3. The synthetic method comprises the following steps: dropwise adding an aqueous solution of monomethylamine hydrochloride-D3 into a solution of dithio carboxylic acid dimethylster, carrying out reaction to obtain an intermediate of S-methyl-N-methyl carboxylic acid dimethylster, slowly injecting Cl2 into the intermediate at a low temperature, successively adding an acid-binding agent and metacresol, and preparing the isotopically labeled tsumacide-D3. Compared with the prior art, the deuterium isotopic labeling process in the invention has a simple process and high yield, isotopic abundance is undiluted, and the method is suitable for laboratory production of tsumacide-D3.
Owner:SHANGHAI RES INST OF CHEM IND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com