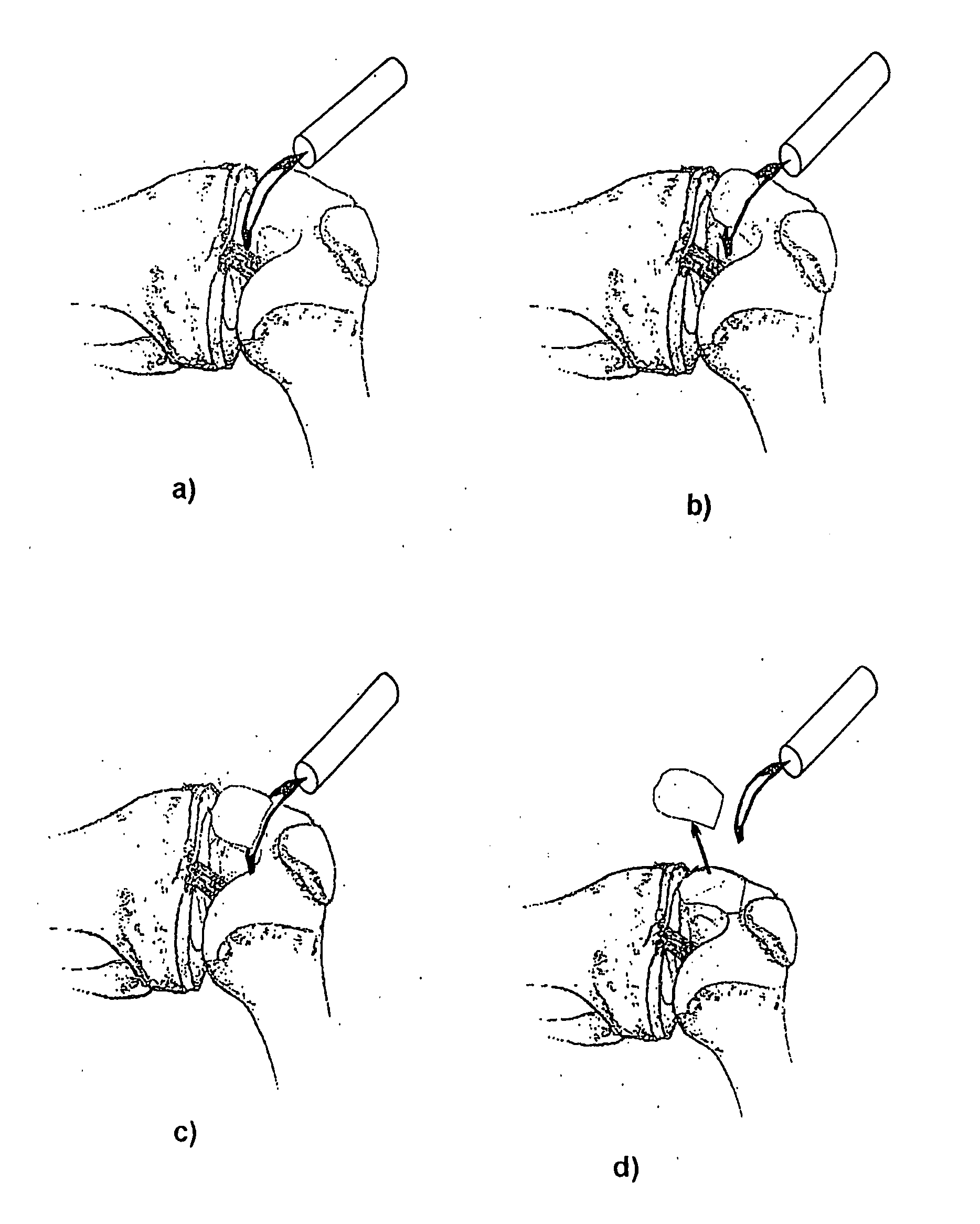
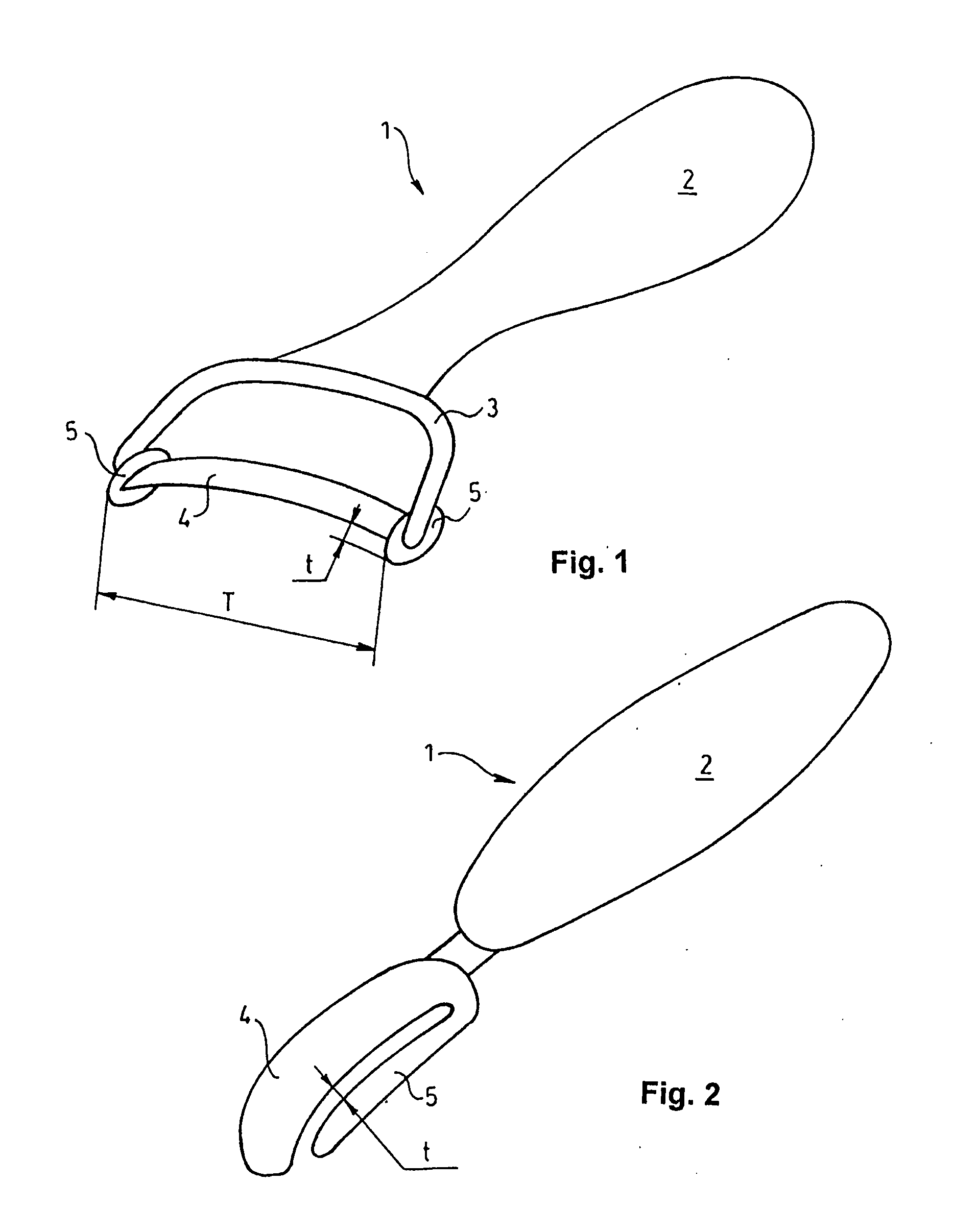
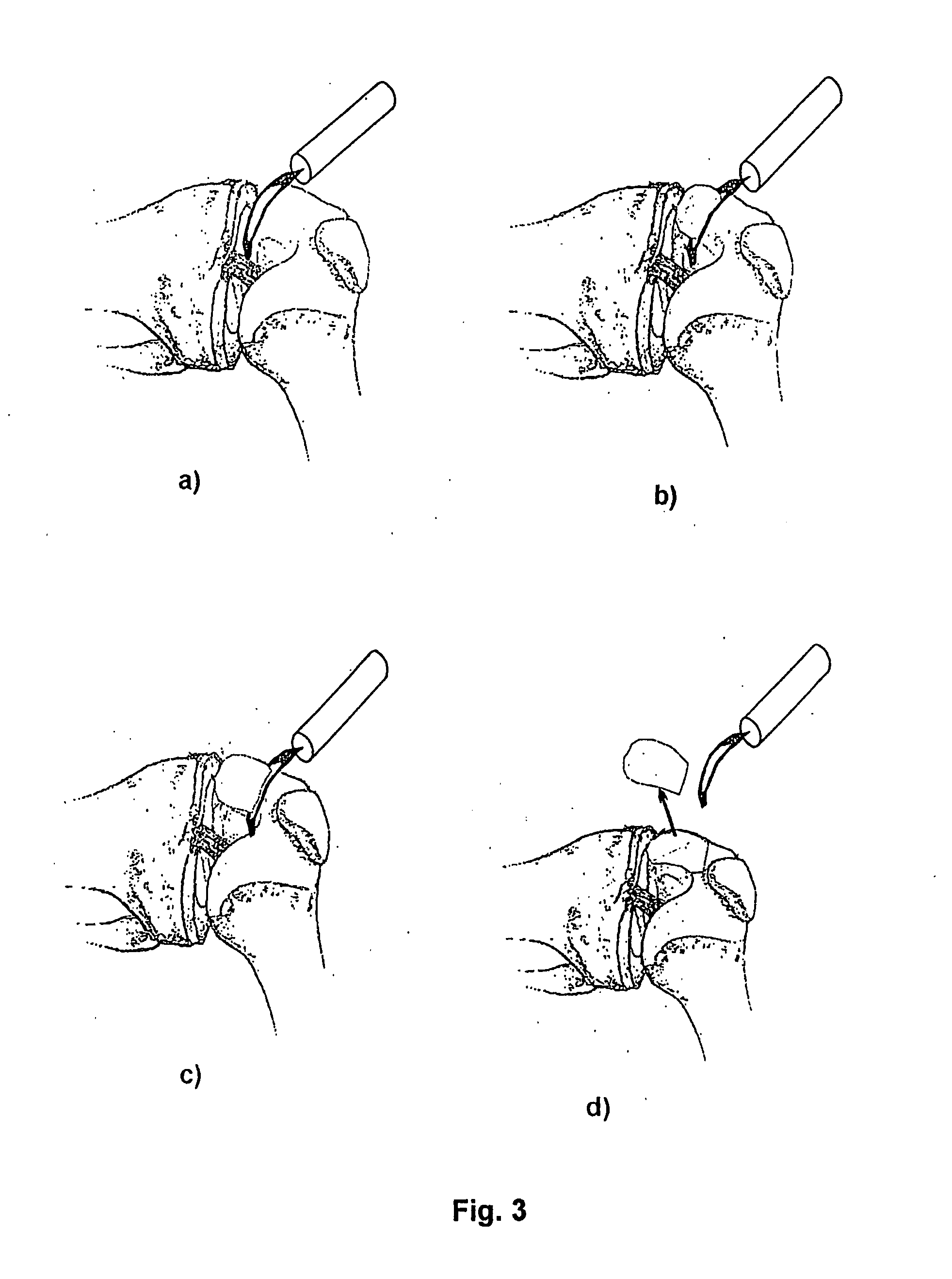
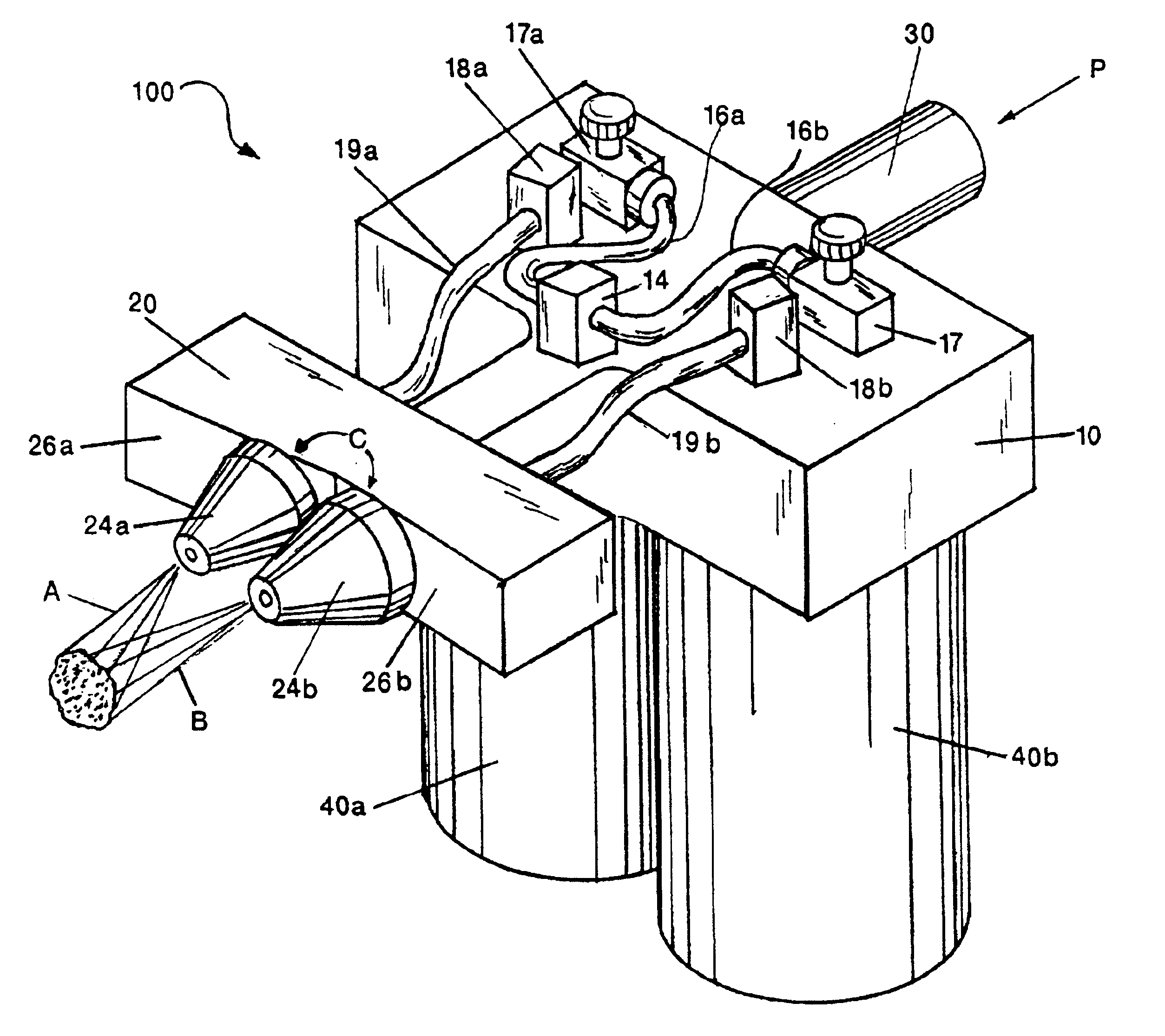
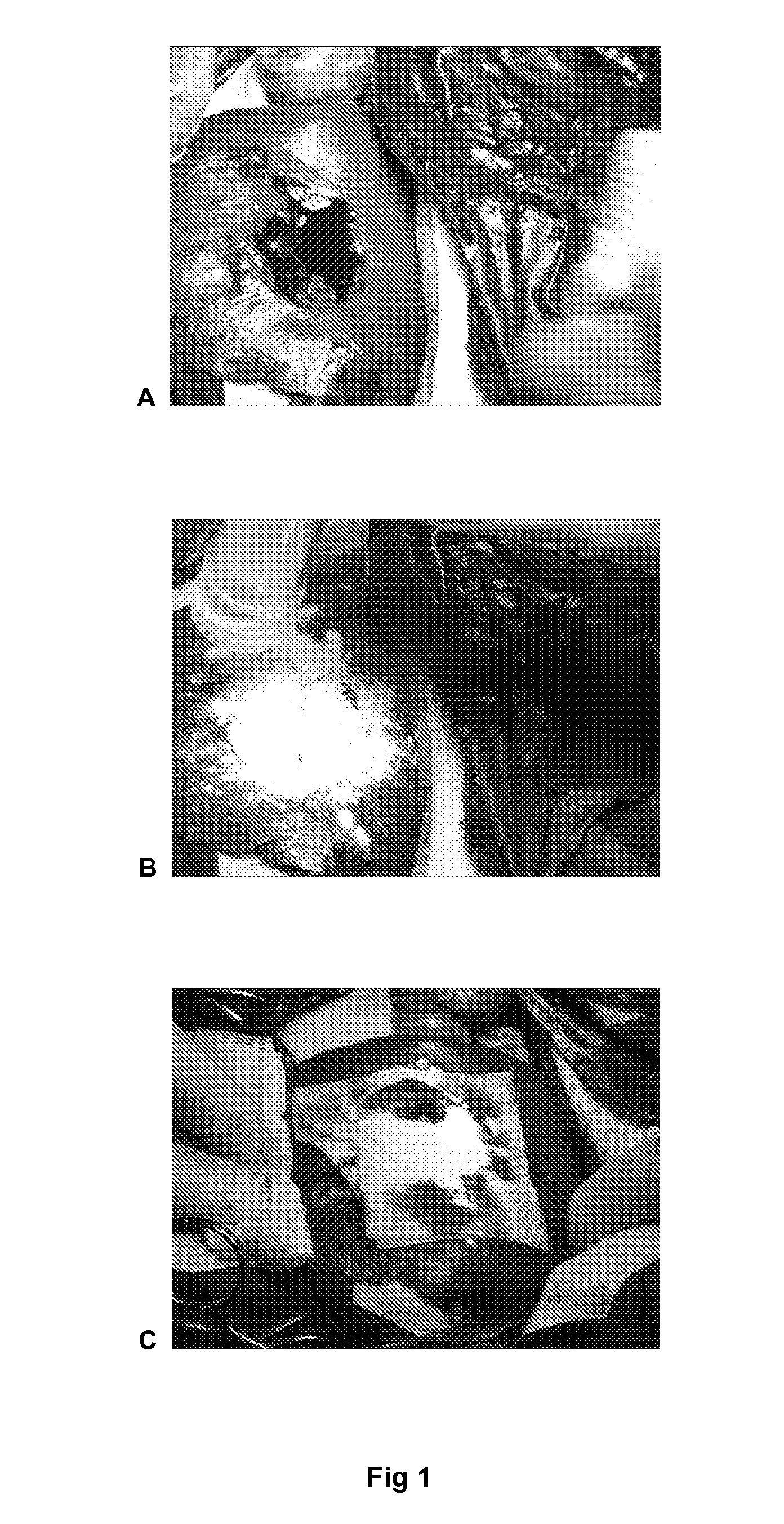
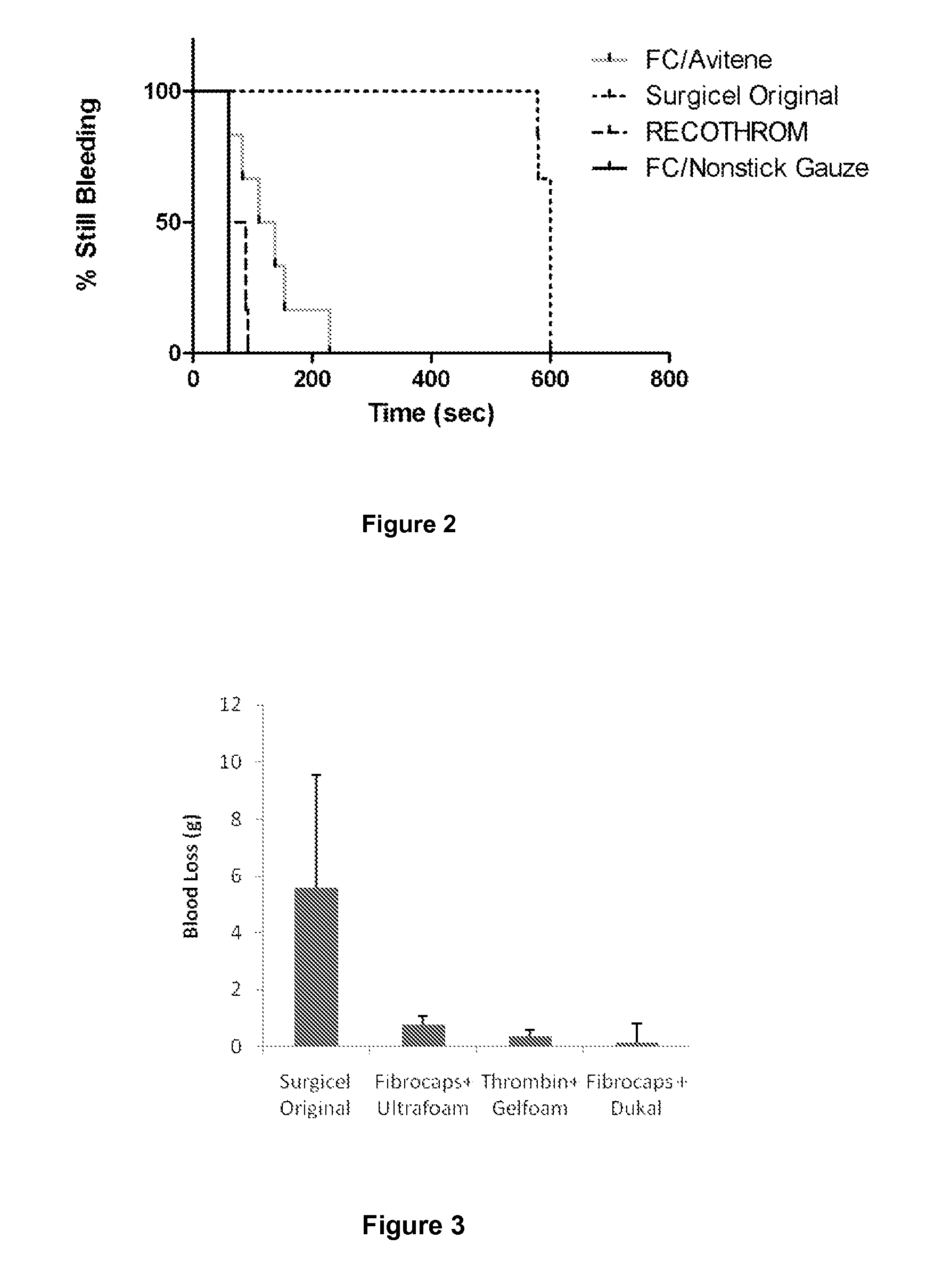
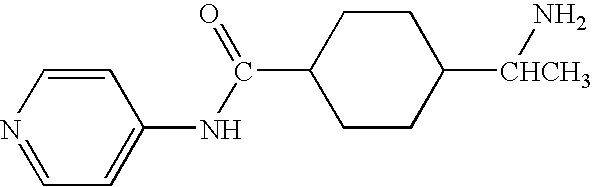
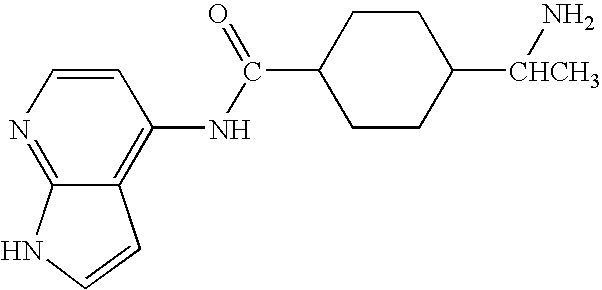
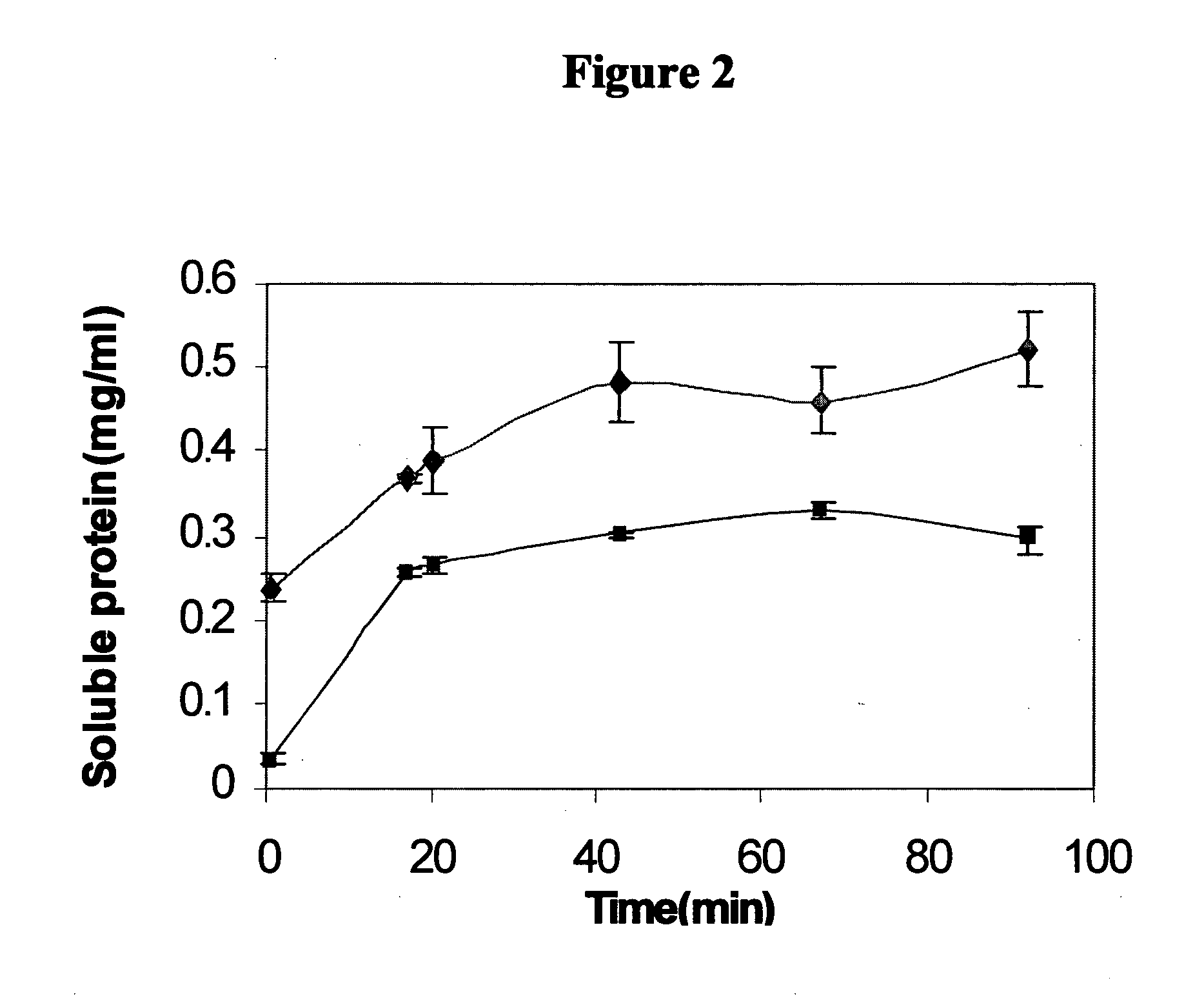
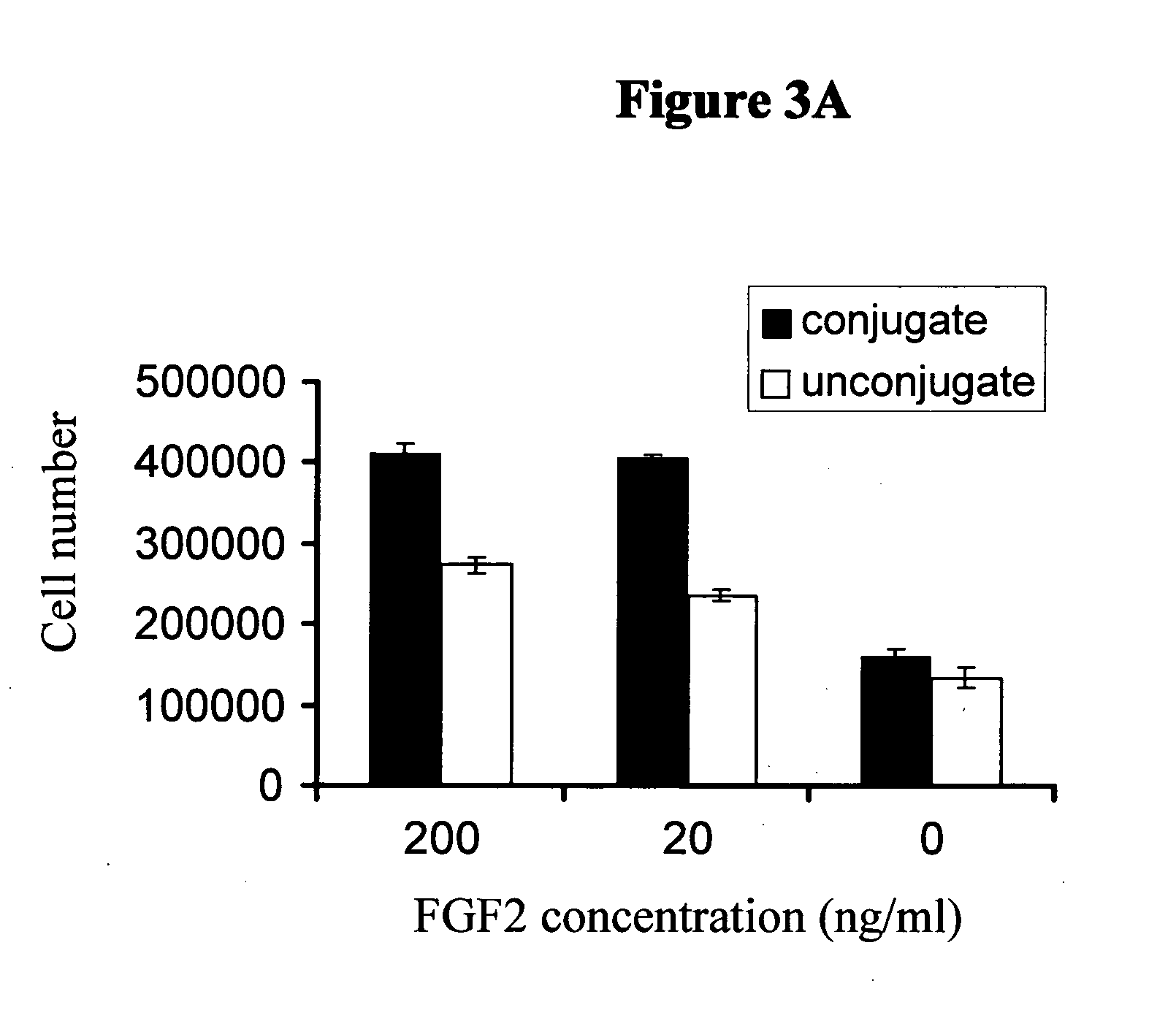
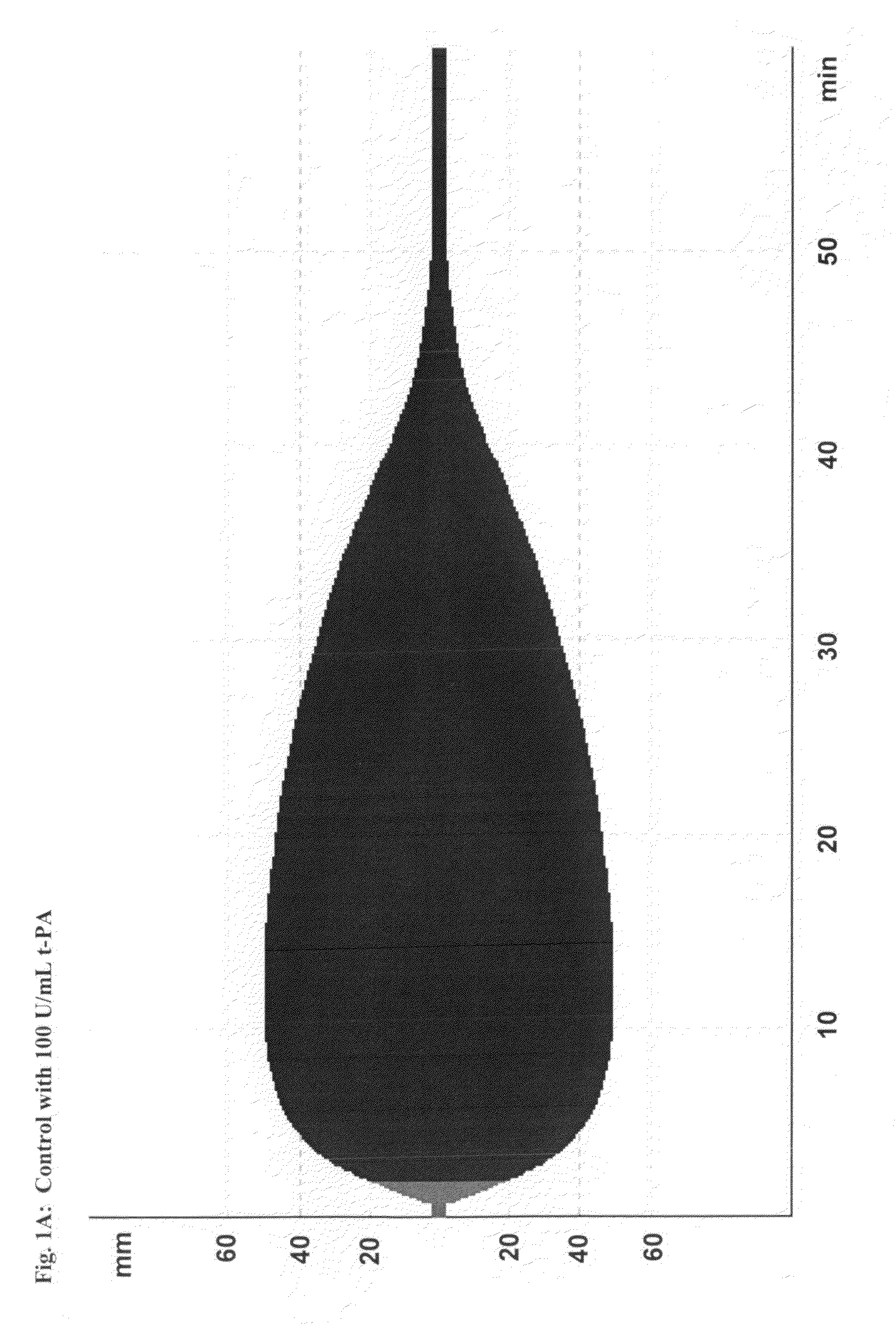
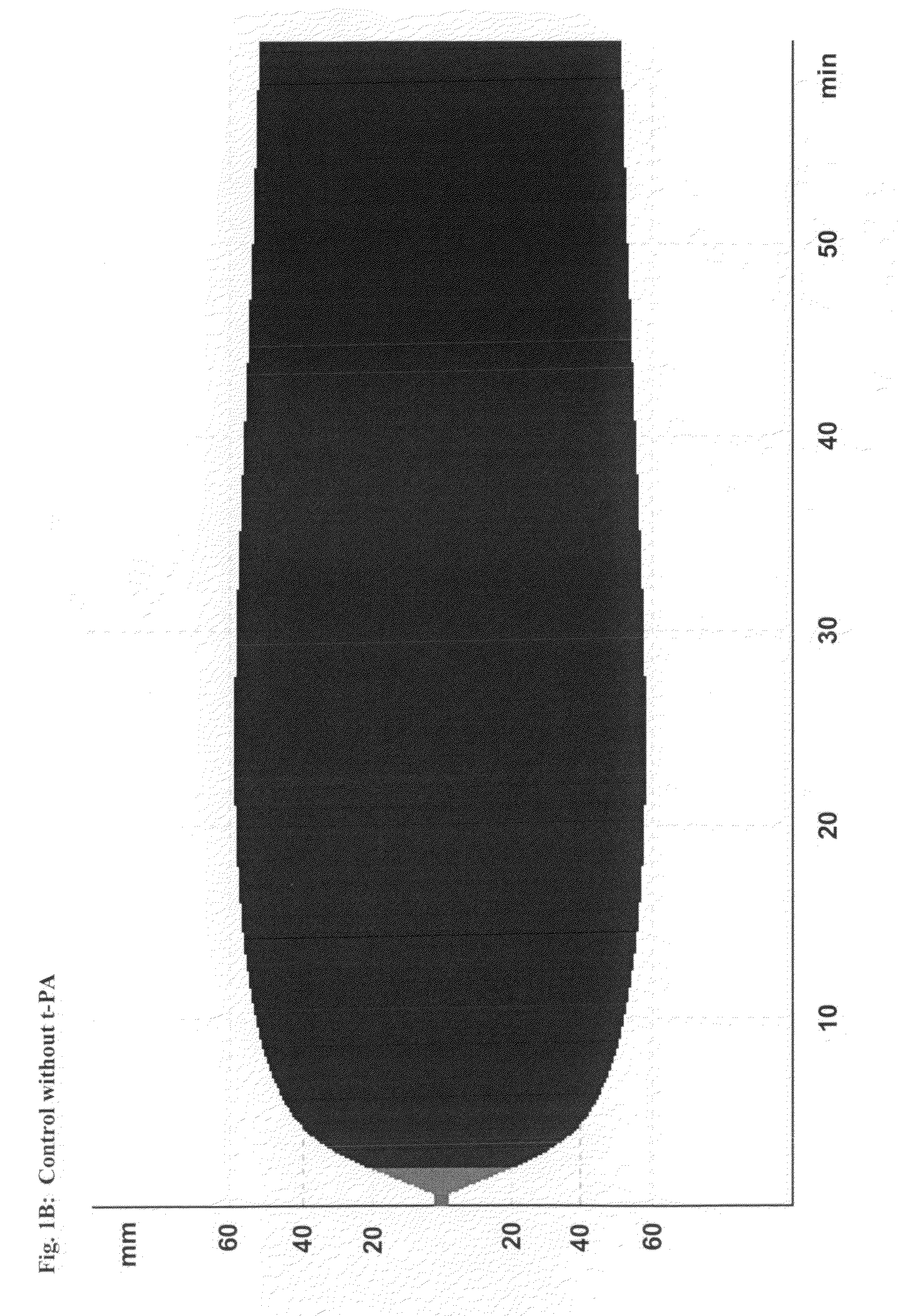
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
180 results about "Fibrin adhesive" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Systems and methods for preparing autologous fibrin glue
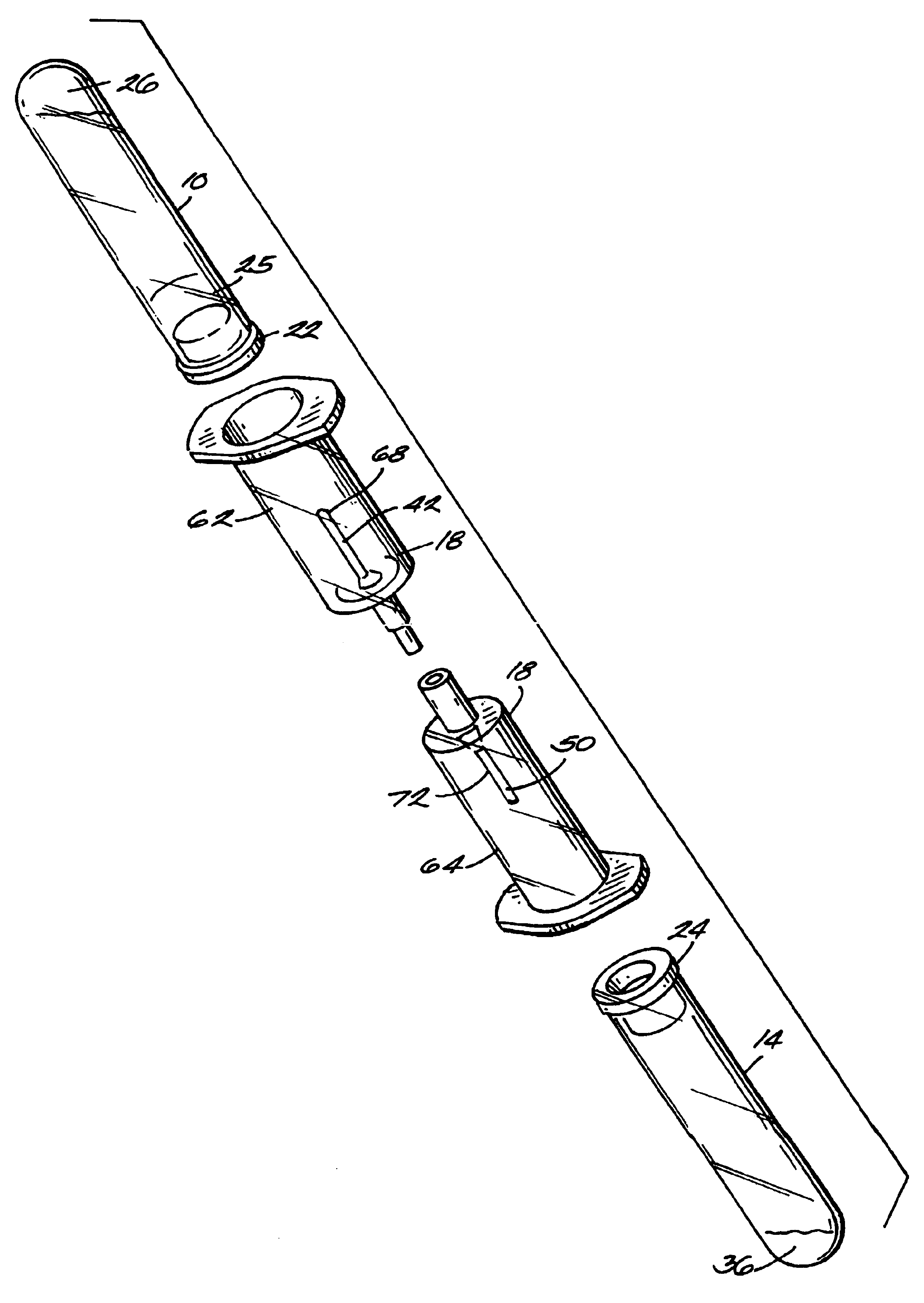
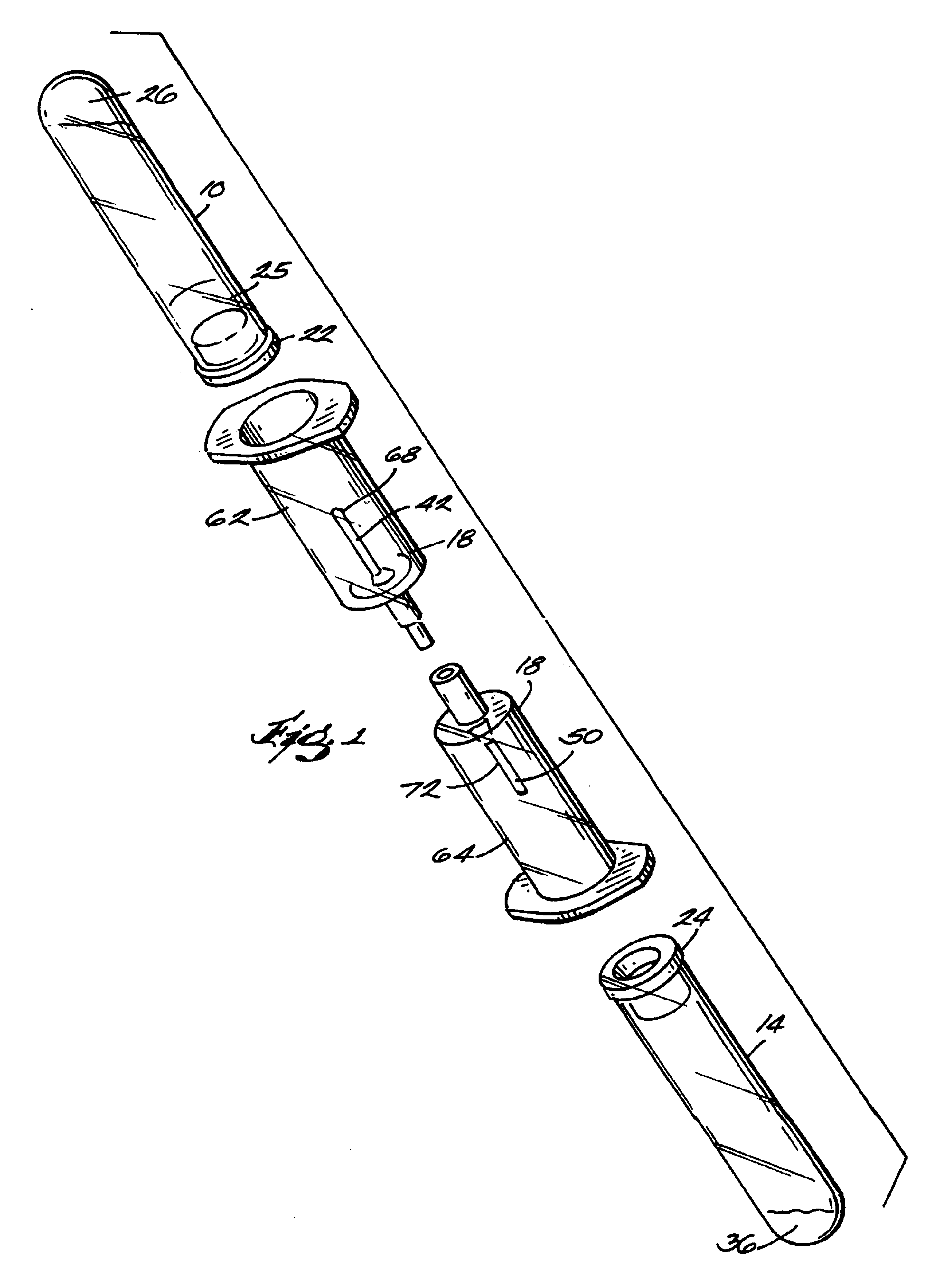
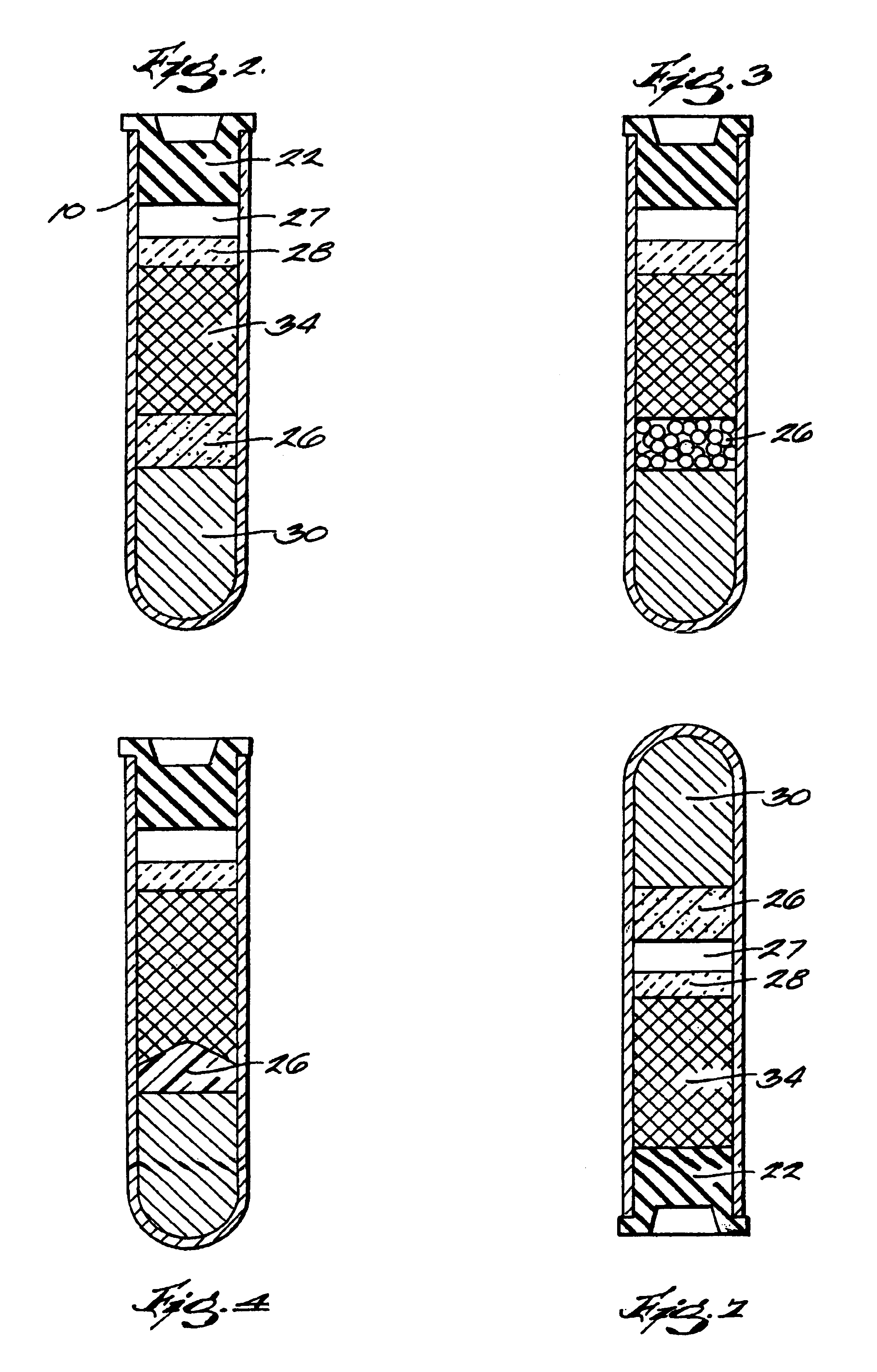
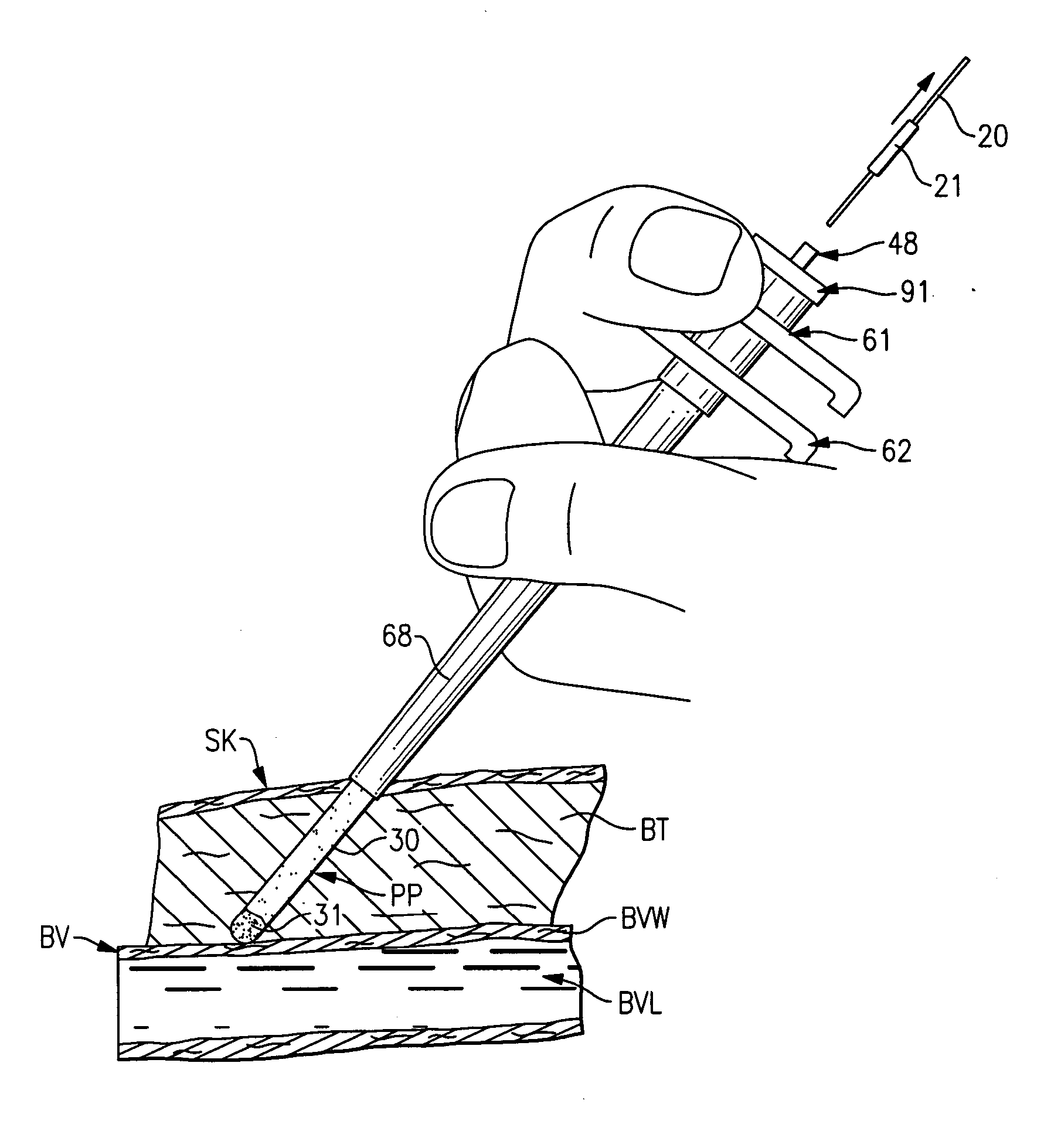
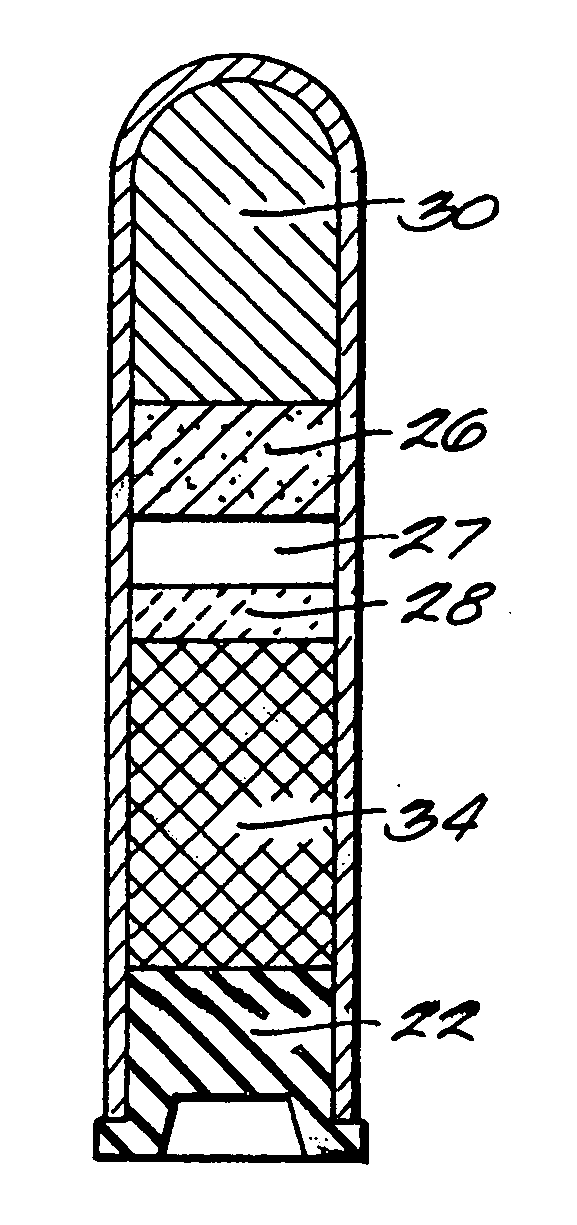
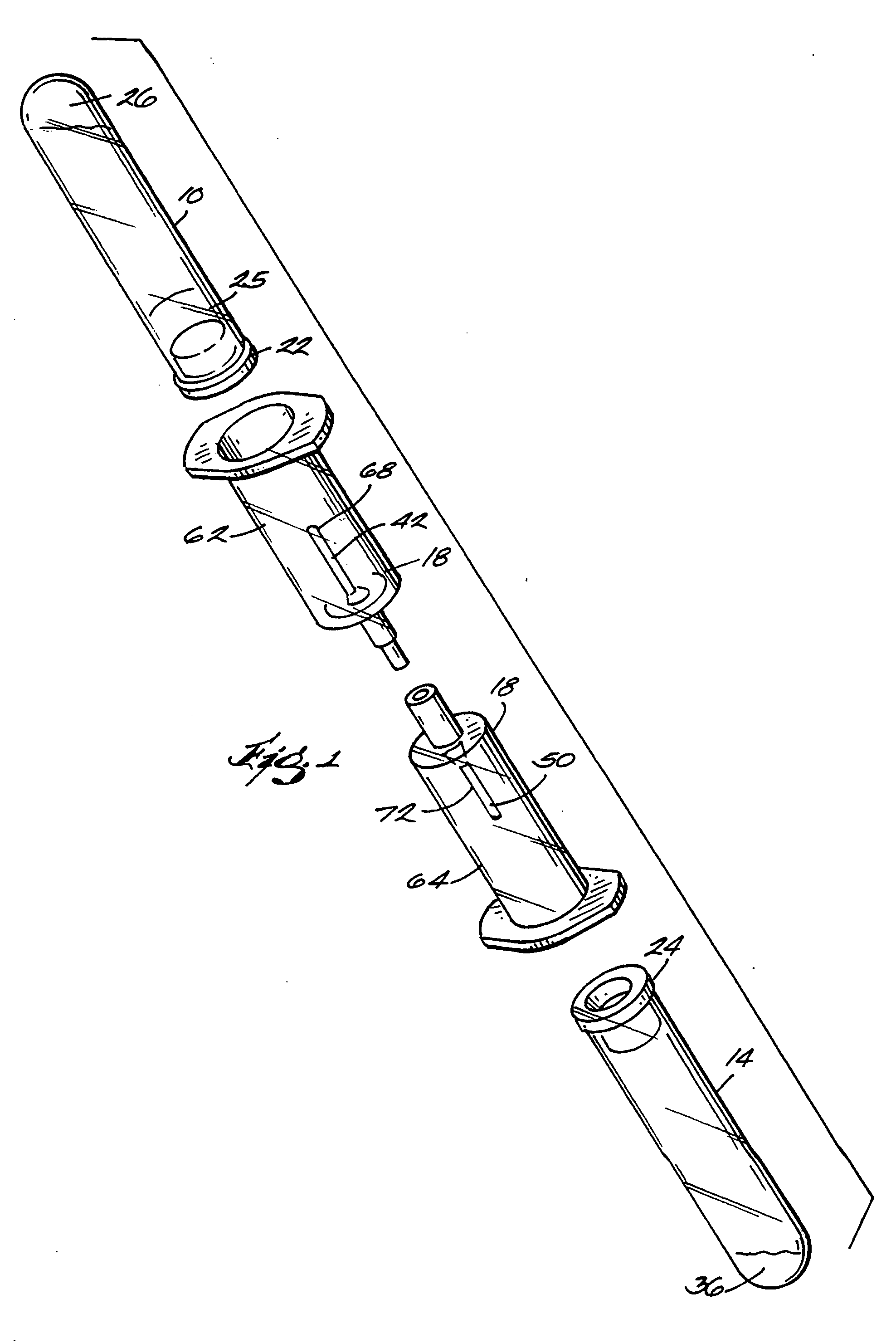
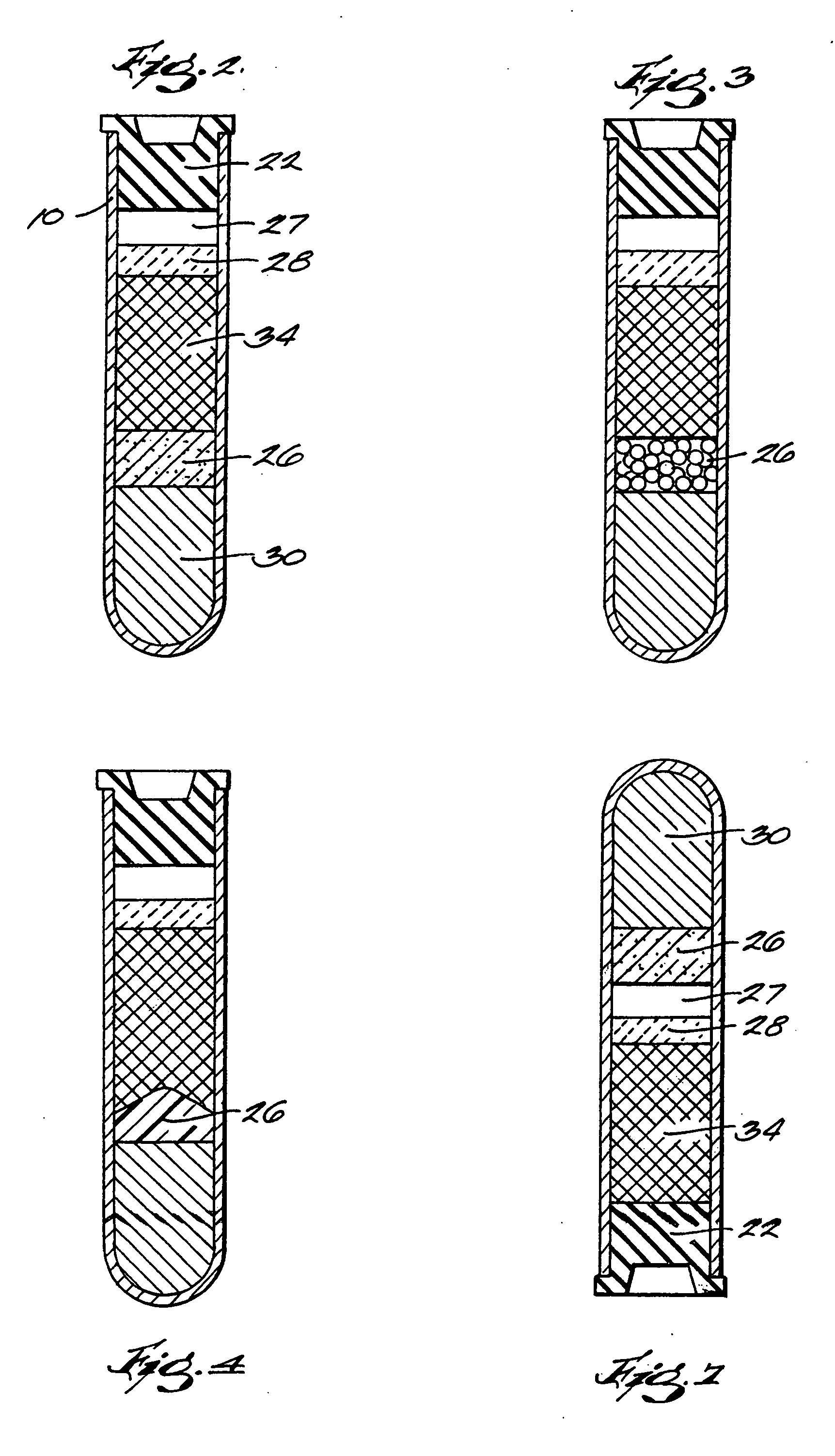
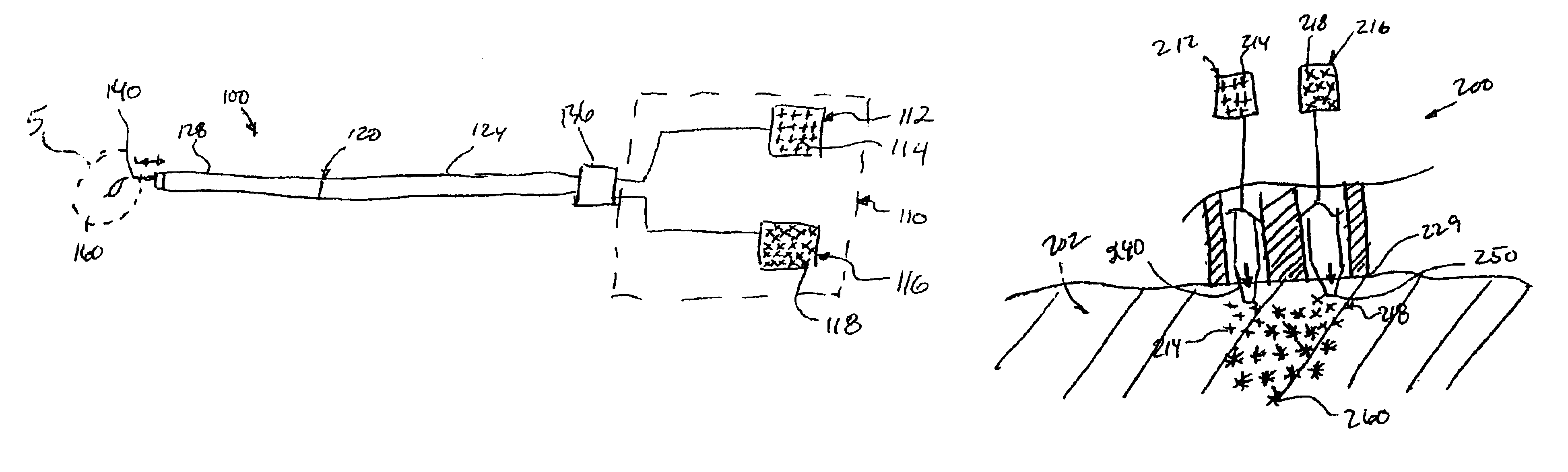
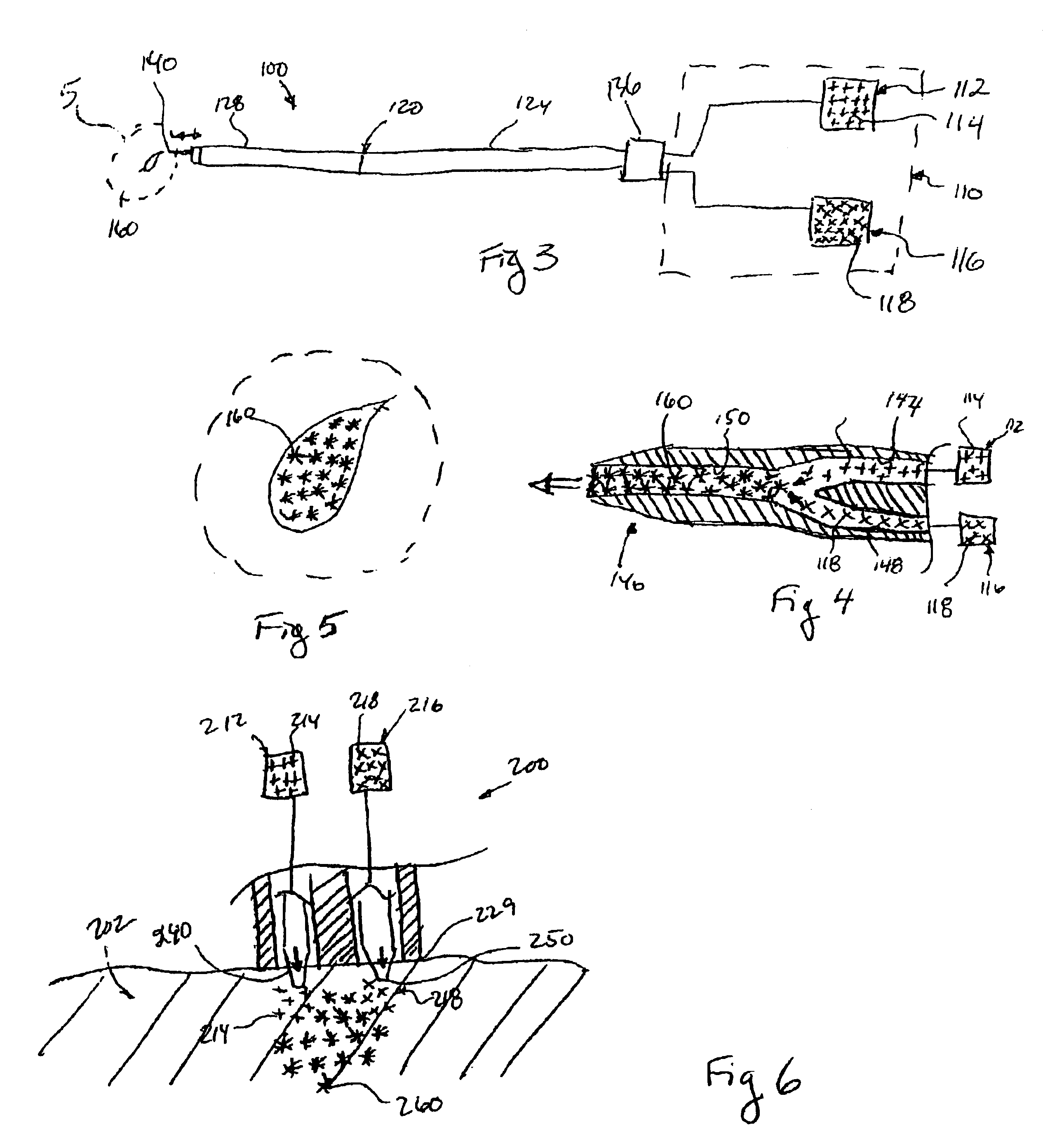
The invention provides a system for preparing an autologous solid-fibrin web suitable for regenerating tissue in a living organism. The system includes a sealed primary container containing a separation medium and a low-density high-viscosity liquid. The separation medium separates red blood cells from plasma when the container contains blood and is centrifuged, and the primary container has a first pressure. The system further includes a sealed secondary container containing a calcium-coagulation activator. The secondary container has a second pressure that is less than the first pressure. The system also comprises a transfer device including a cannula having a first end and a second end. The first and second ends puncture the sealed primary and secondary containers in order to provide fluid communication between the first and second containers. The low-density high-viscosity liquid of the primary container blocks flow through the cannula upon entering therein.
Owner:CASCADE MEDICAL ENTERPRISES
Fibrin glue without fibrinogen and biosealant compositions and methods
InactiveUS6168788B1Stop blood flowPrevents unwantedSurgical adhesivesPeptide/protein ingredientsFibrin glueClot formation
The invention is a fibrin glue that avoids the use of fibrinogen and thus eliminates the need for premixing and premature clot formation. The fibrin glue of the invention comprises thrombin, thromboplastin and calcium and may have clotting Factors, VII, IX and X, and the like. The invention also comprises a biosealant for use with the fibrin glue without fibrinogen or for use alone. The biosealant is a two component mixture of gelatin / resorcinol and glyoxal / glutaraldehyde / 4-(p-maleimidophenyl) butyric acid. The two components are mixed on use.
Owner:WORTHAM LEON
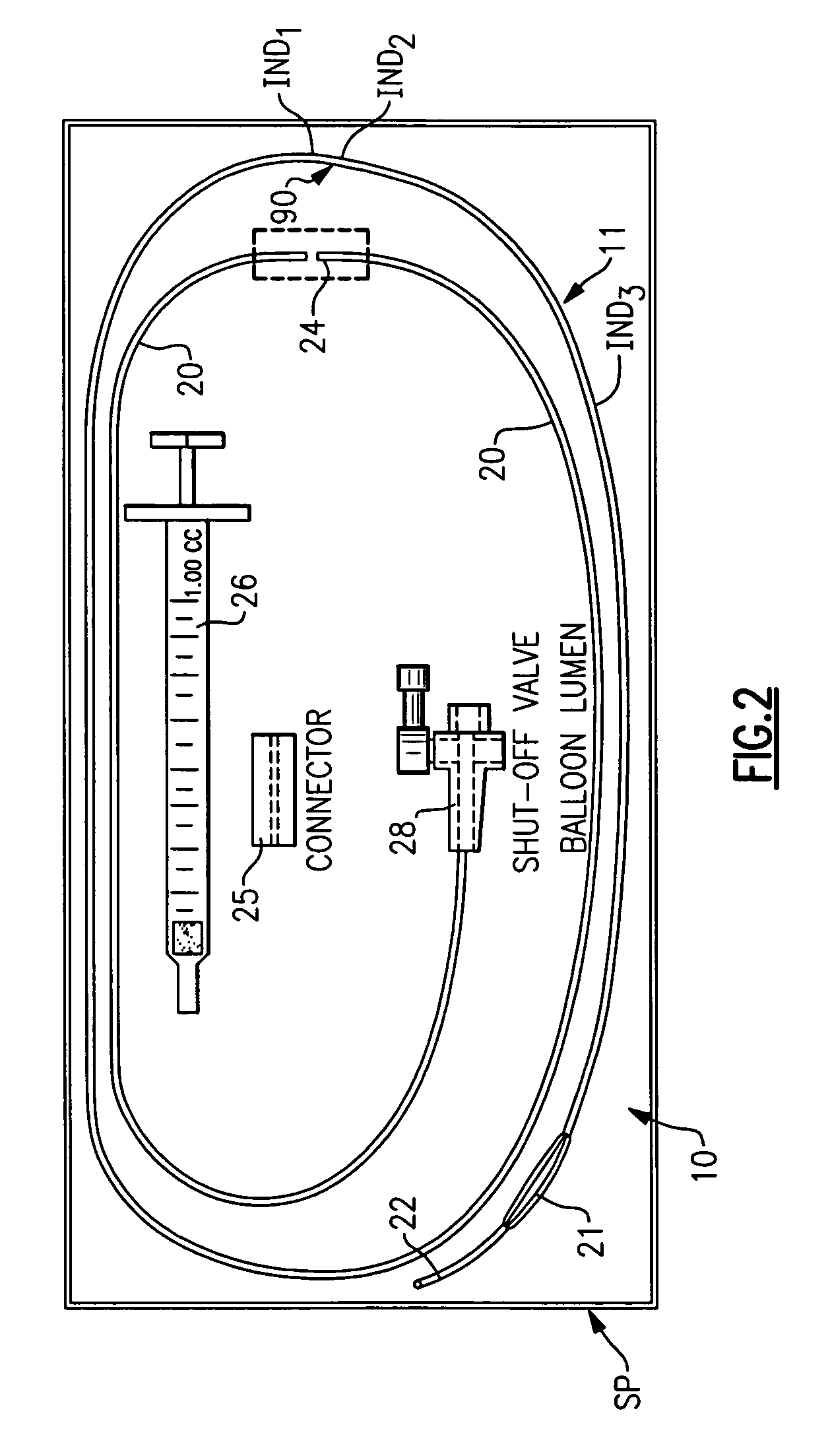
Percutaneous puncture sealing system
InactiveUS20050065549A1Obstruct passageImprove sealingSurgical veterinaryWound clampsBody cavity useSurgery
A method of sealing percutaneous punctures in a patient's body that open into an internal body cavity using a sealing material such as a fibrin adhesive while preventing the sealing material from entering the body cavity. An apparatus for delivering the sealing material is also disclosed.
Owner:CATES CHRISTOPHER U +2
Material compositions and related systems and methods for treating cardiac conditions
InactiveUS20050271631A1Preventing negative remodelingHigh retention rateBiocideSurgical adhesivesInjectable polymersFibrin glue
A medical condition associated with a cardiac structure is treated by injecting an injectable polymer agent into the cardiac structure such that a therapeutic mechanical scaffolding is formed within the cardiac structure itself. In particular, the injectable scaffolding agent is a fibrin glue agent and is injected into regions of damaged myocardium such as ischemic tissue or infarct. LV wall dysfunction may also be treated by injecting the scaffolding agent into the LV wall. Cell therapy may be combined with the injection of fibrin glue or other injectable polymer scaffold agent. The polymeric forms of the agent may be injectable as precursor materials that polymerize as a scaffold in-situ within the cardiac structure. In other modes, polymer agents are injected in order to provide therapeutic angiogenesis, or to induce deposition of cells within the injected area, such as by providing the polymer with fragment E or RDG binding sites, respectively.
Owner:RGT UNIV OF CALIFORNIA
Biocolloid hemostatic prepared by aldehyde-modified sodium alginate and amine-modified gelatine
InactiveCN101716366AEasy to prepareRapid hemostasisAbsorbent padsBandagesFibrin glueBiocompatibility Testing
The invention relates to a biocolloid hemostatic prepared by aldehyde-modified sodium alginate and amine-modified gelatine. Sodium alginate and gelatine as raw materials; according to ratio, aldehyde-modified sodium alginate solution with the concentration of 5-25% and the same volume of amine-modified gelatine solution with the concentration of 5-40% are mixed; sodium alginate is modified by aldehyde through sodium periodate oxidation; and then gel is obtained by mixing with gelatine modified by ethanediamine. The adopted gelatine and sodium alginate are safe and stable, have good biocompatibility and can be absorbed and degraded by organism. Gel is rapidly formed through Schiff base reaction of aldehyde-modified sodium alginate and amine-modified gelatine, and the gel has the same gel-forming time as fibrin glue but better adhesion property and adhesion strength, can be acted on any part of body through simple local spraying or injection, and can be used as substitute product of fibrin glue.
Owner:TIANJIN UNIV
Method of preparing a collagen sponge, a device for extracting a part of a collagen foam, and an elongated collagen sponge
InactiveUS7098315B2Improve featuresHigh densitySurgical adhesivesPeptide/protein ingredientsAprotininFibrin glue
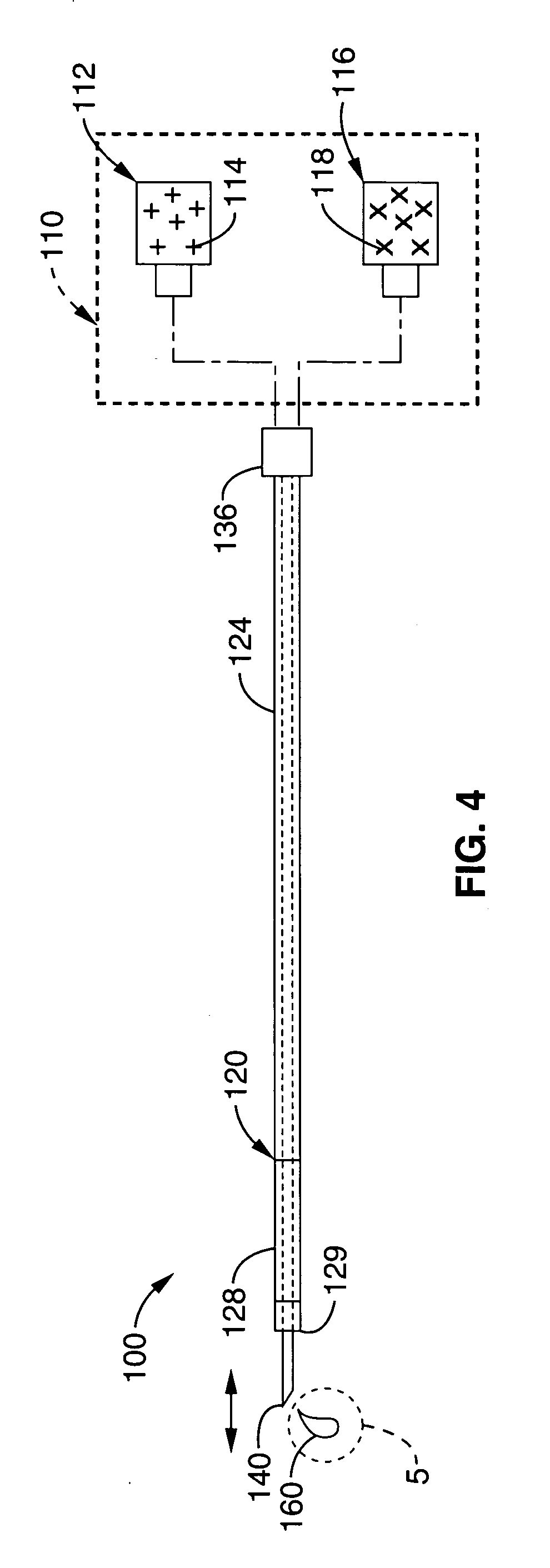
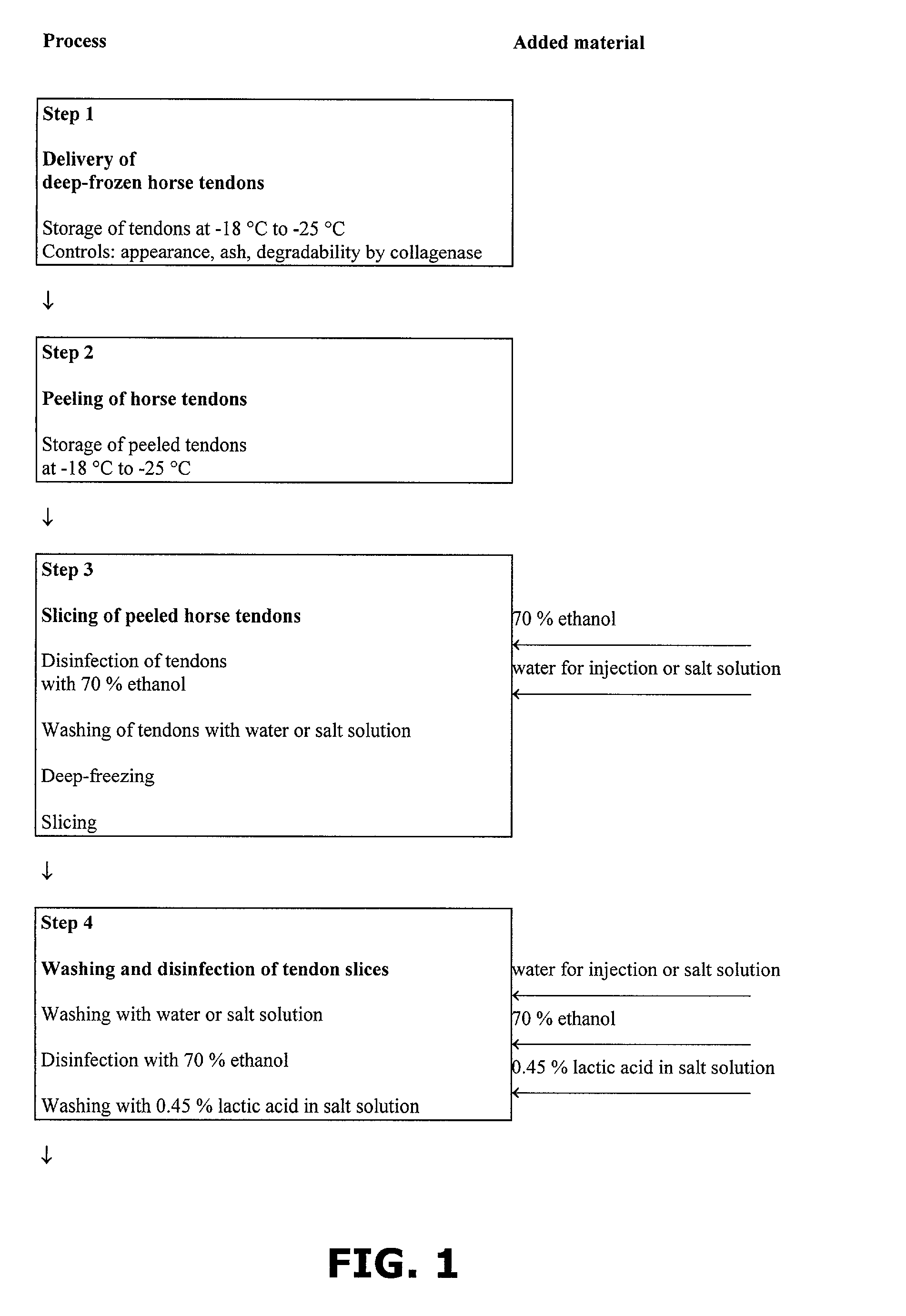
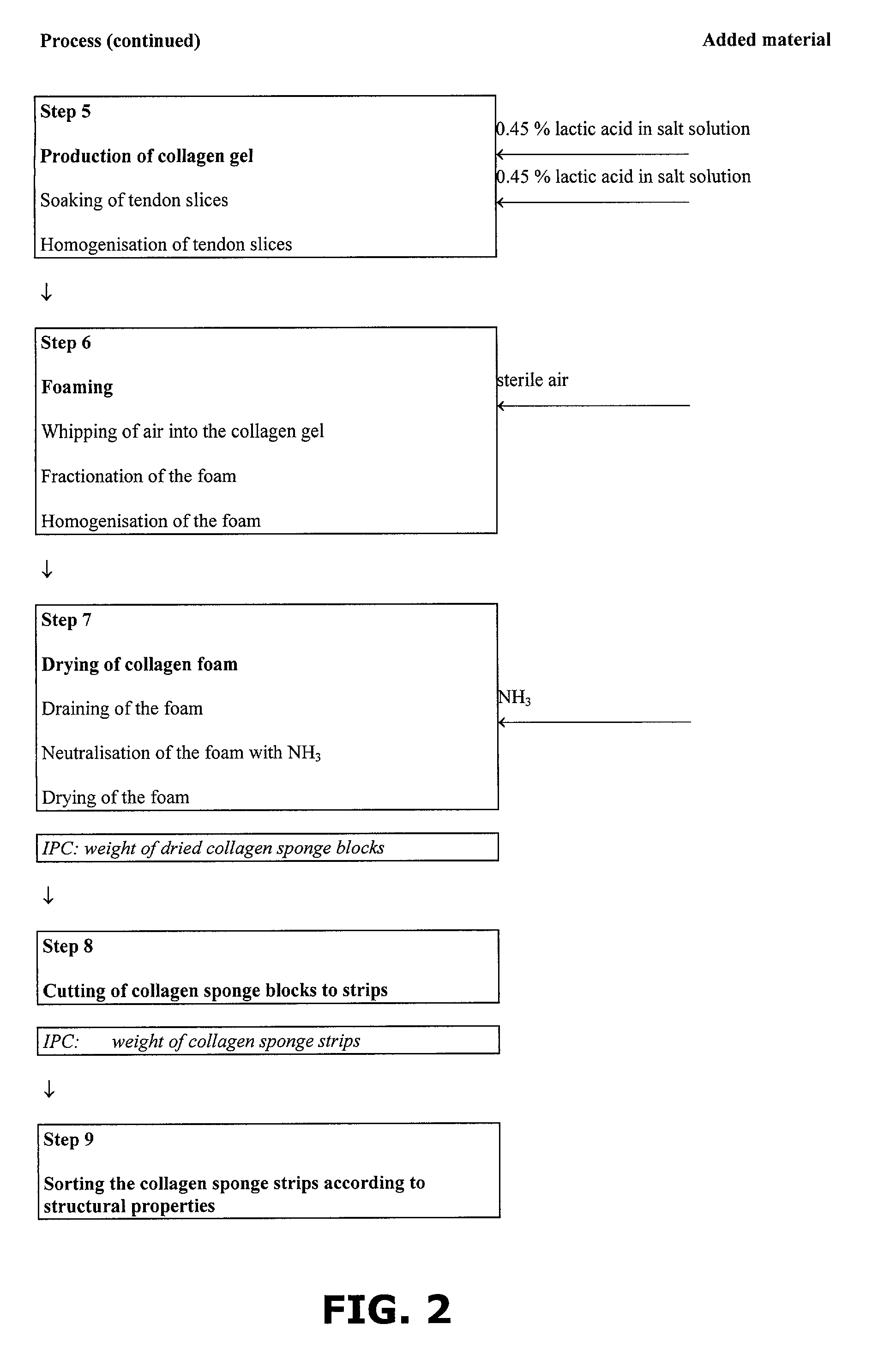
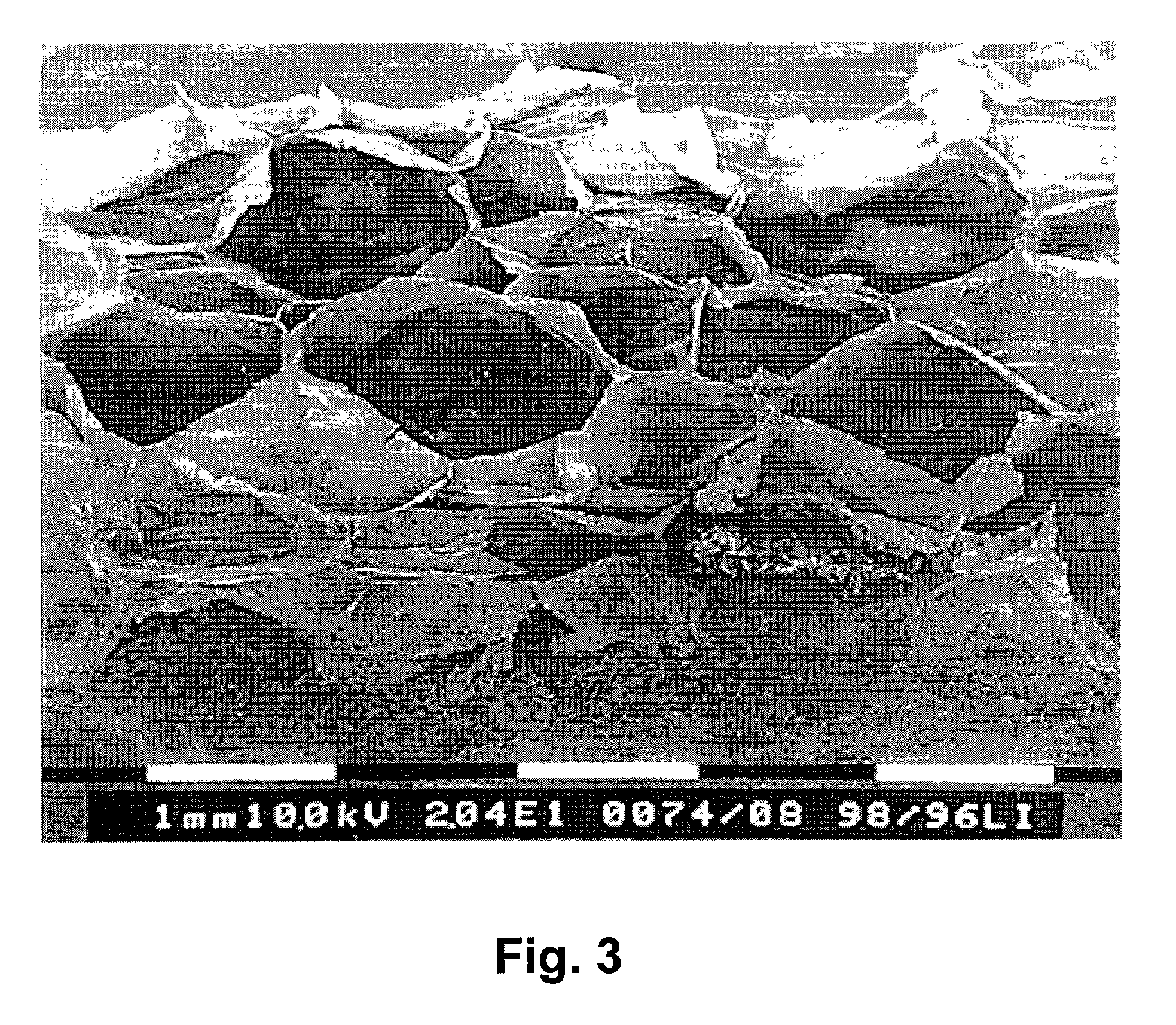
A method of preparing a collagen sponge comprises mixing air into a collagen gel, so as to obtain a collagen foam which is dried. From the dried product thereby obtained, collagen sponge is obtained by isolating parts of sponge with a chamber diameter of more than 0.75 mm and less than 4 mm, or parts with an average chamber diagonal dimension of 3 mm. The collagen sponge may be used as a material for sealing wounds, possibly with a coating comprising a fibrin glue, such as a combination of fibrinogen, thrombin and aprotinin. A device for extracting a part of a collagen foam and for degenerating another part of the collagen foam to a collagen gel is disclosed. An elongated collagen sponge having a through-going hole or bore and a flexible wall may be used for re-establishing walls in a mammalian gastrointestinal funnel or trachea system.
Owner:TOPAZ INVESTMENT AS
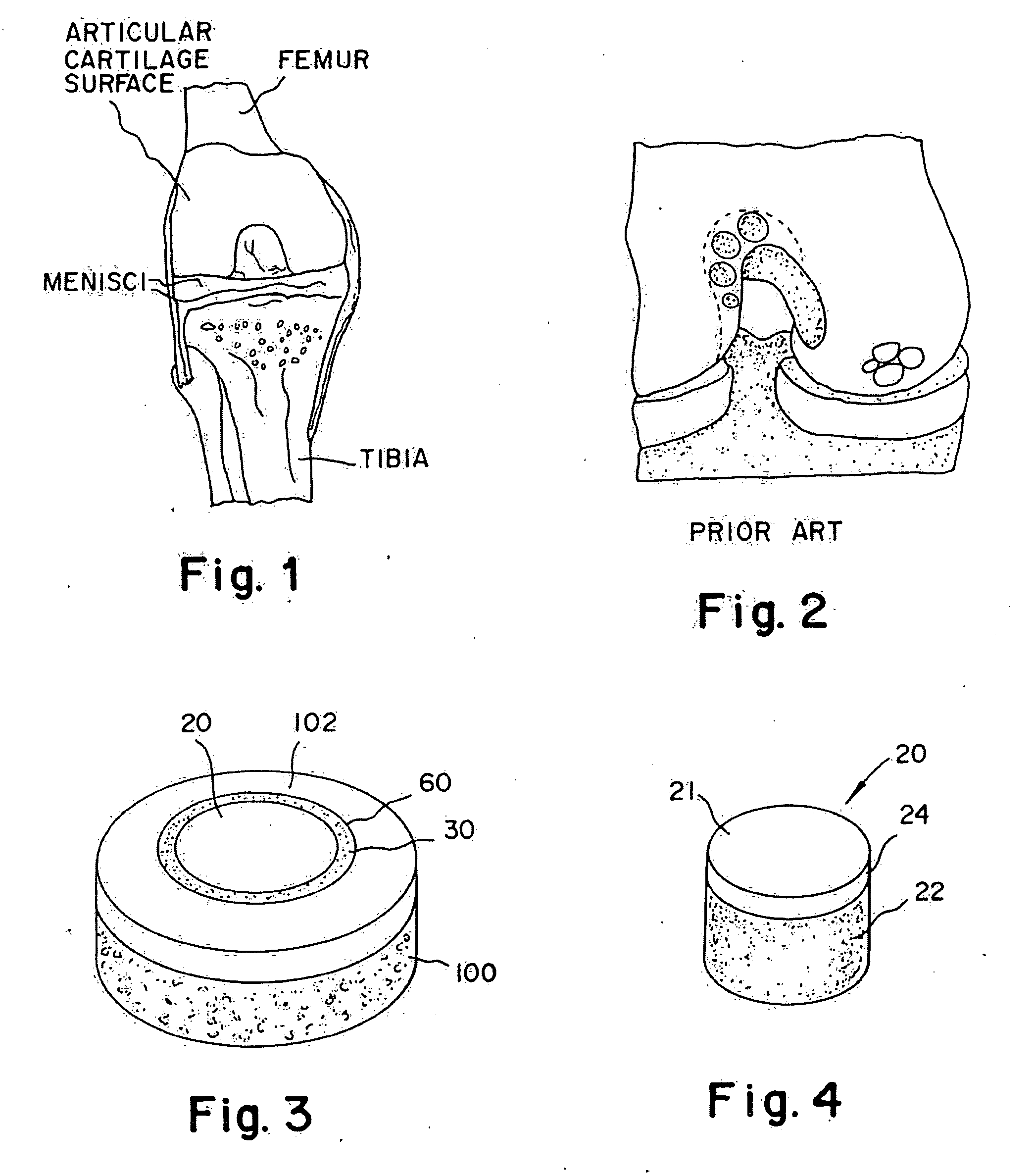
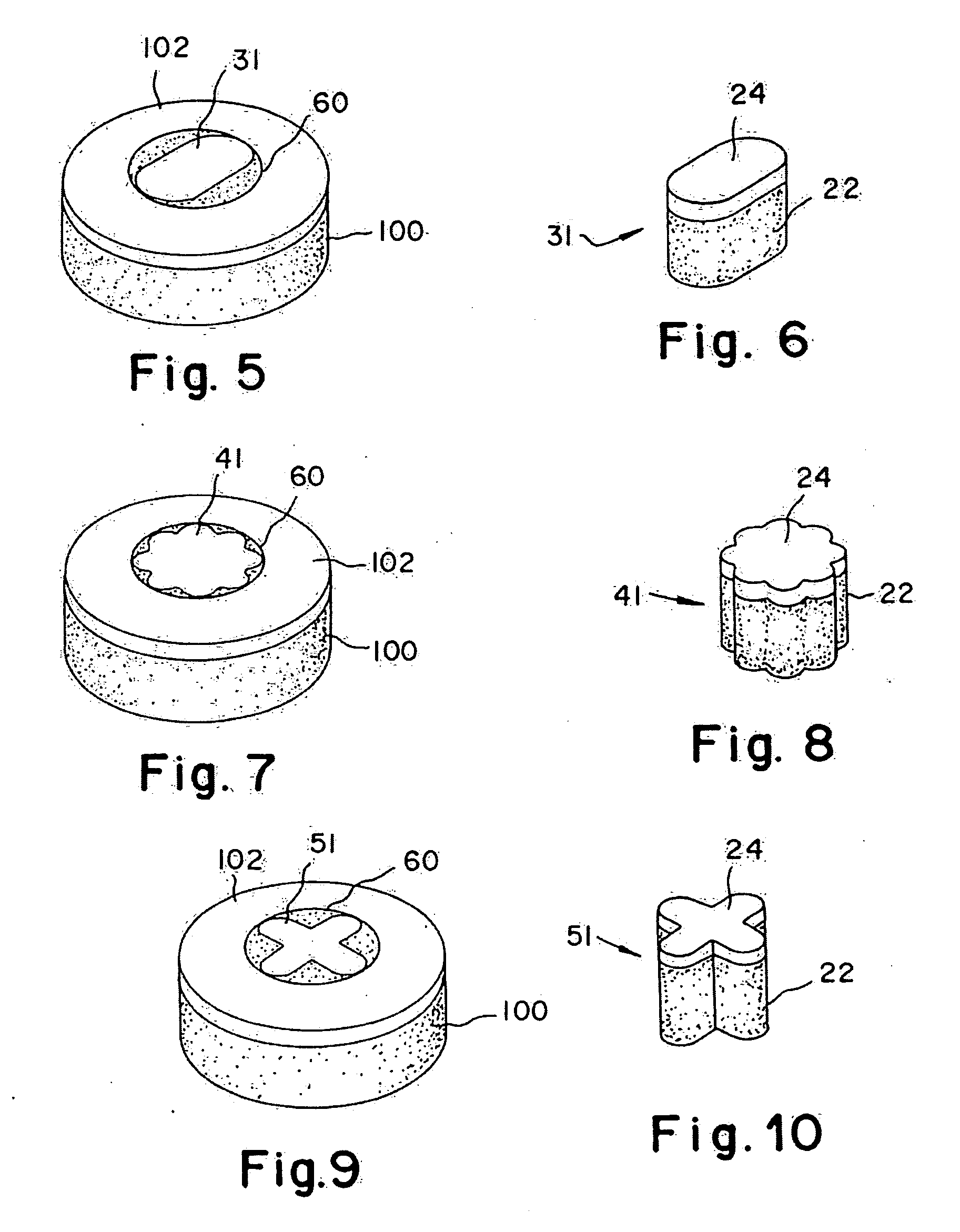
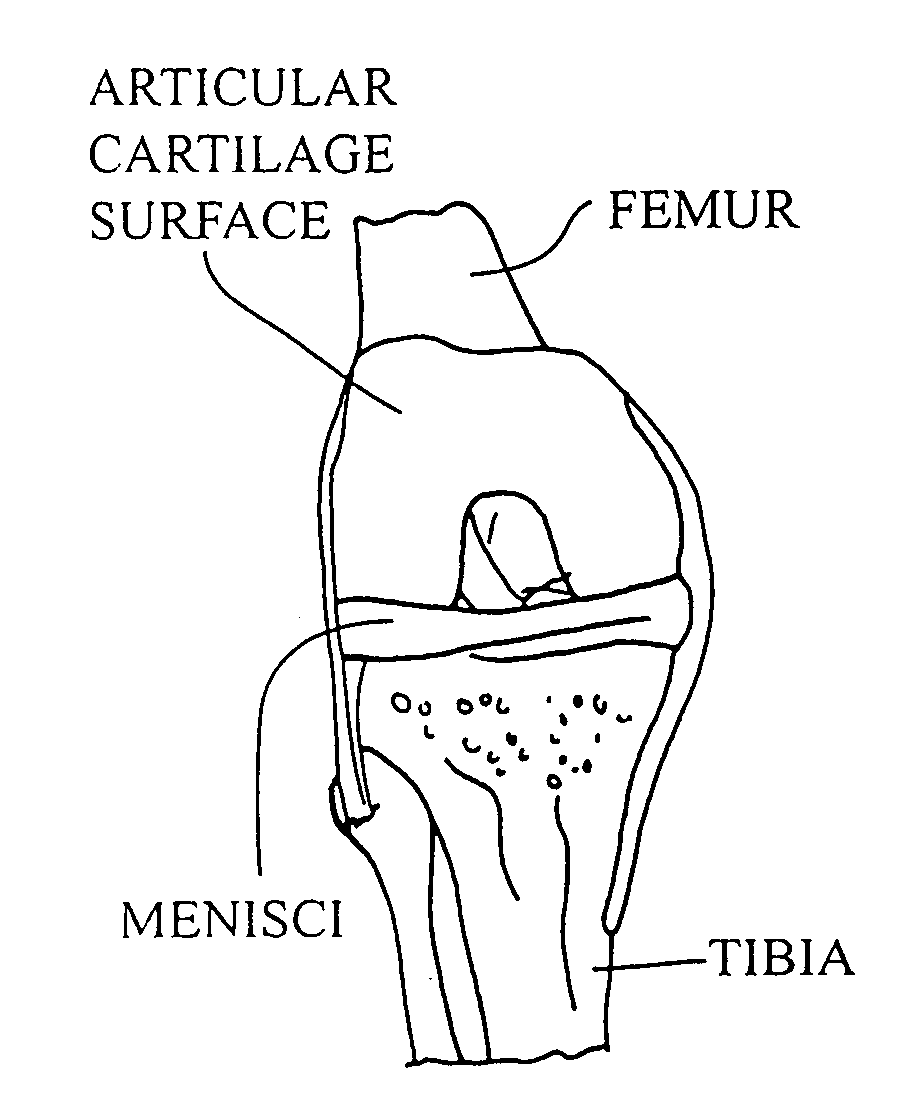
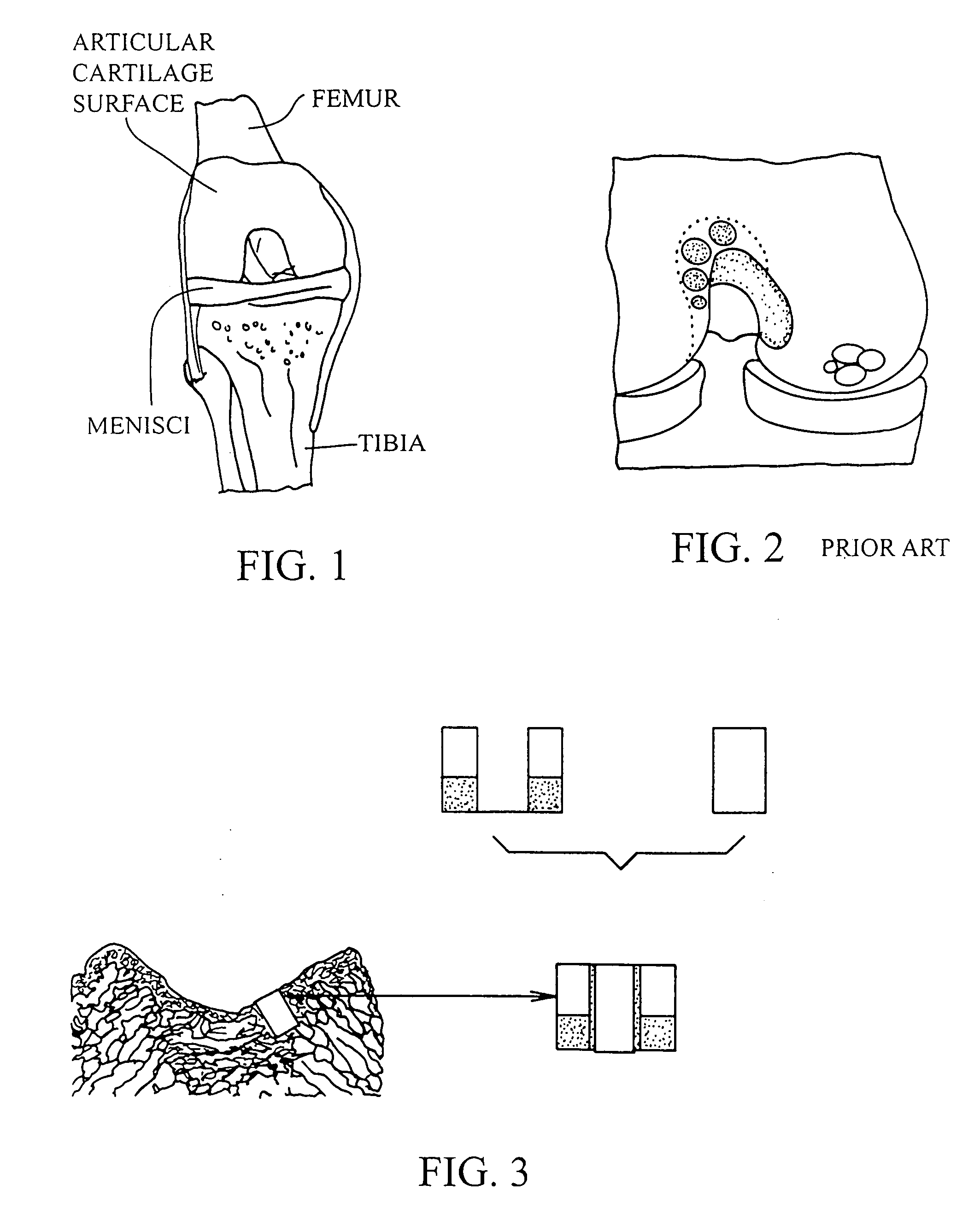
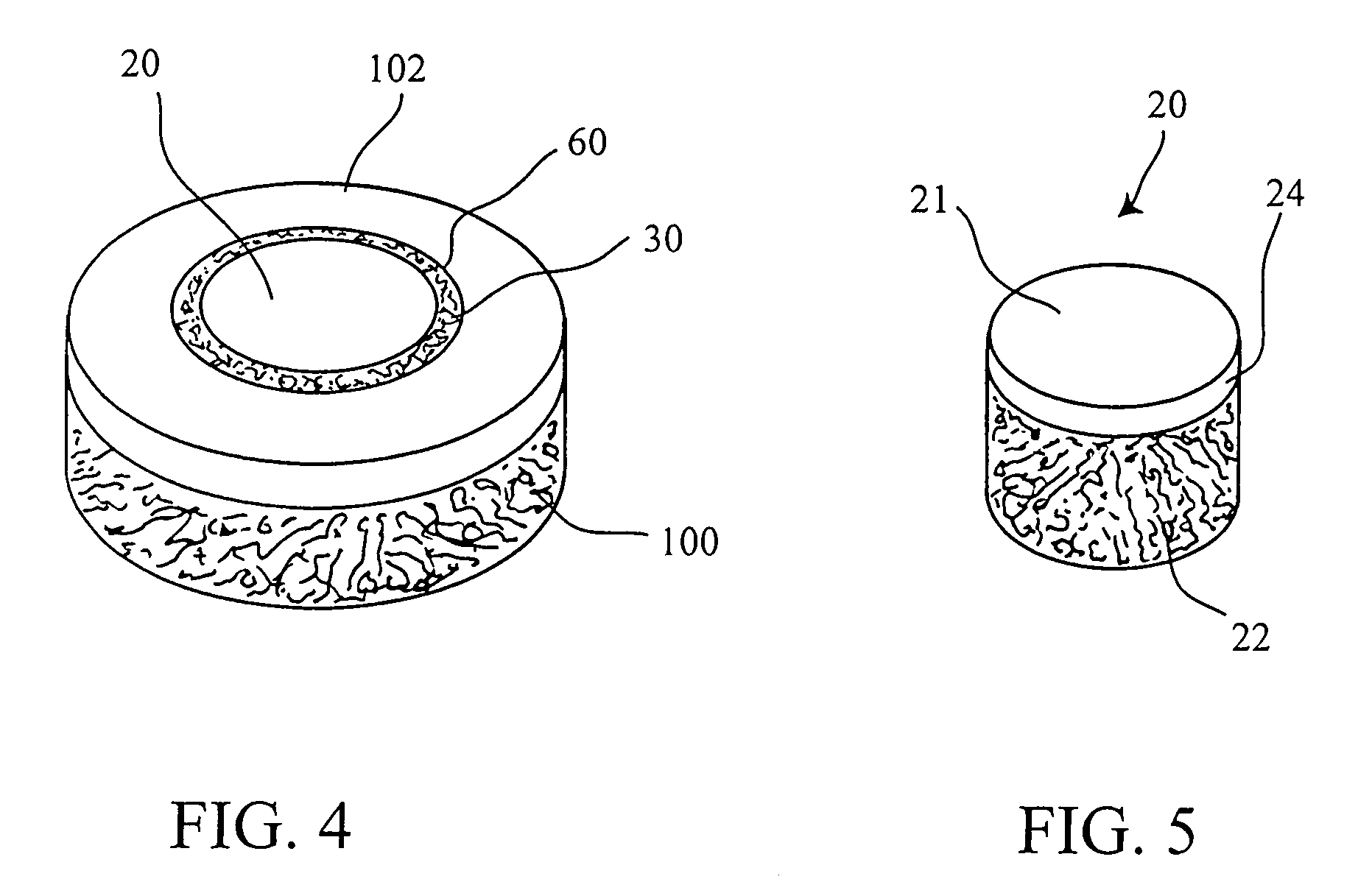
Articular cartilage, device and method for repairing cartilage defects
InactiveUS20100211173A1Easy to useSimple structureSuture equipmentsInternal osteosythesisYarnCartilage cells
The articular cartilage according to the invention is made of pure cartilage and is provided with incisions (12) on the surface facing the bone. The cartilage cells are preferably seeded on the surface provided with incisions (12). The method for producing the articular cartilage comprises the step of collecting cartilage from joints, wherein pure cartilage is collected without bone, and incisions are made on the surface of the cartilage intended to face the bone. It is preferably fresh frozen until use. The device for harvesting articular cartilage, comprises handle and cutting blade, wherein the cutting blade (4) is curvilinear and is provided with spacer elements (5), meanwhile the device for producing incisions in articular cartilages comprises handle (2) and a bridge (3) connected to said handle (2) and being provided with one or more cutting blade(s) (4). During the method for applying the articular cartilage the articular cartilage is fixed by thin surgical yarn stitches, by fibrin glue or by small anchors (FIG. 8).
Owner:PECSI TUDOMANYEGYETEM
Fibrin applicator pistol
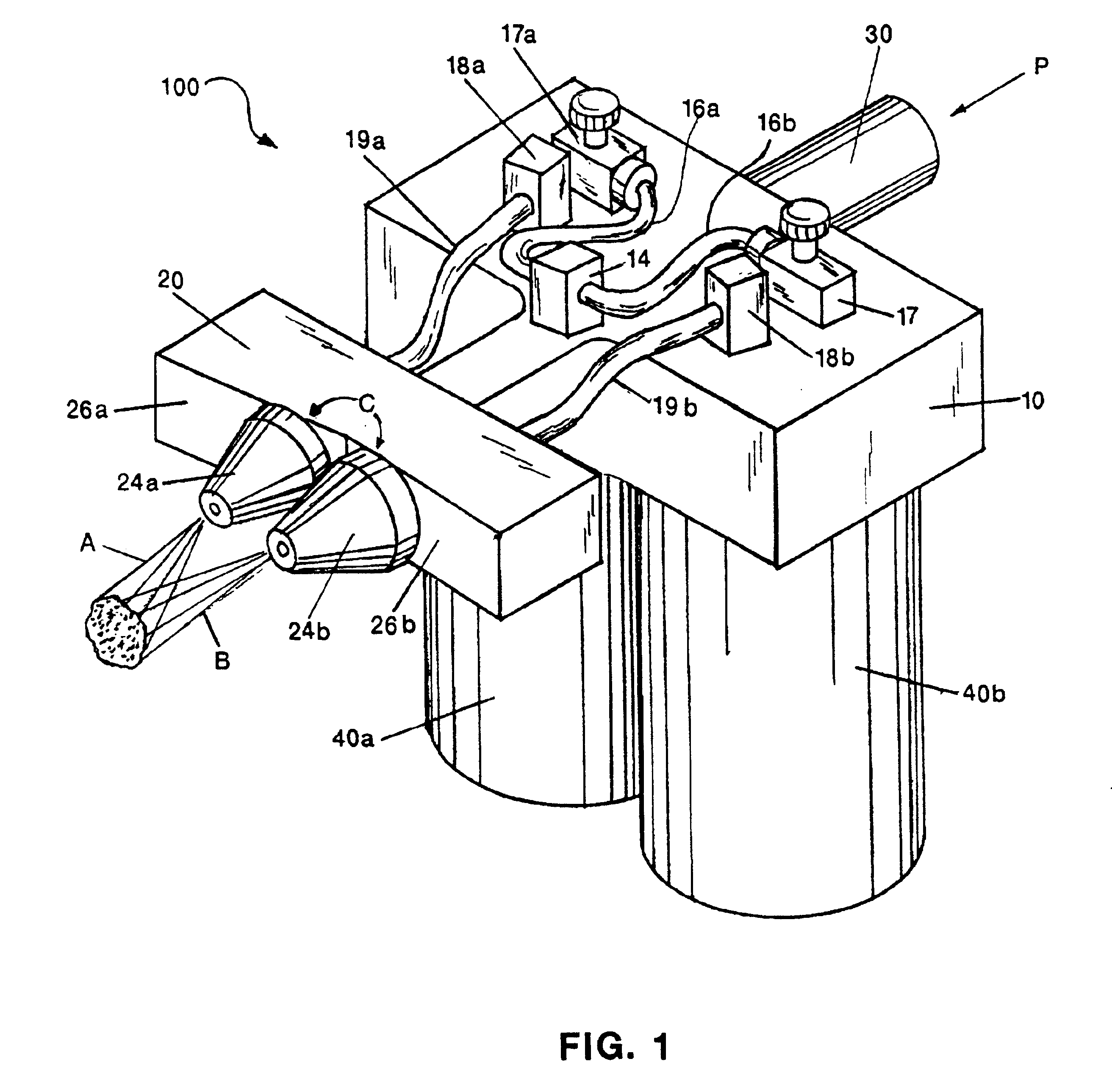
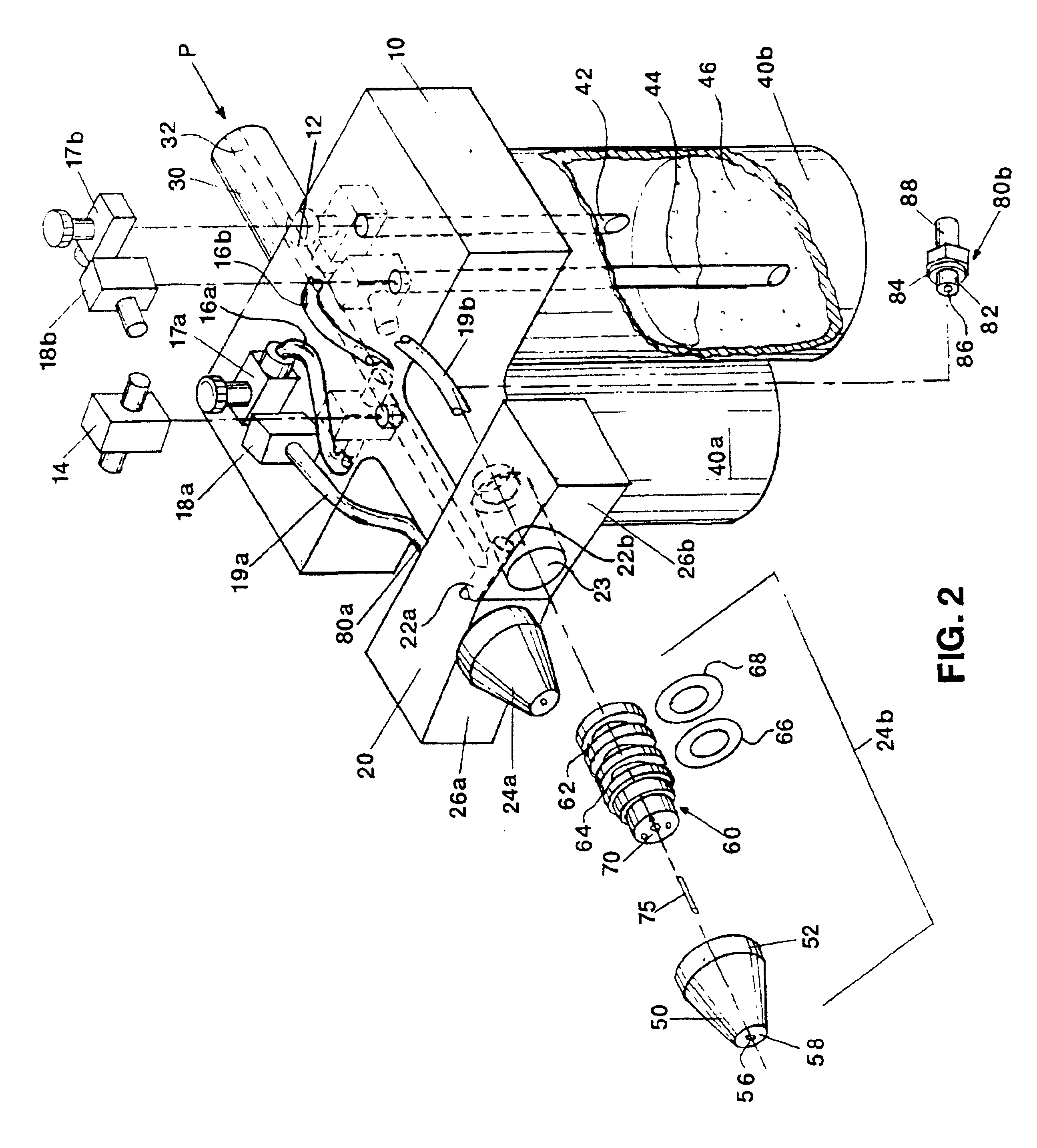
InactiveUS6863660B2Reliable deliveryNanotechPeptide/protein ingredientsSuspended particlesFibrin glue
An applicator for dispensing a first and second component of a biological adhesive, such as fibrin glue. At least one of said components may contain a suspension of fibrin microbeads (FMB) or a suspension of cells. The present invention uses a single supply of pressurized gas to force the components from the applicator using positive fluid pressure and to atomize them into a convergent spray. Another embodiment of the present invention also provides for the endoscopic application of the biological adhesive directly to tissue defects. The application of positive pressure allows precise metering of the components and application of the adhesive, prevents internal coagulation of the fibrin or clogging by suspended particles and reduces waste and contamination of the components.
Owner:HAPTO BIOTECH
Biologic partial meniscus and method of preparation
InactiveUS20130304209A1Provide biomechanical strengthMetal rolling stand detailsSurgeryFibrin glueAdhesive
Biologic partial menisci (biologic partial meniscal replacements / constructs) used to replace at least a part of a meniscus, and methods of forming such biologic partial menisci. Meniscal cartilage is employed to form a moldable allograft paste. A sterile mold that replicates a meniscus (for example, the medial or lateral meniscus) is provided in various sizes and is used as a biologic mold to recreate the anatomic shape of the meniscus. The moldable paste is inserted (for example, injected) into the mold and allowed to set into a stable, anatomically shaped meniscus (the biologic partial meniscus). Fibrin glue or other biologic adhesives or strengtheners may be optionally added to provide further biomechanical strength. Once the biologic partial meniscus is removed from the mold, it is provided at the surgical site and attached to the excised meniscus (placed into the correct anatomical shape) to complete the meniscal repair.
Owner:ARTHREX
Double-layer artificial nerve catheter and preparation method thereof
The invention discloses an artificial nerve conduit of double layer and the method for preparing same. The said nerve conduit comprises an inner conduit and an outer layer, which are made from degradable and adsorptive material in living body. The slit between said inner conduit and outer conduit is filled with fiber albumen glue (used for regeneration of nervous tissue and blood vessel). The artificial nerve conduit of double layer can be used for bypass surgery for damaged round nerve and spinal marrow, and it will be degraded and adsorbed in living body after nerve regeneration. The said artificial nerve conduit possesses good elasticity and strength, and the degradation time is adaptive to growing speed of nervous fiber (about 1-6 months).
Owner:首都医科大学北京神经科学研究所
Autologous fibrin sealant and method for making the same
InactiveUS20050152886A1Eliminate riskBiocideOrganic active ingredientsBlood plasmaPlasma rich platelet
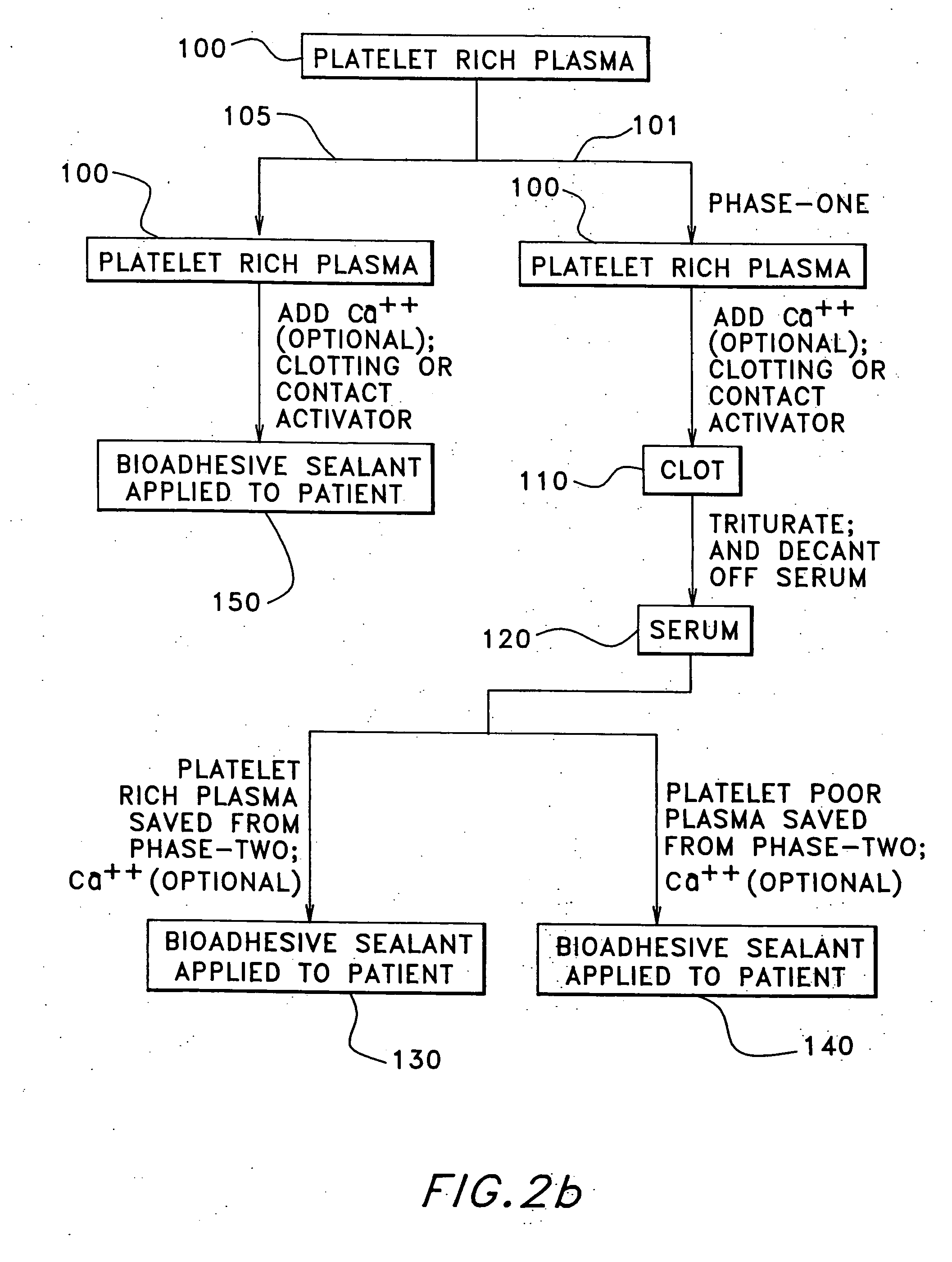
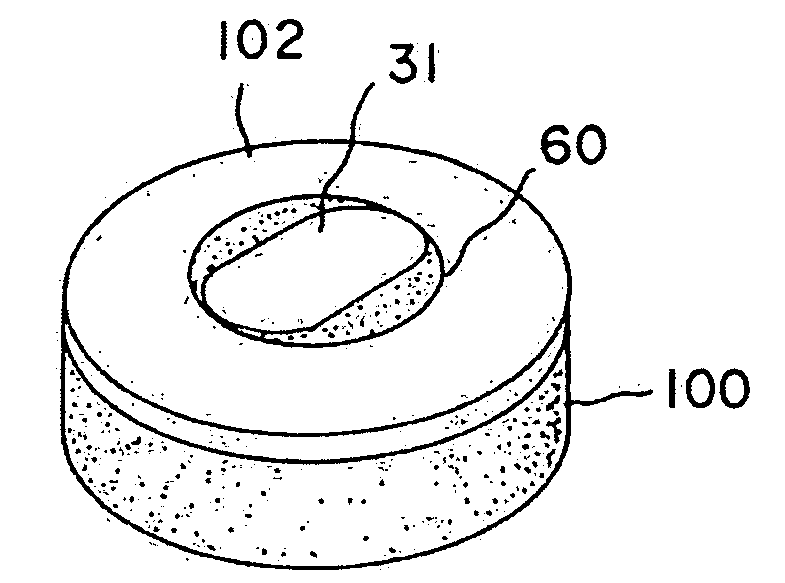
The present relates to an autologous bioadhesive sealant composition or fibrin glue prepared by a two-phase method, wherein all of the blood components for the bioadhesive sealant are derived from a patient to whom the bioadhesive sealant will be applied. A platelet rich plasma and a platelet poor plasma are formed by centrifuging a quantity of anticoagulated whole blood that was previously drawn from the patient. In one embodiment, the platelet rich plasma is divided into two portions. In phase one, a compound that reverses the effect of the anticoagulant is added to the first portion and a clot is allowed to form. The clot is then triturated, and the resulting serum containing autologous thrombin is collected. In phase two, the serum obtained from phase one is mixed with the second portion of the platelet rich plasma to form the bioadhesive sealant of the present invention.
Owner:ARTERIOCYTE MEDICAL SYST
Cartilage implant plug with fibrin glue and method for implantation
Owner:MASSACHUSETTS INST OF TECH +1
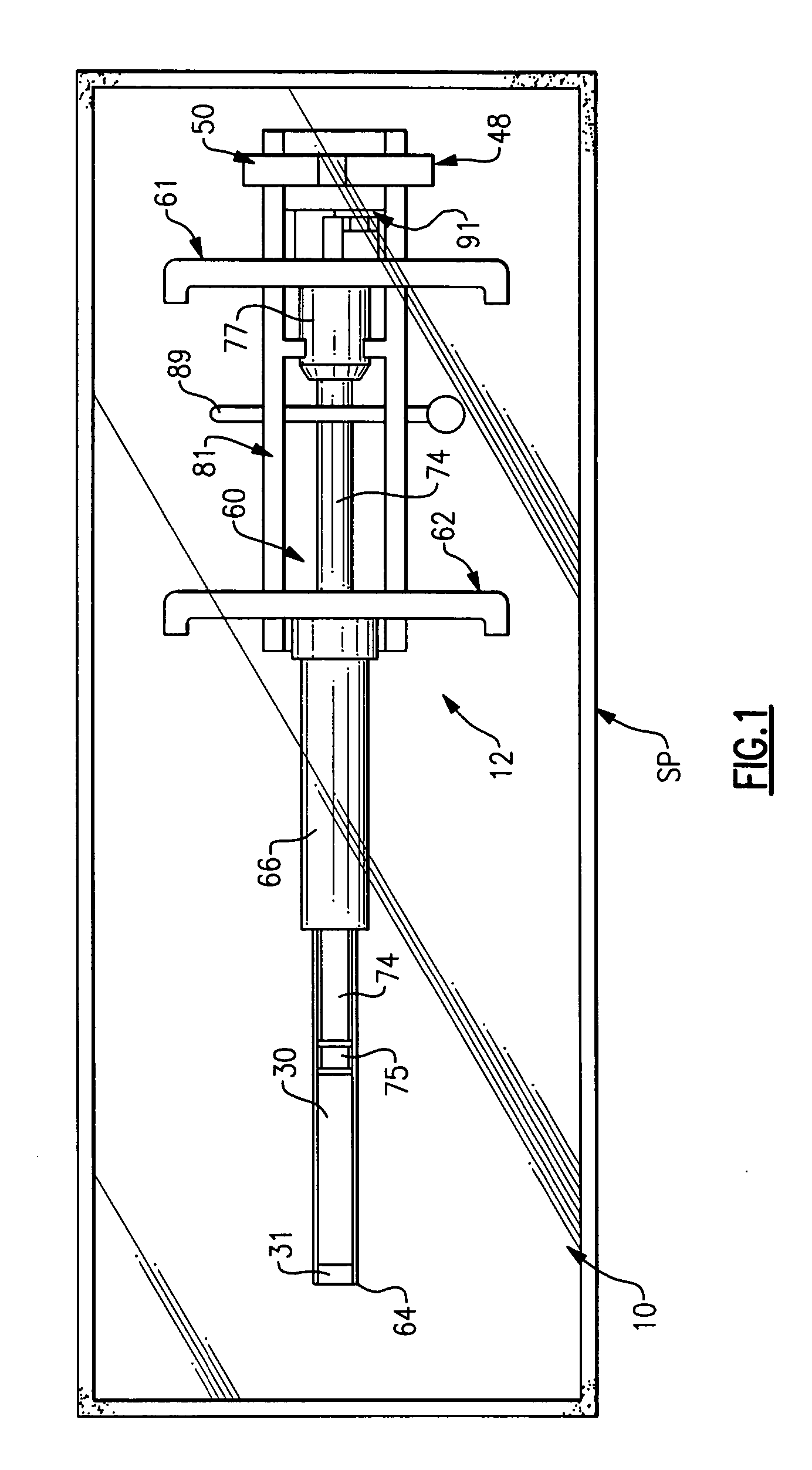
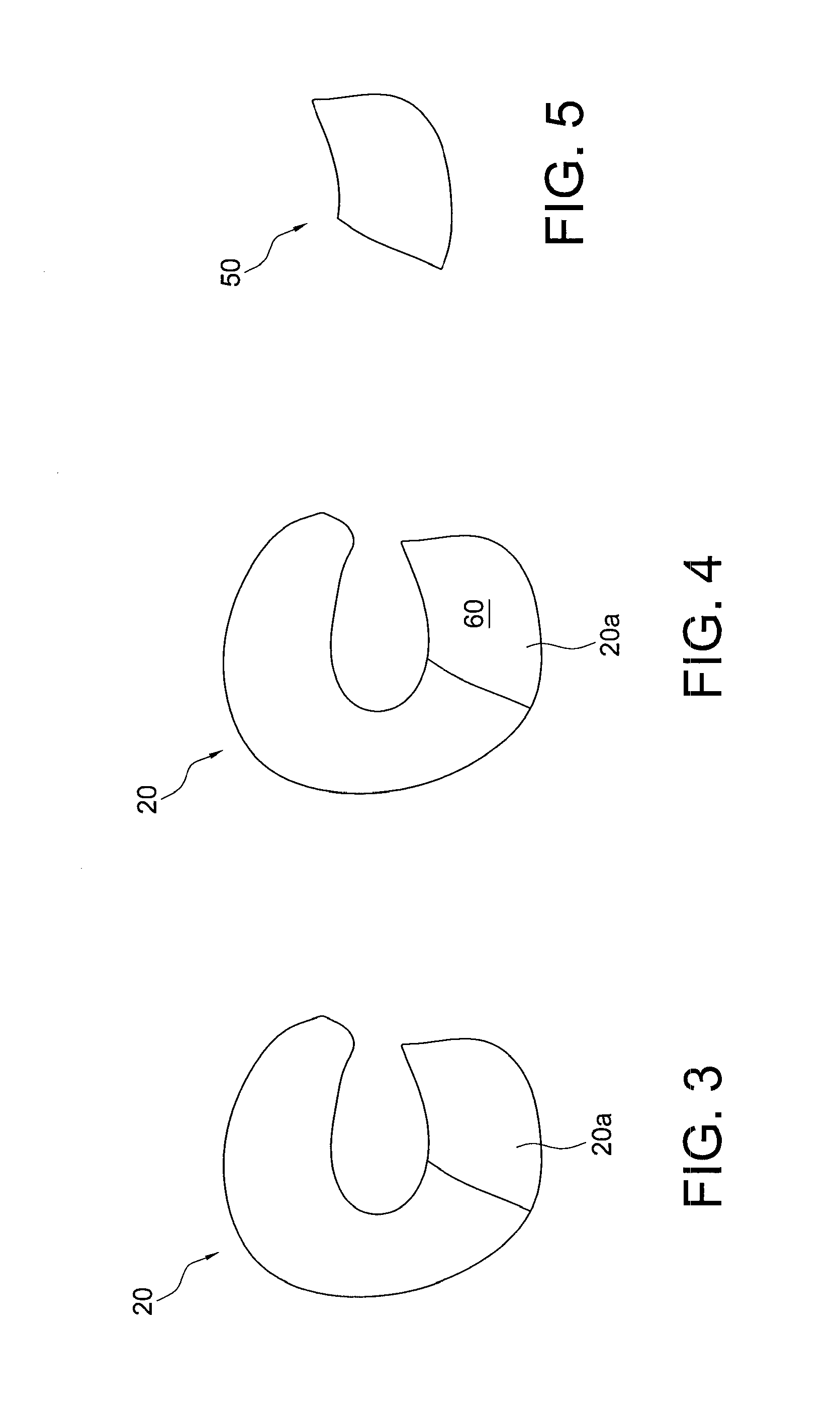
Systems and methods for preparing autologous fibrin glue
Owner:CASCADE MEDICAL ENTERPRISES +1
Method of arthroscopic osteochondral resurfacing using PRP strengthened with fibrin glue
Methods of arthroscopic resurfacing of a joint utilizing a biological component strengthened with fibrin glue. The biological component is selected from the group consisting of PRP, bone marrow aspirate (BMA) and autologous conditioned plasma (ACP). The biological component / fibrin glue composition may be inserted (by injection or by employing a biologic resurfacing mold, for example) into a transosseous tunnel in the vicinity of the defect to be repaired. Upon insertion at the defect site, the biological component / fibrin glue composition is designed to coagulate and solidify within few minutes, to advance the healing of the damaged tissue and tissue growth. The biological component / fibrin glue composition may optionally comprise components such as growth factors, antiseptic chemicals and / or antibiotics and / or electrolytes, or hormones or site-specific hybrid proteins, among others.
Owner:ARTHREX
Cartilage implant plug with fibrin glue and method for implantation
InactiveUS20080274157A1Guaranteed functionEasy to placeSurgical adhesivesPeptide/protein ingredientsSubchondral boneFibrin glue
The invention is directed toward a cartilage repair assembly comprising a shaped structure of subchondral bone with an integral overlying cartilage cap which is treated to remove cellular debris and proteoglycans and milled cartilage in a bioabsorbable carrier. The shaped structure is dimensioned to fit in a drilled bore in a cartilage defect area so that said shaped bone and cartilage cap when centered in the bore does not engage the side wall of the bore and is positioned from the side wall of the bone a distance ranging from 10 microns to 1000 microns and is surrounded by milled cartilage and a fibrin thrombin glue. A method for inserting the assembly into a cartilage defect area is disclosed.
Owner:MASSACHUSETTS INST OF TECH +1
Dry powder fibrin sealant
ActiveUS8846105B2Increase absorbanceSpeed up the flowSurgical adhesivesPeptide/protein ingredientsFibrinogenFibrin adhesive
Owner:MALLINCKRODT PHARMA IP TRADING D A C
Suspension comprising fibrinogen, thrombin and alcohol, a method for preparing such a suspension, a method for coating a carrier with such a suspension, a method of drying a coating of a carrier, and a coated collagen sponge
InactiveUS20050214277A1Easy to identifySatisfy fixationSurgical adhesivesFibrinogenFiberMean diameter
A suspension of fibrinogen, thrombin, alcohol and optionally aprotinin is obtained by mixing fibrinogen in alcohol with thrombin in alcohol. The suspension contains fibrinogen and thrombin particles with a Folk Ward mean diameter of 25-100 μm. The thrombin may be human, bovine or recombinant. The fibrinogen may be human or recombinant. A method for coating a carrier, such as a collagen sponge, with the suspension, and a method for drying the coating is disclosed. The coated collagen carrier may be used as a ready-to-use absorbable composition for tissue gluing, tissue sealing and hemostasis wherein the carrier is coated with solidly fixed components of fibrin glue, i.e. fibrinogen and thrombin.
Owner:SCHAUFLER ALFRED
Double-component medical adhesive based on glucan and chitosan and preparation method of double-component medical adhesive
ActiveCN107496974ALow priceGood biocompatibilitySurgical adhesivesPharmaceutical delivery mechanismEthylenediamineFibrin glue
The invention relates to a double-component medical adhesive based on glucan and chitosan and a preparation method of the double-component medical adhesive. The double-component medical adhesive comprises formylated glucan and aminated carboxymethyl chitosan, wherein the mass ratio of the formylated glucan to the aminated carboxymethyl chitosan is (1.67-3.33):1. The preparation method includes: adding a sodium periodate solution into a glucan solution under a dark condition to perform dialysis, and performing freeze drying to obtain the formylated glucan; mixing carboxymethyl chitosan, ethylenediamine and water-soluble carbodiimide, and performing dialysis and freeze drying to obtain the aminated carboxymethyl chitosan; mixing the formylated glucan and aminated carboxymethyl chitosan which are identical in volume, and performing Schiff base reaction to form hydrogel to obtain the medical adhesive. The double-component medical adhesive and the preparation method thereof have the advantages that the raw materials of the double-component medical adhesive are nontoxic and odorless, cheap and good in biocompatibility; the preparation method is simple; the prepared medical adhesive is good in adhesive strength and capable of shortening gelation time, the medical adhesive can act on any body parts through simple local spraying or injection, and the medical adhesive is an ideal replacement product of commercially available fibrin glue.
Owner:DONGHUA UNIV
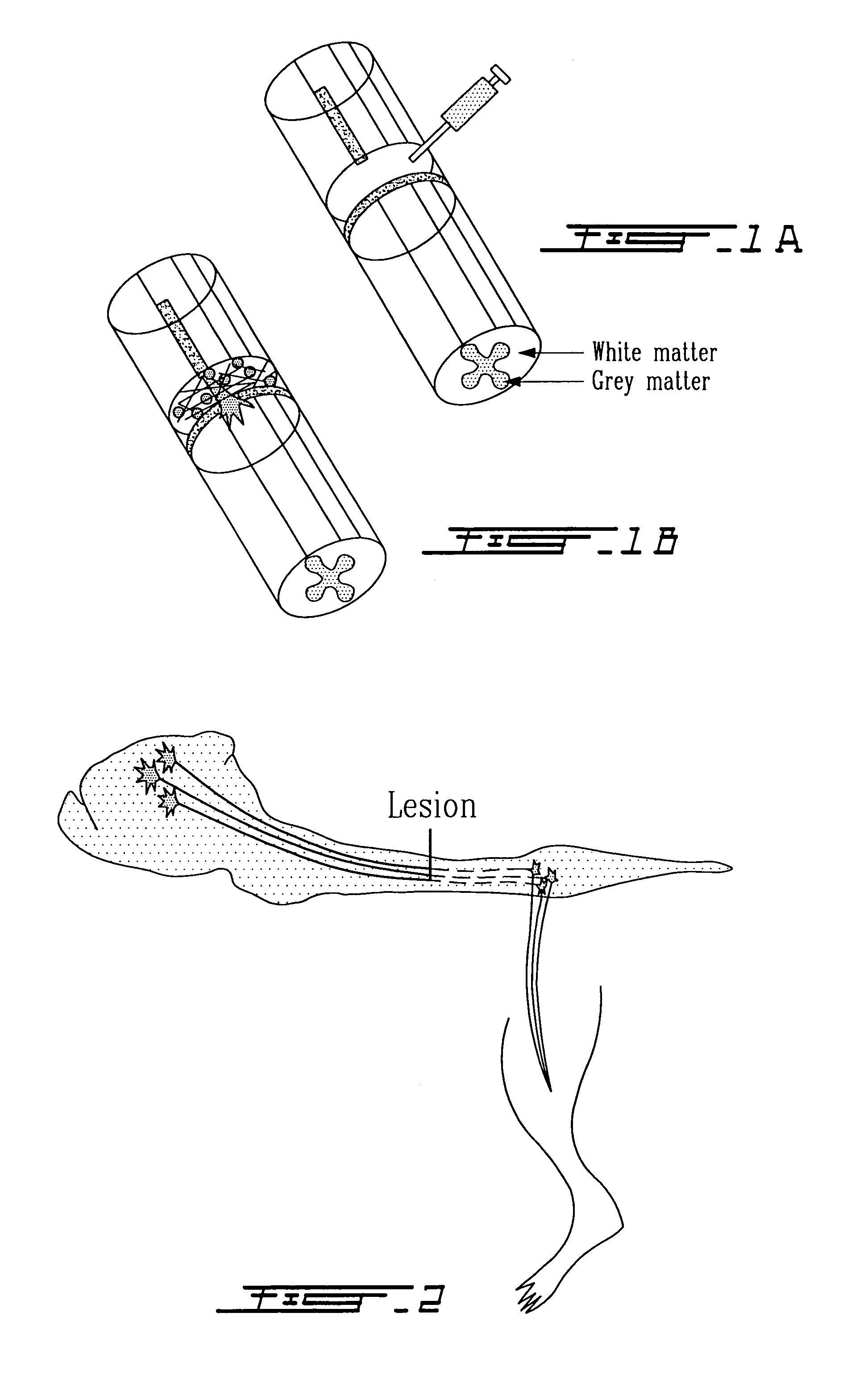
Methods for making and delivering rho-antagonist tissue adhesive formulations to the injured mammalian central and peripheral nervous systems and uses thereof
InactiveUS7141428B2Avoid crackingReduce deliveryOrganic active ingredientsPowder deliveryNervous systemFibrin glue
The present invention provides methods for making, delivering and using formulations that combine a therapeutically active agent(s) (such as for example a Rho antagonist(s)) and a flowable carrier component capable of forming a therapeutically acceptable matrix in vivo (such as for example tissue adhesives), to injured nerves to promote repair and regeneration and regrowth of injured (mammalian) neuronal cells, e.g. for facilitating axon growth at a desired lesion site. Preferred active agents are known Rho antagonists such as for example C3, chimeric C3 proteins, etc. or substances selected from among known trans-4-amino(alkyl)-1-pyridylcarbamoylcyclohexane compounds or Rho kinase inhibitors. The system for example may deliver an antagonist(s) in a tissue adhesive such as, for example, a fibrin glue or a collagen gel to create a delivery matrix in situ. A kit and methods of stimulating neuronal regeneration are also included.
Owner:BIOAXONE BIOSCI +1
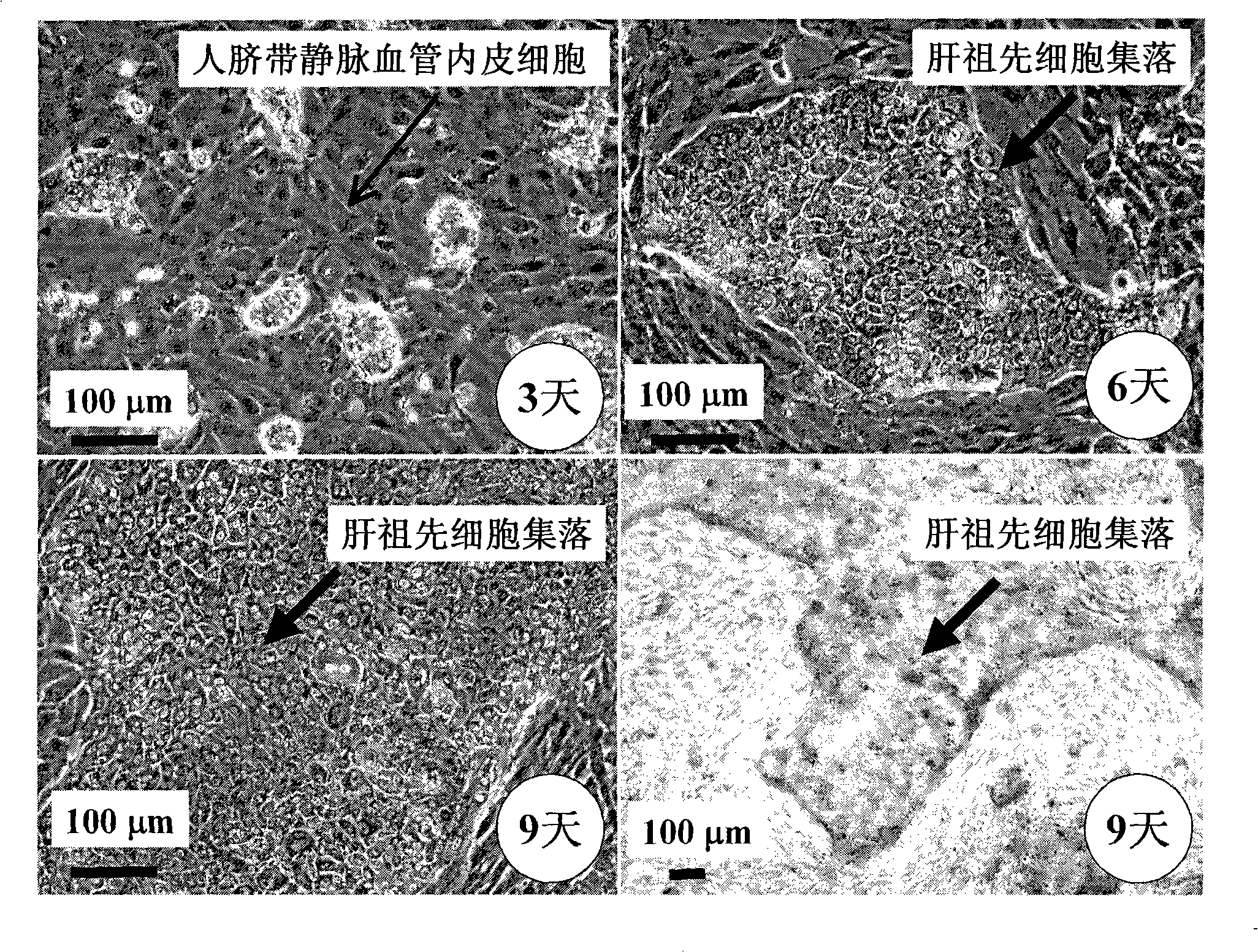
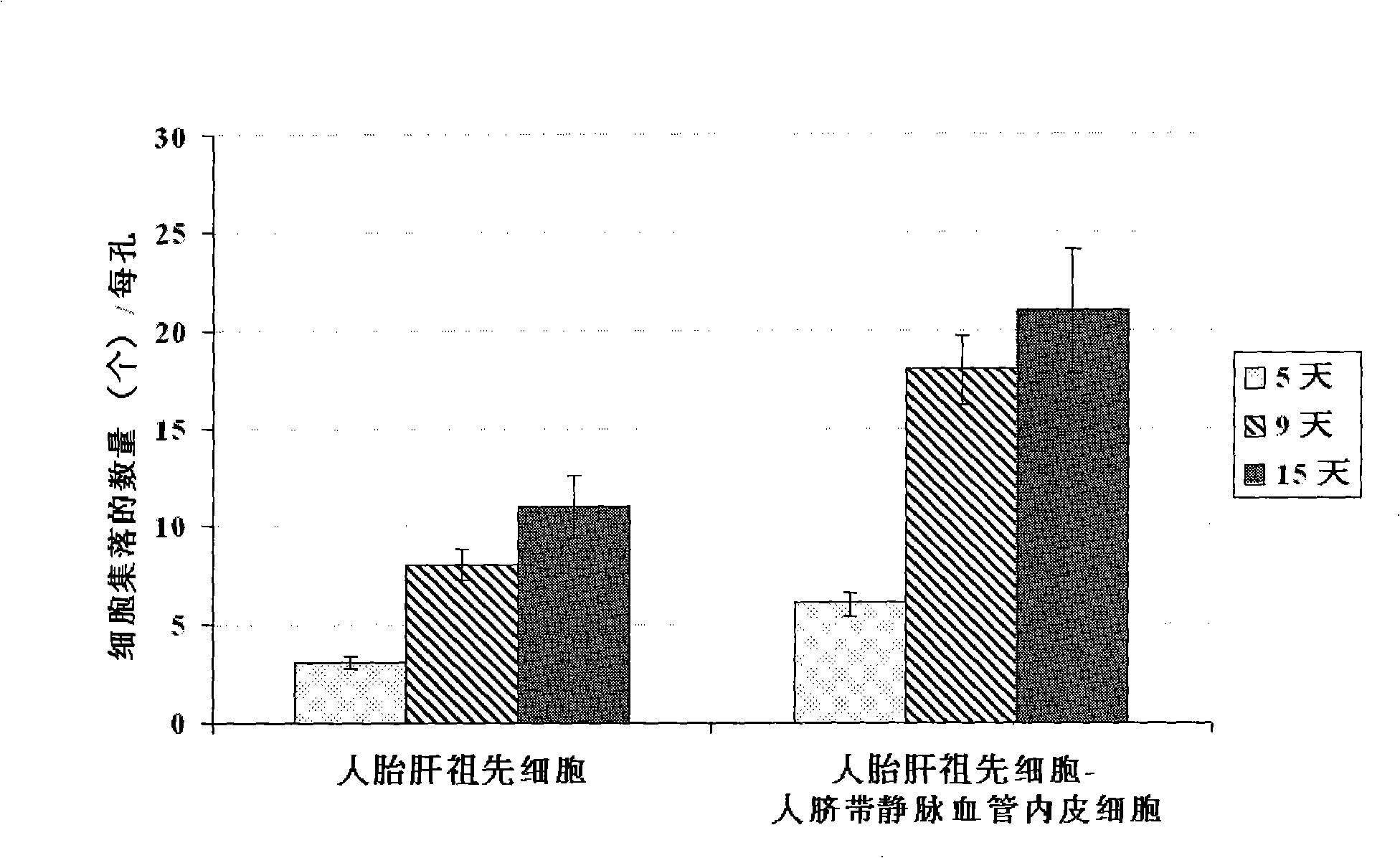
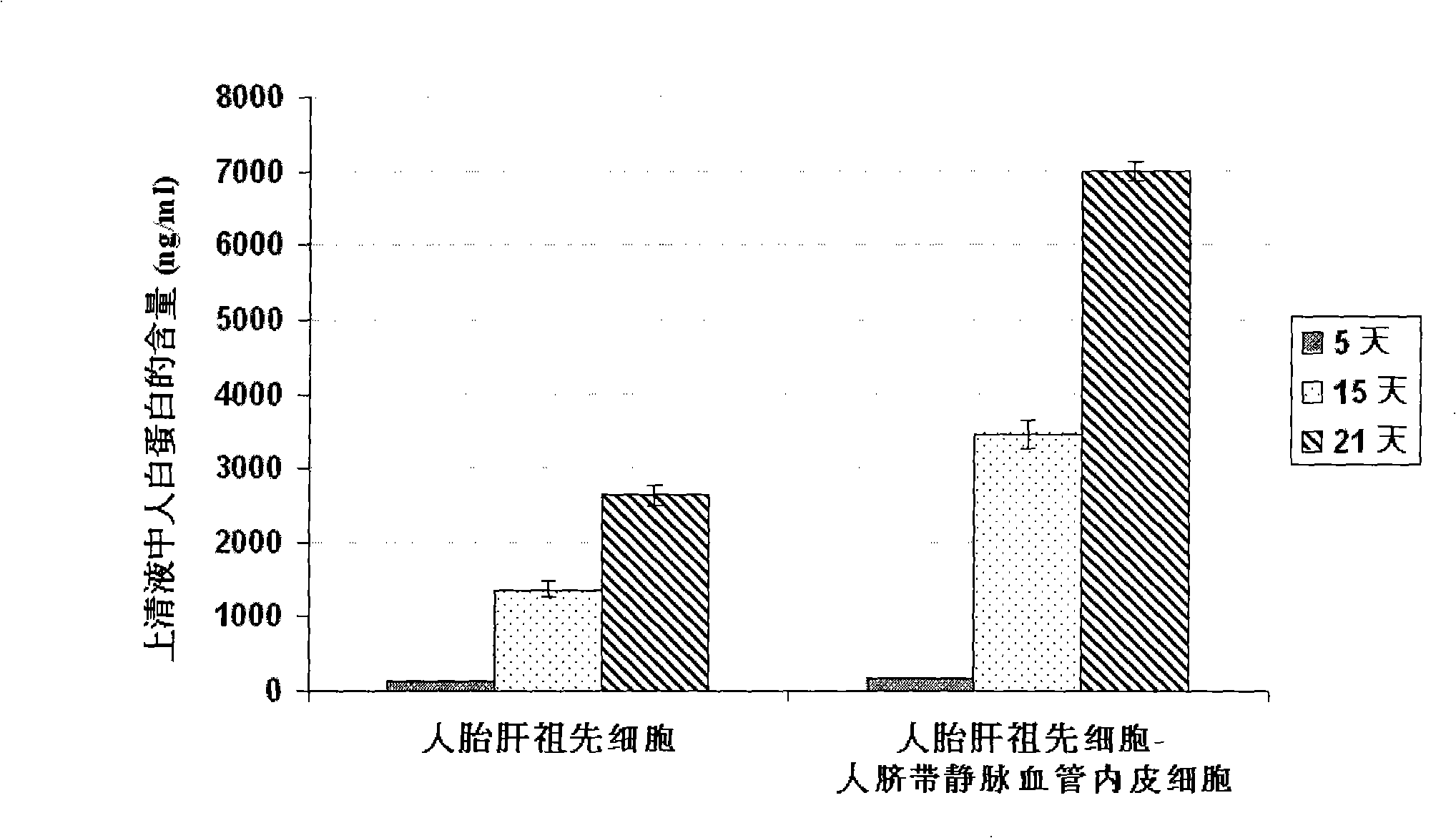
In vitro culture-amplified human liver progenitor cell and preparation thereof
InactiveCN101275121AAccelerate self-renewalConvenient sourceArtificially induced pluripotent cellsNon-embryonic pluripotent stem cellsCell-Extracellular MatrixECM Protein
The present invention provides a preparing method of a human hepatic progenitor cell which is amplified and cultivated in vitro, including: a. separating the human hepatic progenitor cell; b. co-cultivating a feeder cell and the human hepatic progenitor cell separated from the step a by a medium having no serum on an extracellular matrix containing human fibrin sealant or other analogues, obtaining a human hepatic progenitor cell colony by amplification. The human hepatic progenitor cell colony is easy to separate from the surface of human fibrin sealant by the simple gelatinolytic band process, purified or / and subcultured by further single cell preparation technology process such as enzymatic degradation etc. The human hepatic progenitor cell prepared by the method is hepatic progenitor cell treatment, including a cell transplantation and a bioartificial liver support system, providing excellent human hepatocyte source for cytotoxicity test platform in the drug screening, virus infection and drug screening platform etc.
Owner:芦银雪
System and method for forming a non-ablative cardiac conduction block
A system forms a cardiac conduction block at a location in a heart of a patient without substantially ablating cardiac tissue. The system includes a delivery system coupled to a source of material that is substantially non-ablative with respect to cardiac tissue. The delivery system delivers the material to the location, and the material at the location forms a conduction block without ablating the cardiac cells there. The material may include living cells, such as for example skeletal myocytes, and / or may include a non-living matter such as biopolymers such as a fibrin glue agent, or collagen agents. An expandable member with needle assembly is used to deliver the material so as to form a non-ablative circumferential conduction block at a location where a pulmonary vein extends from an atrium.
Owner:RGT UNIV OF CALIFORNIA
Water soluble reactive derivatives of carboxy polysaccharides and fibrinogen conjugates thereof
ActiveUS20100086594A1Inhibit productionHigh yieldAntibacterial agentsPowder deliveryTissue repairWater soluble
The present invention provides water-soluble reactive esters of carboxy polysaccharides and derivatives thereof. The reactive carboxy polysaccharide derivatives are useful per se in aqueous solutions or specifically for the formation of water-soluble covalent fibrinogen conjugates. A preferred conjugate is a hyaluronic acid-fibrinogen conjugate and fibrin adhesive, clot or matrix derived from it. Methods of preparation and methods of use in tissue repair and regeneration are also disclosed.
Owner:HEPACORE LTD
Producing method for double-layer artificial nerve catheter
The invention discloses an artificial nerve conduit of double layer and the method for preparing same. The said nerve conduit comprises an inner conduit and an outer layer, which are made from degradable and adsorptive material in living body. The slit between said inner conduit and outer conduit is filled with fiber albumen glue (used for regeneration of nervous tissue and blood vessel). The artificial nerve conduit of double layer can be used for bypass surgery for damaged round nerve and spinal marrow, and it will be degraded and adsorbed in living body after nerve regeneration. The said artificial nerve conduit possesses good elasticity and strength, and the degradation time is adaptive to growing speed of nervous fiber (about 1-6 months).
Owner:首都医科大学北京神经科学研究所
Trypsin-like serine protease inhibitors, and their preparation and use
InactiveUS20100022781A1Good effectAvoid constantOrganic compound preparationPeptidesMedicineOrgan transplanting
The invention relates to inhibitors of trypsin-like serine proteases of the general formula (I) which, as well as plasmin, also inhibit plasma kallikrien, and to their preparation and use as medicaments, preferably for treatment of blood loss, especially in the case of hyperfibrinolytic states, in organ transplants or heart surgery interventions, in particular with a cardiopulmonary bypass, or as a constituent of a fibrin adhesive.
Owner:THE MEDICINES CO (LEIPZIG) GMBH
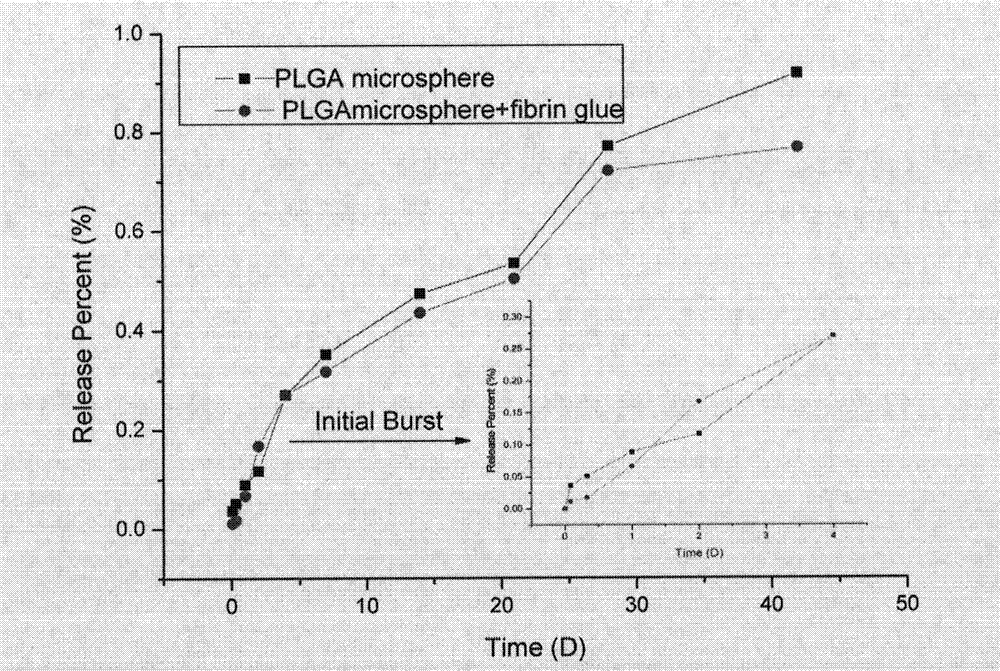
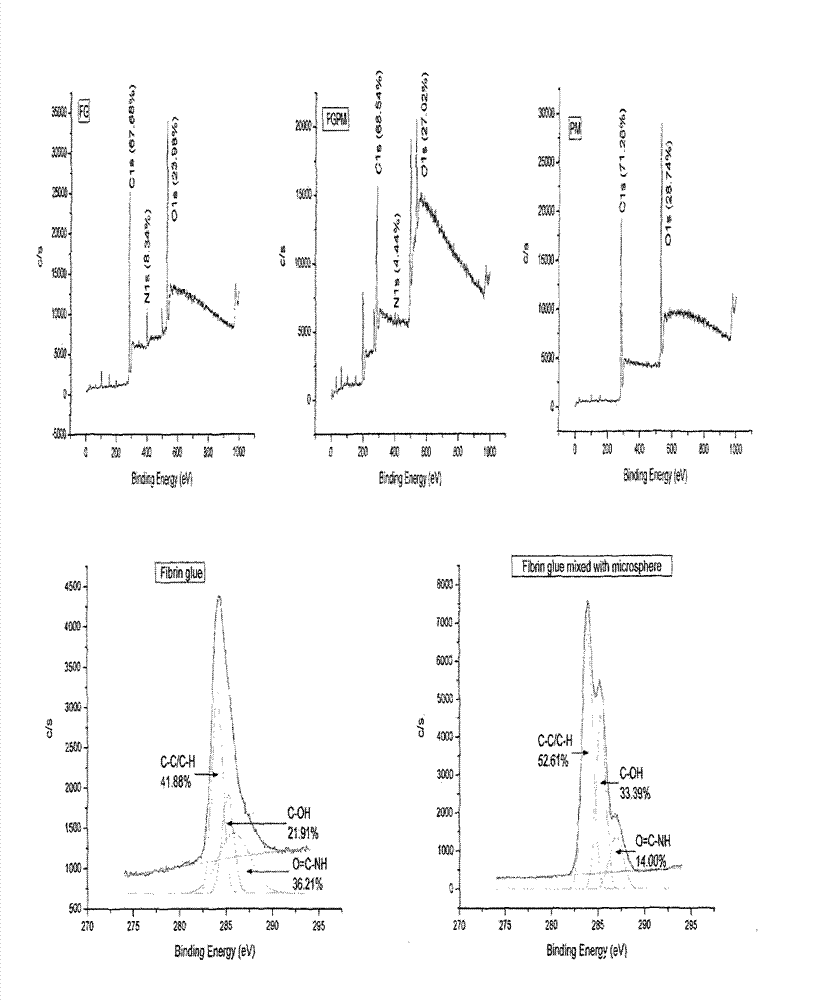
Preparation method and application of fibrin glue composite recombinant human bone morphogenetic protein-2 (rhBMP-2) microsphere
The invention relates to a preparation method and application of a fibrin glue composite recombinant human bone morphogenetic protein-2 (rhBMP-2) microsphere. The preparation method comprises the steps of: preparing a slow release microsphere with a proper particle diameter; and then constructing an rhBMP-2 / PLGA microsphere / fibrin glue composite material. The rhBMP-2 / PLGA microsphere fibrin glue composite material can be used through local injection, surgical trauma can be reduced, a healing process of bone fracture and nonunion is accelerated by continuously supplementing the local bone morphogenetic protein, thus the fibrin glue composite recombinant human bone morphogenetic protein-2 is a bone repair material with excellent degradability and osteogenic activity.
Owner:姚琦 +2
Treatment of peripheral vascular disease using umbilical cord tissue-derived cells
Compositions and methods of using cells derived from umbilical cord tissue, to stimulate and support angiogenesis, to improve blood flow, to regenerate, repair, and improve skeletal muscle damaged by a peripheral ischemic event, and to protect skeletal muscle from ischemic damage in peripheral vascular disease patients are disclosed. In particular, methods of treating a patient having a peripheral vascular disease with umbilical derived cells and fibrin glue are disclosed.
Owner:DEPUY SYNTHES PROD INC
Solid fibrinogen preparation
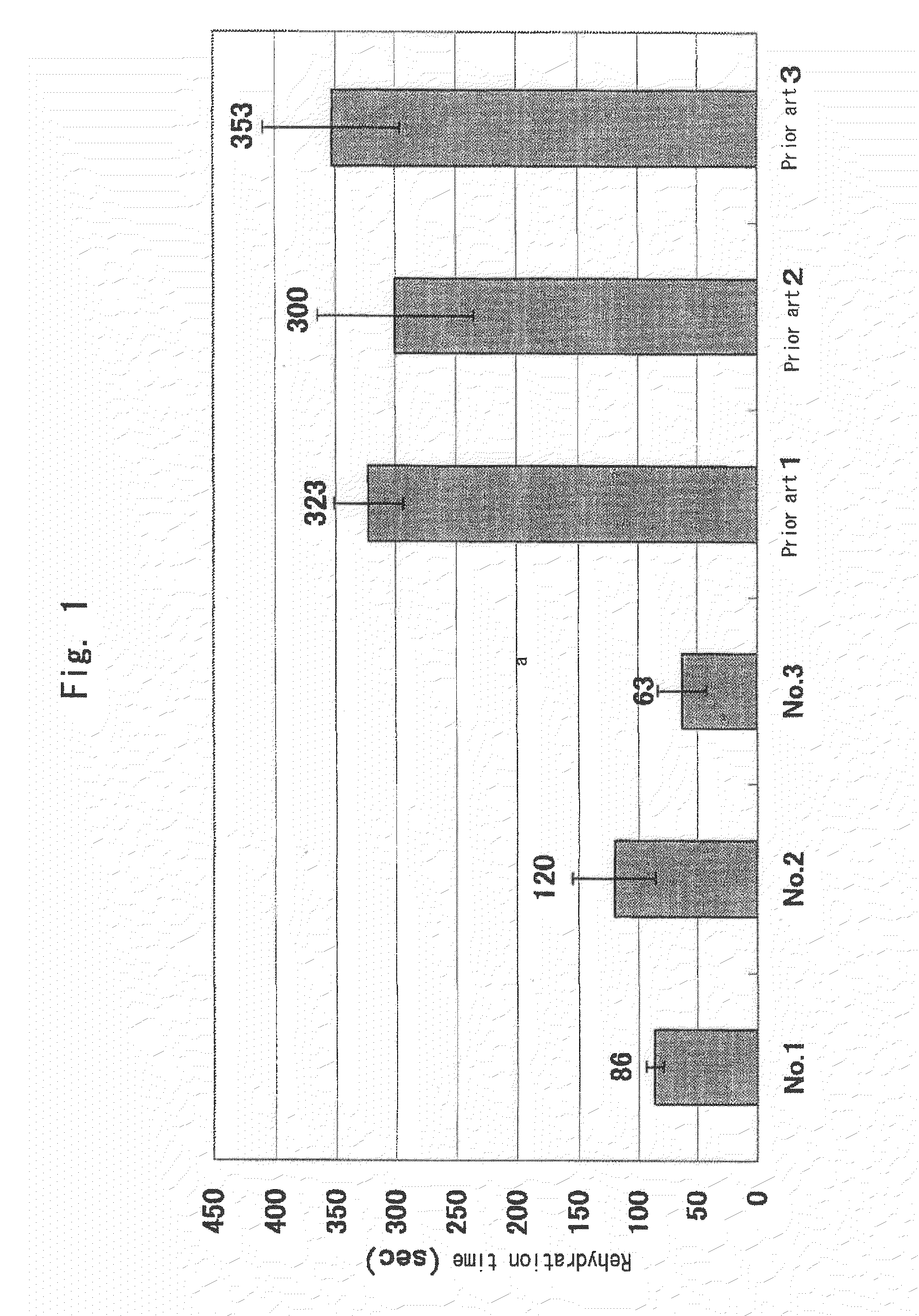
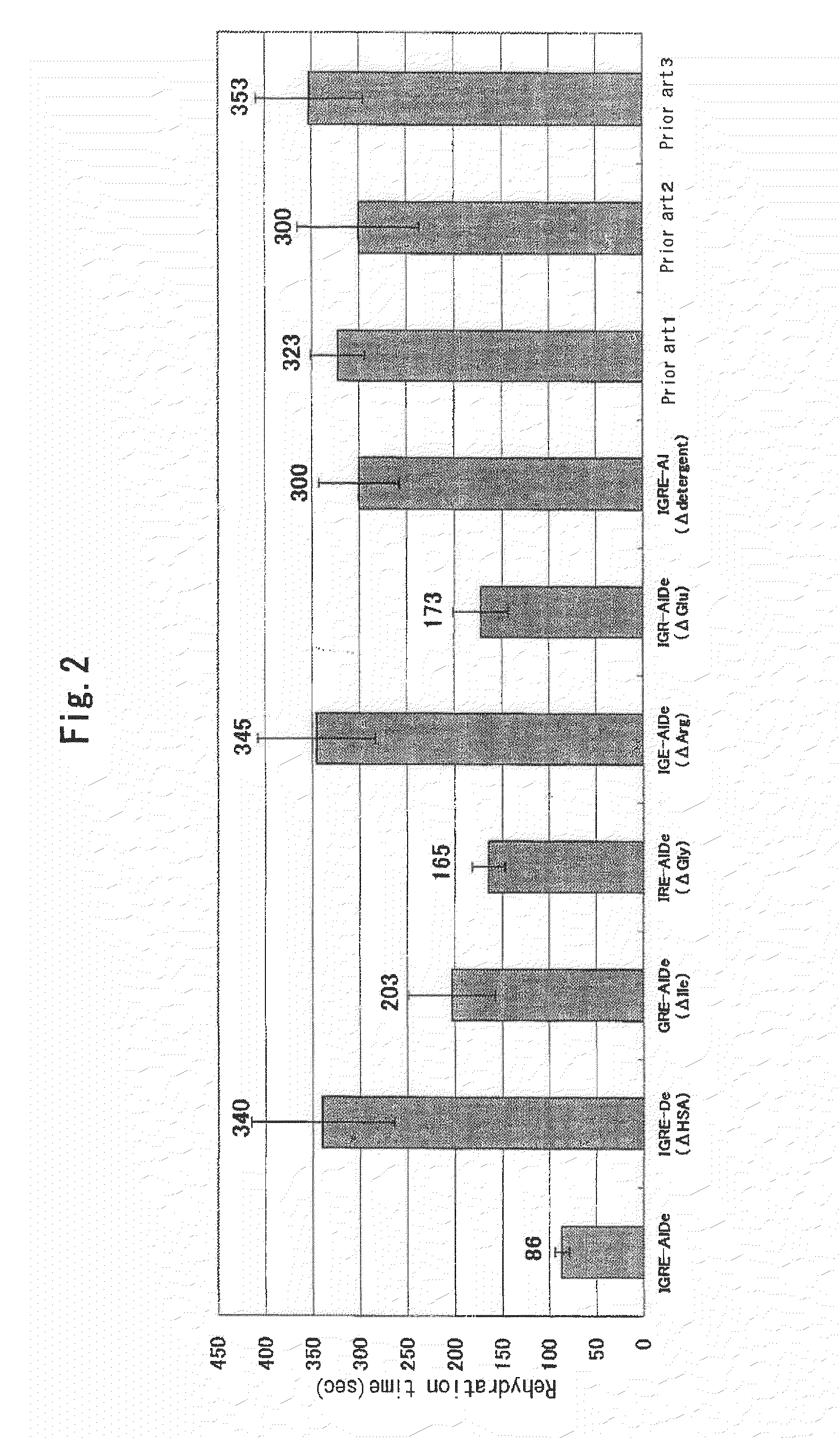
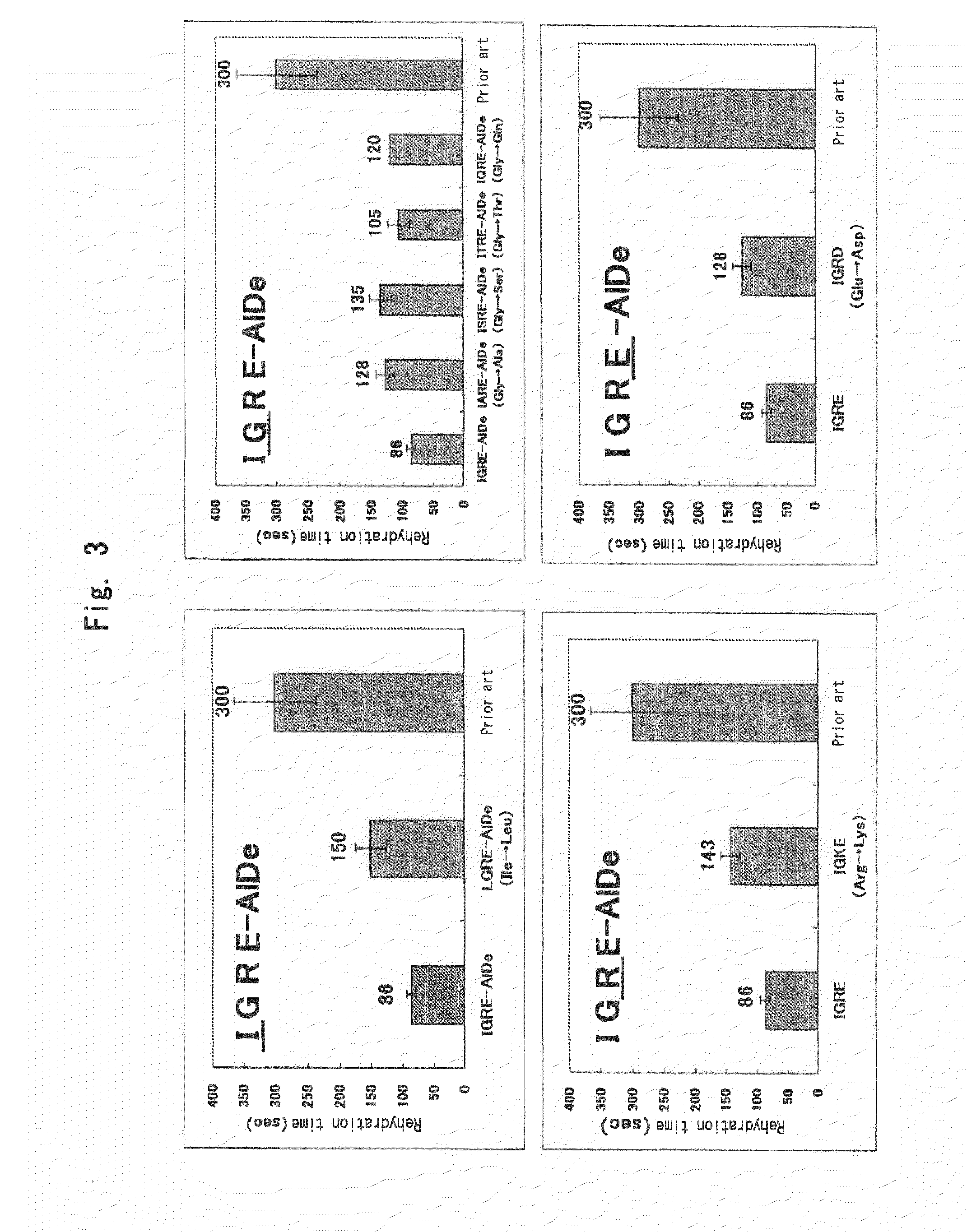
InactiveUS20100119563A1Short timeReduced rehydration timeOrganic active ingredientsBiocideFiberNeutral Amino Acids
A fibrinogen preparation is provided which has an improved solubility, may be prepared within a short time and may be used rapidly in clinical set-up. A solid fibrinogen preparation comprising as a main ingredient fibrinogen and further containing the following components: albumin; a nonionic surfactant; a basic amino acid or a salt thereof; and at least two amino acids or a salt thereof selected from an acidic amino acid or a salt thereof and a neutral amino acid or a salt thereof. The solid fibrinogen composition of the present invention may be held on a medical material to form a supporting material holding fibrinogen. Besides, the supporting material holding fibrinogen may be combined with a component comprising as a main ingredient thrombin to provide a fibrin adhesive.
Owner:KM BIOLOGICS CO LTD
Collagen-chitosan / fibrin glue asymmetric bracket and the preparing method and the application thereof
The invention discloses a collagen-chitose or fibrin glue asymmetric support, preparing method and application, which is characterized by the following: incorporating collagen-chitose porous material layer and fibrin glue layer; arranging the fibrin glue layer on the surface of collagen-chitose porous material layer evenly; forming interface between the fibrin glue layer and the surface of collagen-chitose porous material layer. This product possesses good cell compatibility, which is fit for complexing skin construction. This invention also discloses the preparing method of the asymmetric support and application.
Owner:韩春茂
Medical adhesive
The invention discloses a medical adhesive, comprising the following substances in parts by weight: 65-80 parts of alpha-N-butylcyanoacrylate, 5-8 parts of hydroxypropyl methacrylate, 3-5 parts of sulfur dioxide, 1-2 parts of hydroquinone, 0.5-2 parts of polyurethane, 0.5-1.5 parts of agar, 0.8-1.2 parts of fibrin glue, 2-3 parts of water soluble phenol-formaldehyde resin, and 20-25 parts of deionized water. The medical adhesive has the beneficial effects that the medical adhesive provided by the invention is low in thrill, and excellent in bonding persistence; compared with the traditional common adhesive, the persistence of the medical adhesive can be up to 1.5-3 times; and animal experiments show that the toxic and side effects are small.
Owner:台山市弘毅医疗用品有限公司
Tumor drug-loaded microparticle preparation and preparation method thereof
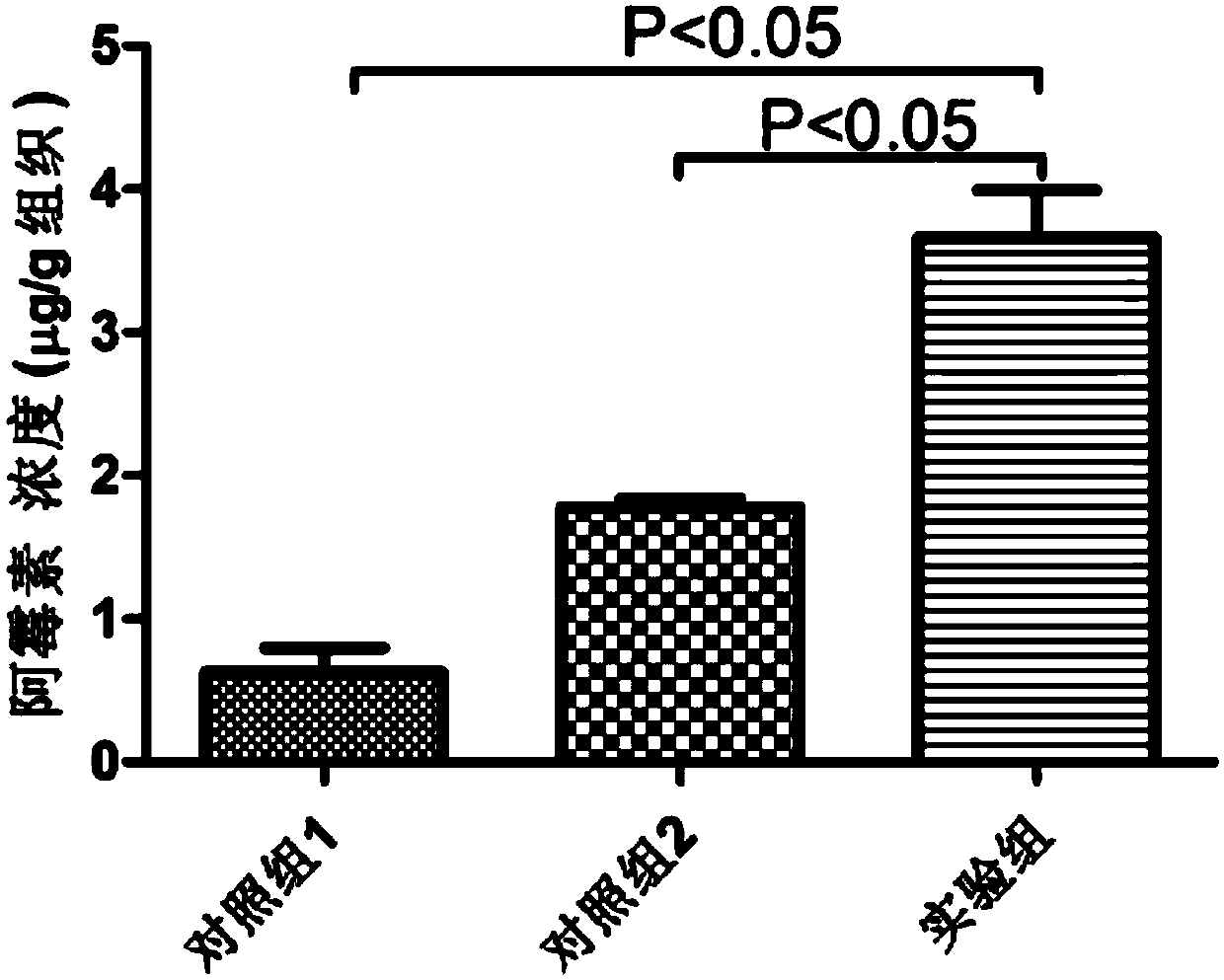
ActiveCN108042805AAvoid side effectsSmall toxicityOrganic active ingredientsPharmaceutical non-active ingredientsCell vesicleSide effect
The invention provides a tumor drug-loaded microparticle preparation and a preparation method thereof. The drug-loaded microparticle preparation includes tumor stem cell apoptosis released cell vesicles and a chemotherapeutic drug wrapped in the cell vesicles as an effective component; and is prepared by mixed culture of a three-dimensional soft fibrin glue and tumor cells in a medium. The tumor drug-loaded microparticle provided by the invention is more beneficial to high enrichment in tumor tissue and deep penetration of tumors and can achieve effective uptake by common tumor cells and tumorstem cells more easily, can improve the killing power of the chemotherapeutic drug to common tumor cells and tumor stem cells, can solve the problem that common tumor cell derived drug-loaded microparticles cannot permeate to the depth parts of tumors to kill a lot of tumor stem cells, and at the same time can reduce the toxic and side effect of the chemotherapeutic drug on the body.
Owner:HUAZHONG UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com