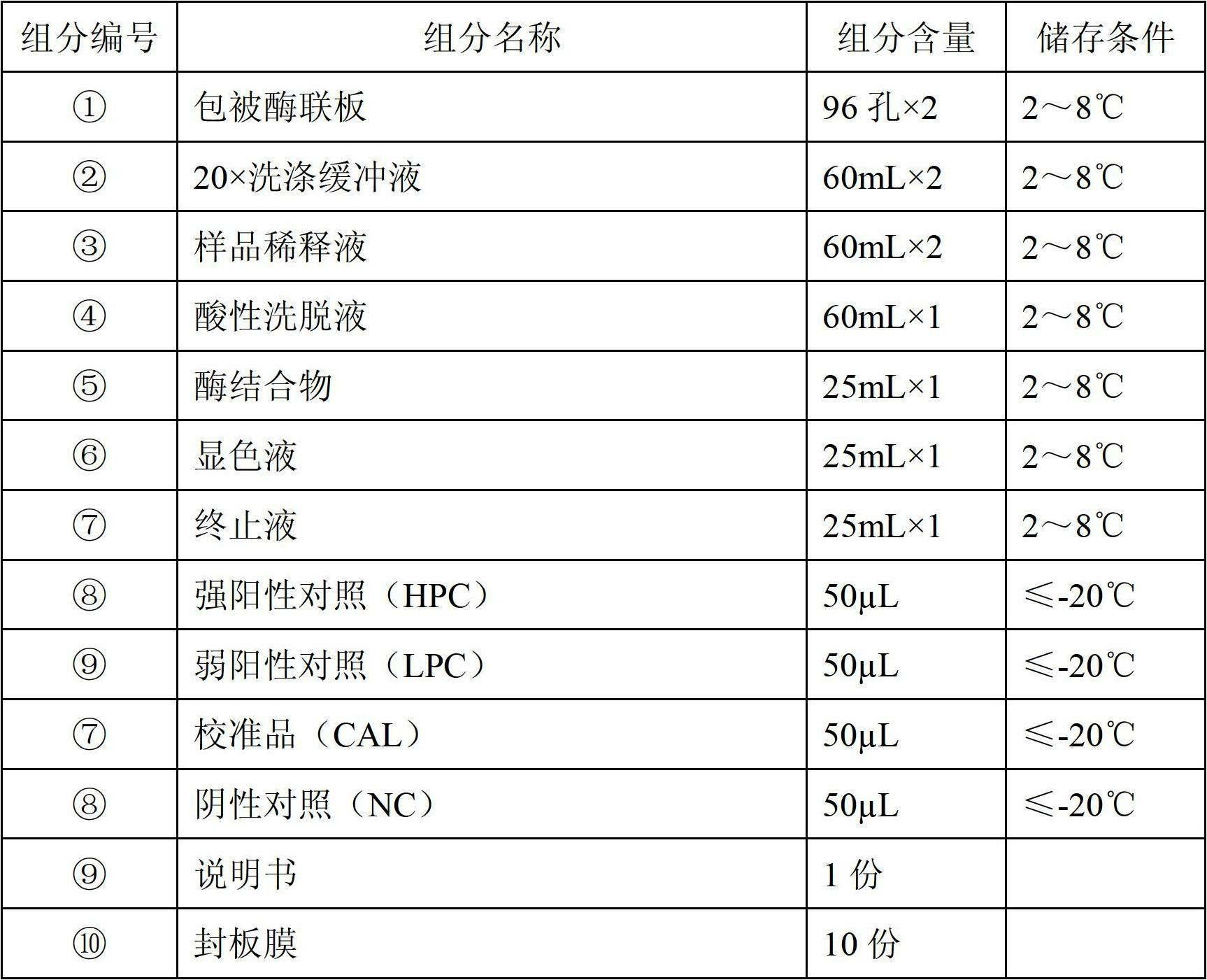
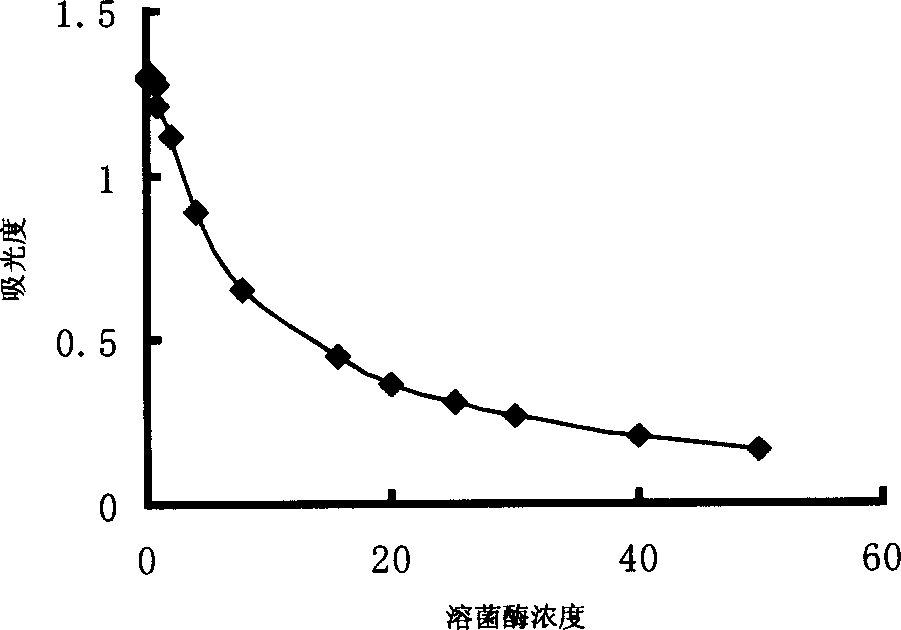
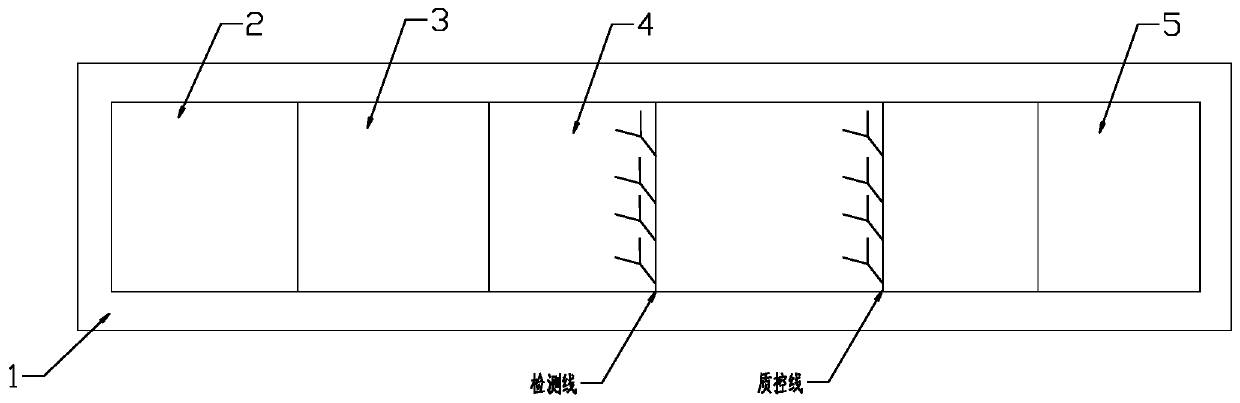
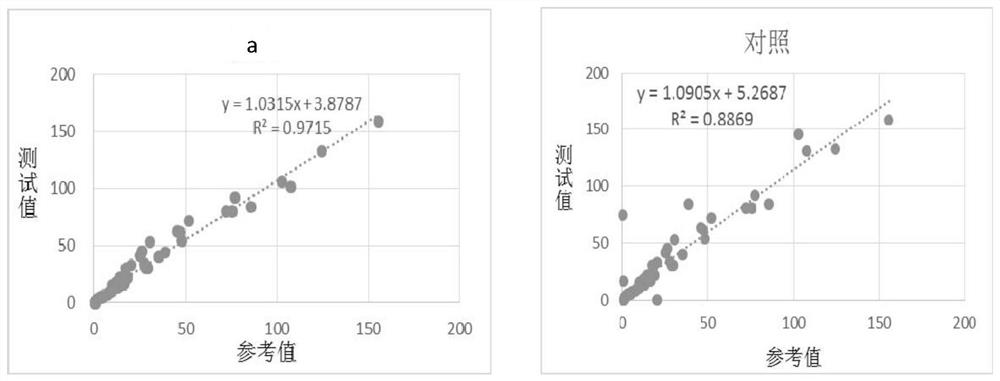
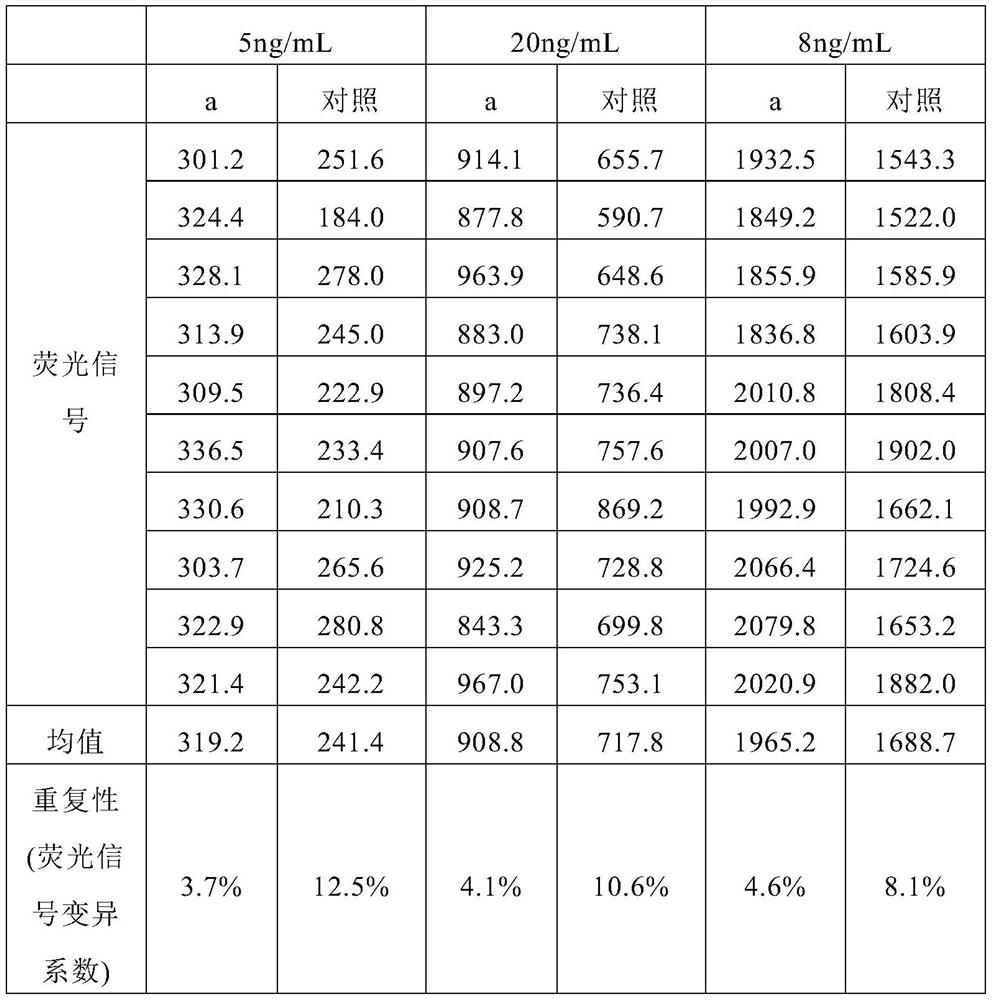
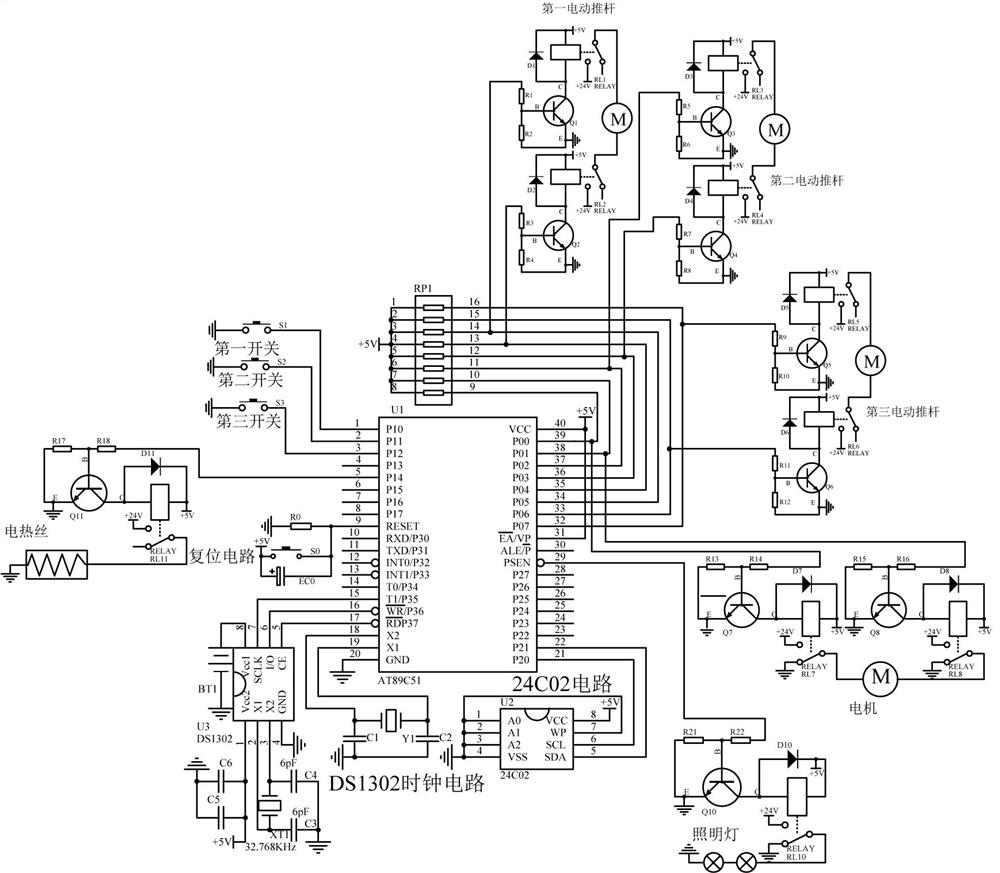
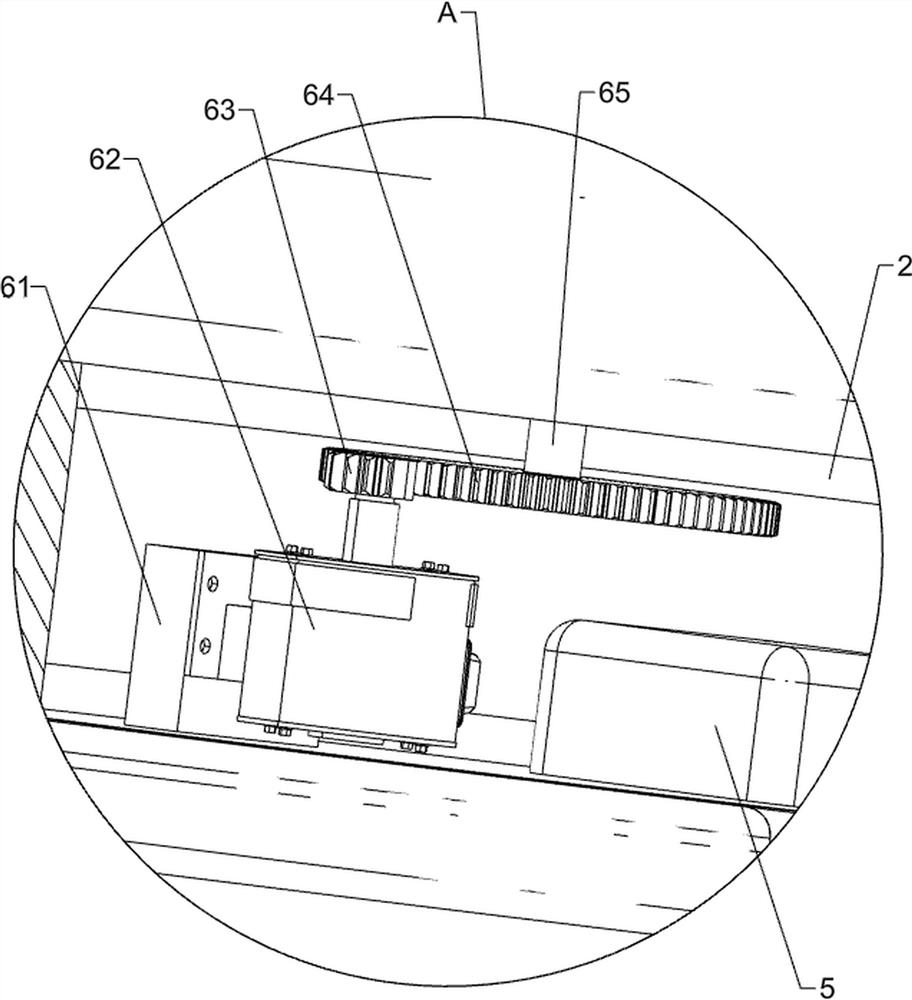
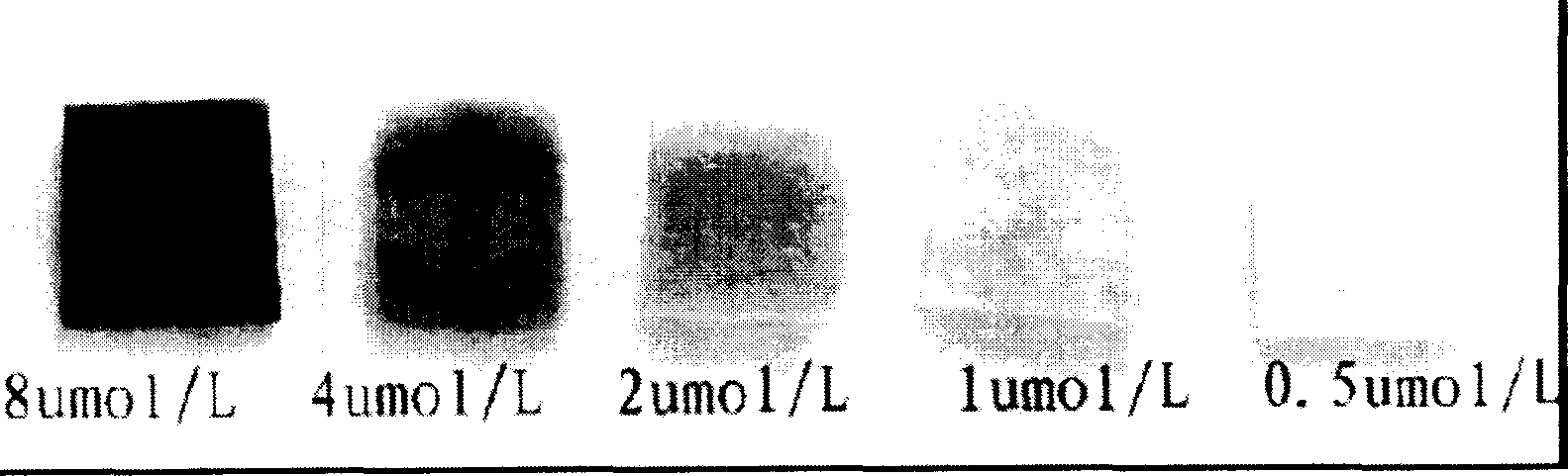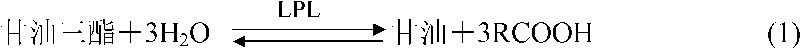Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
42 results about "Enzyme testing" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nicotinamide agent and preparation method thereof
The present invention is reducing nicotine amide coenzyme as enzyme testing reagent for testing body fluid. The enzyme testing reagent of the present invention includes oxidizing nicotine amide coenzyme, corresponding enzyme and substrate. The oxidizing coenzyme is reduced into reducing nicotine amide coenzyme slowly to test the tested matter. The reagent includes alanine aminotransferase reagent, aspartic acid aminotransferase reagent, urea reagent, ammonia reagent, creatinine reagent, CO2 reagent, etc. Owing to constant creation of nicotine amide coenzyme, the present invention has greatly raised reagent stability and greatly lowered reagent cost.
Owner:TECOM SCI CORP
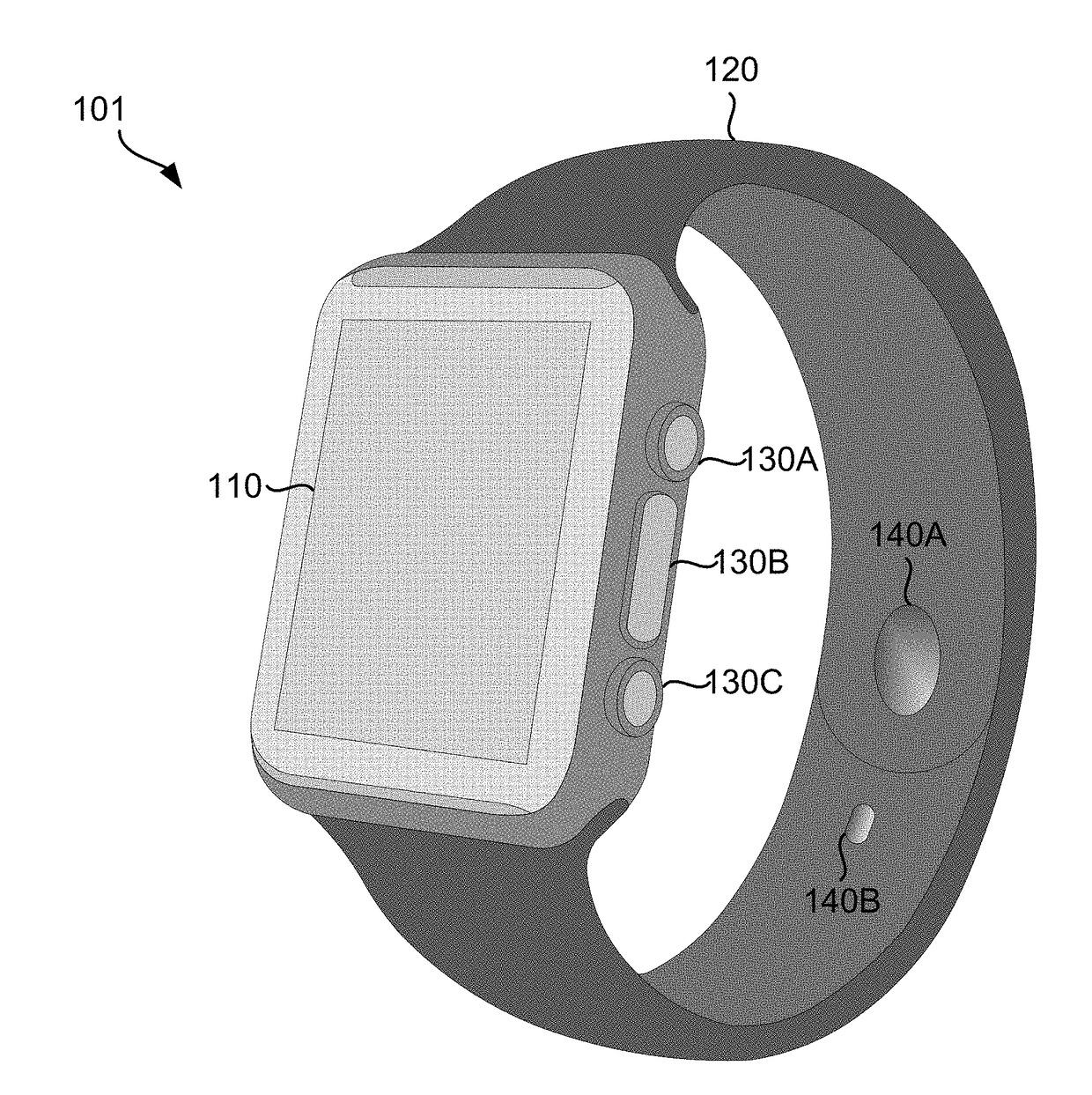
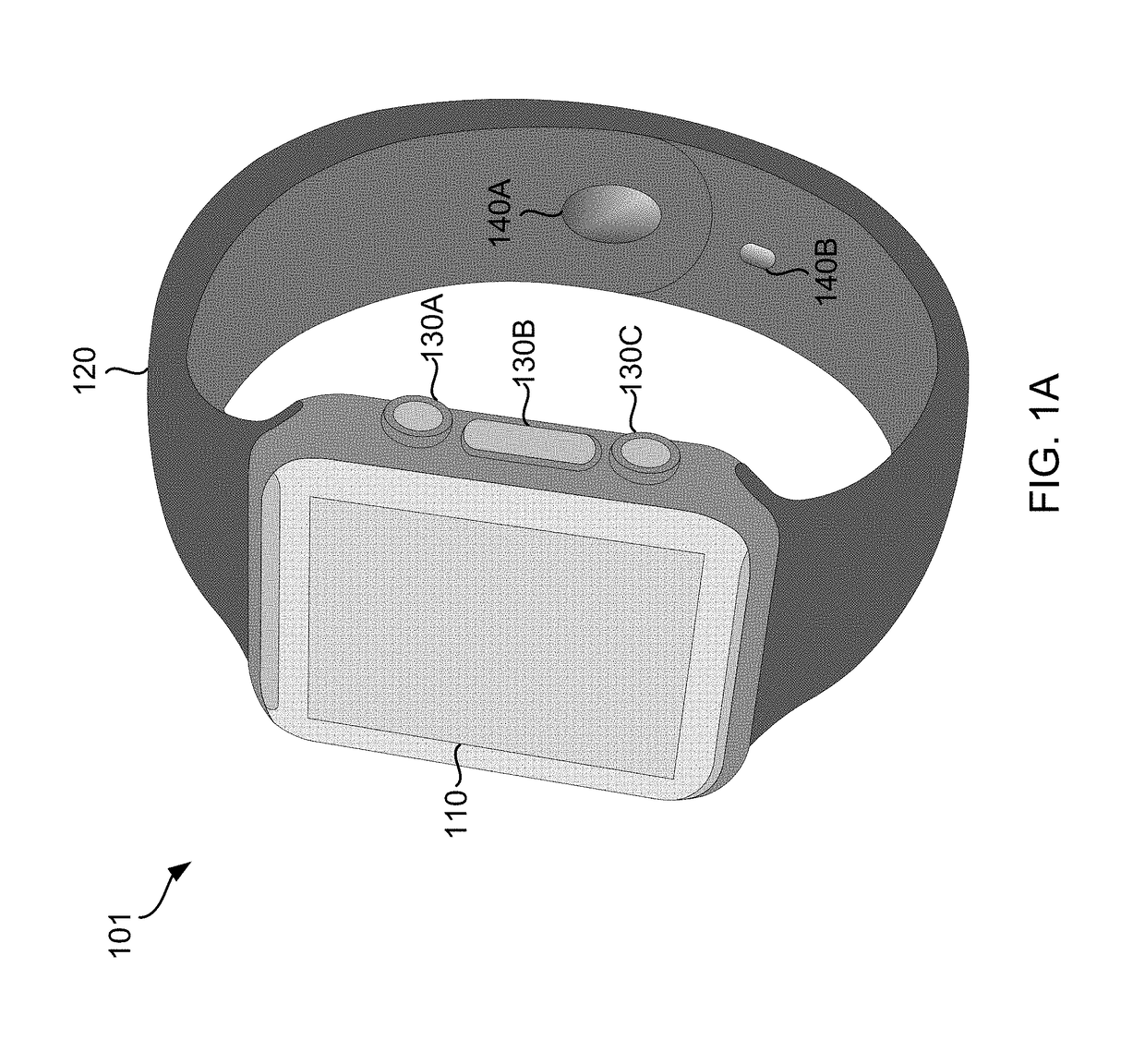
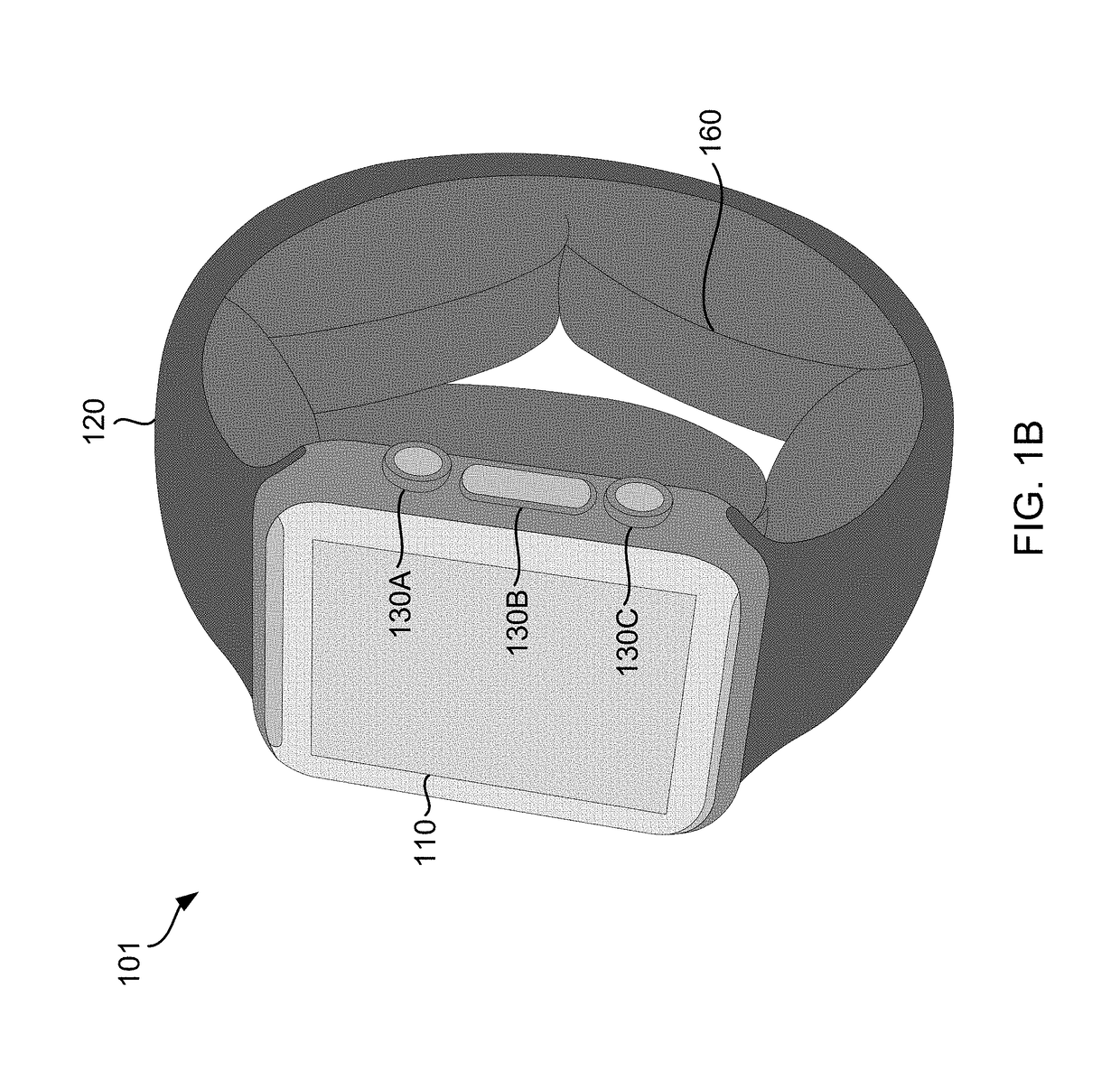
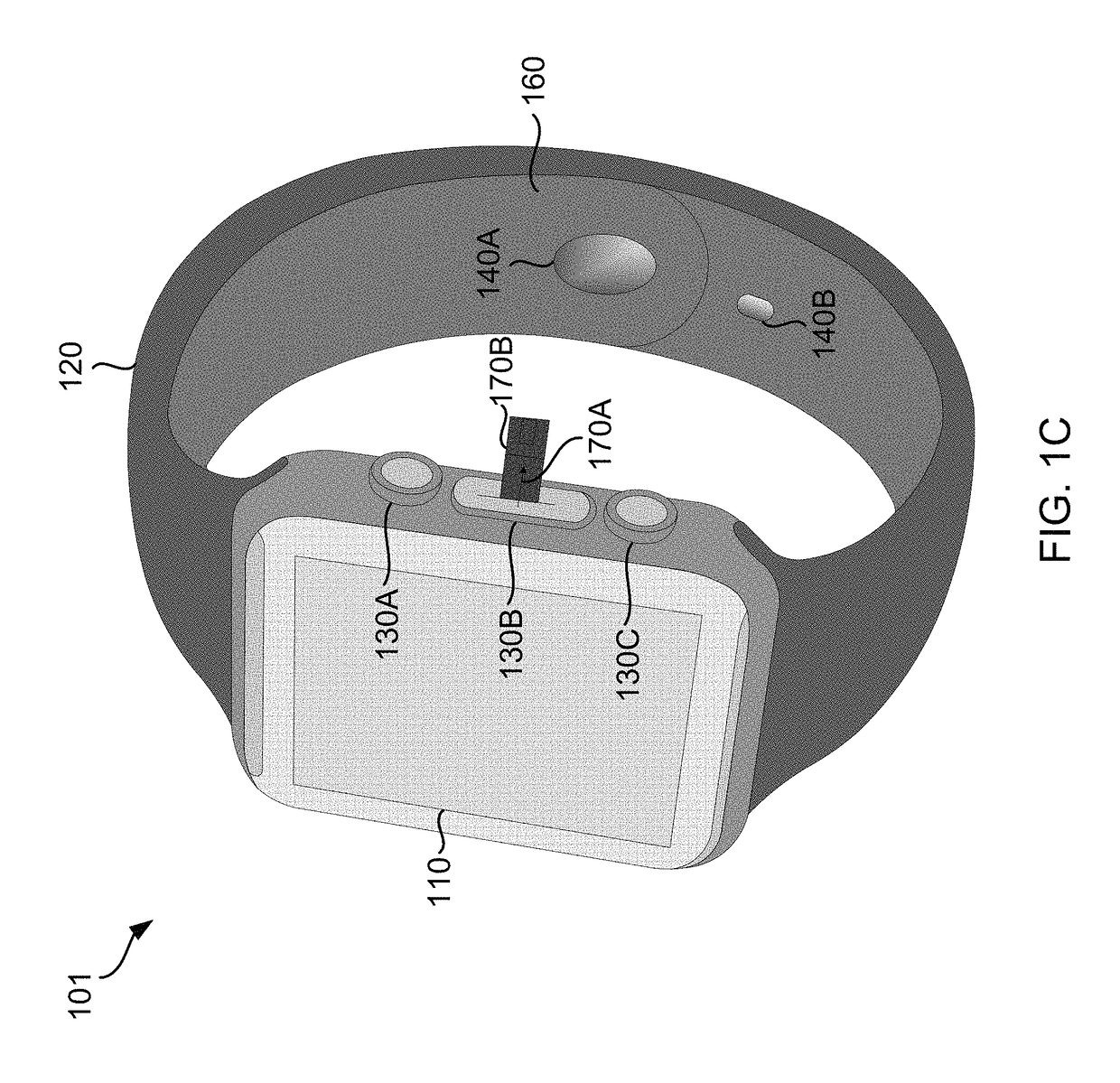
Smart Wearable Device for Health Watch
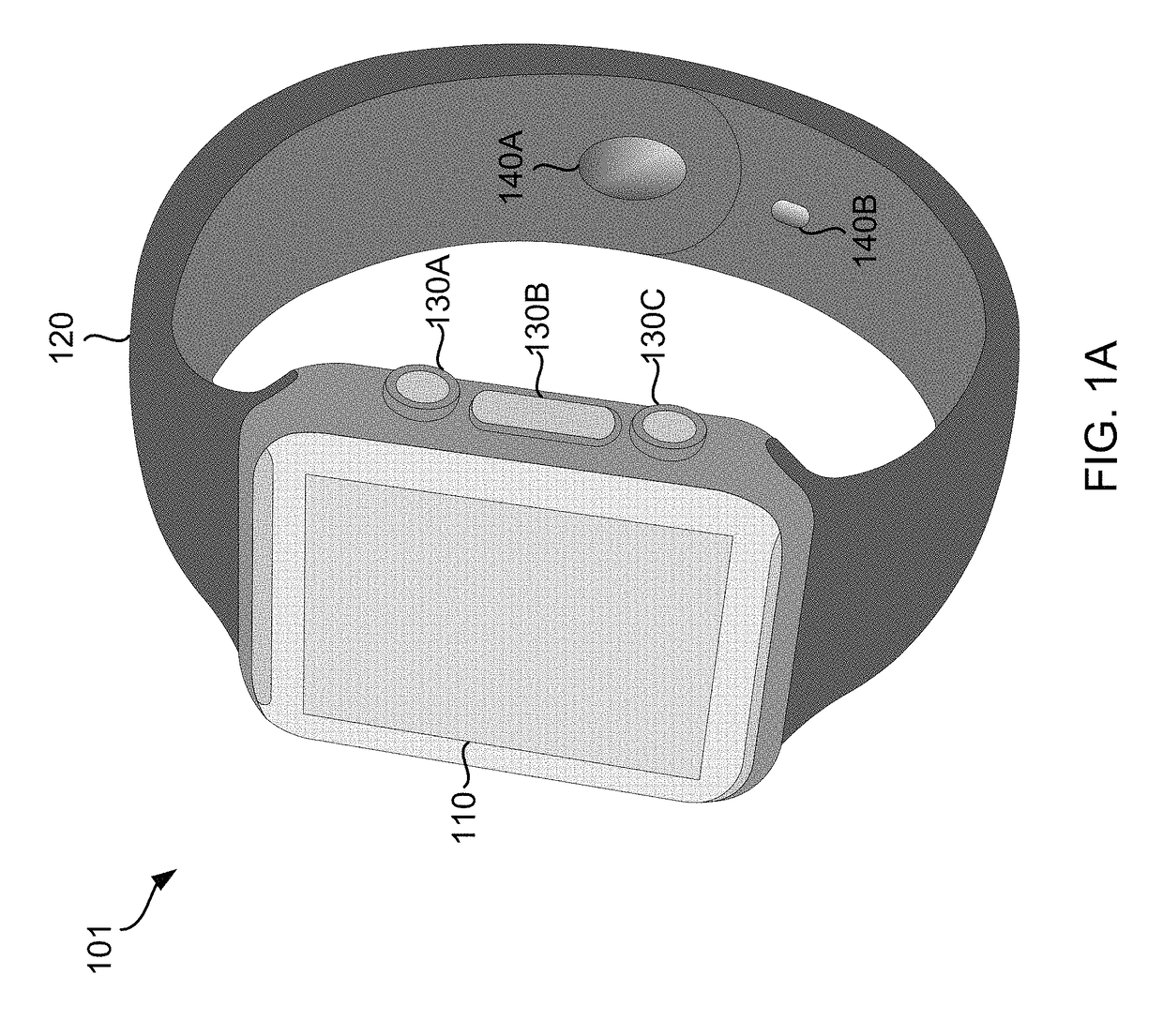
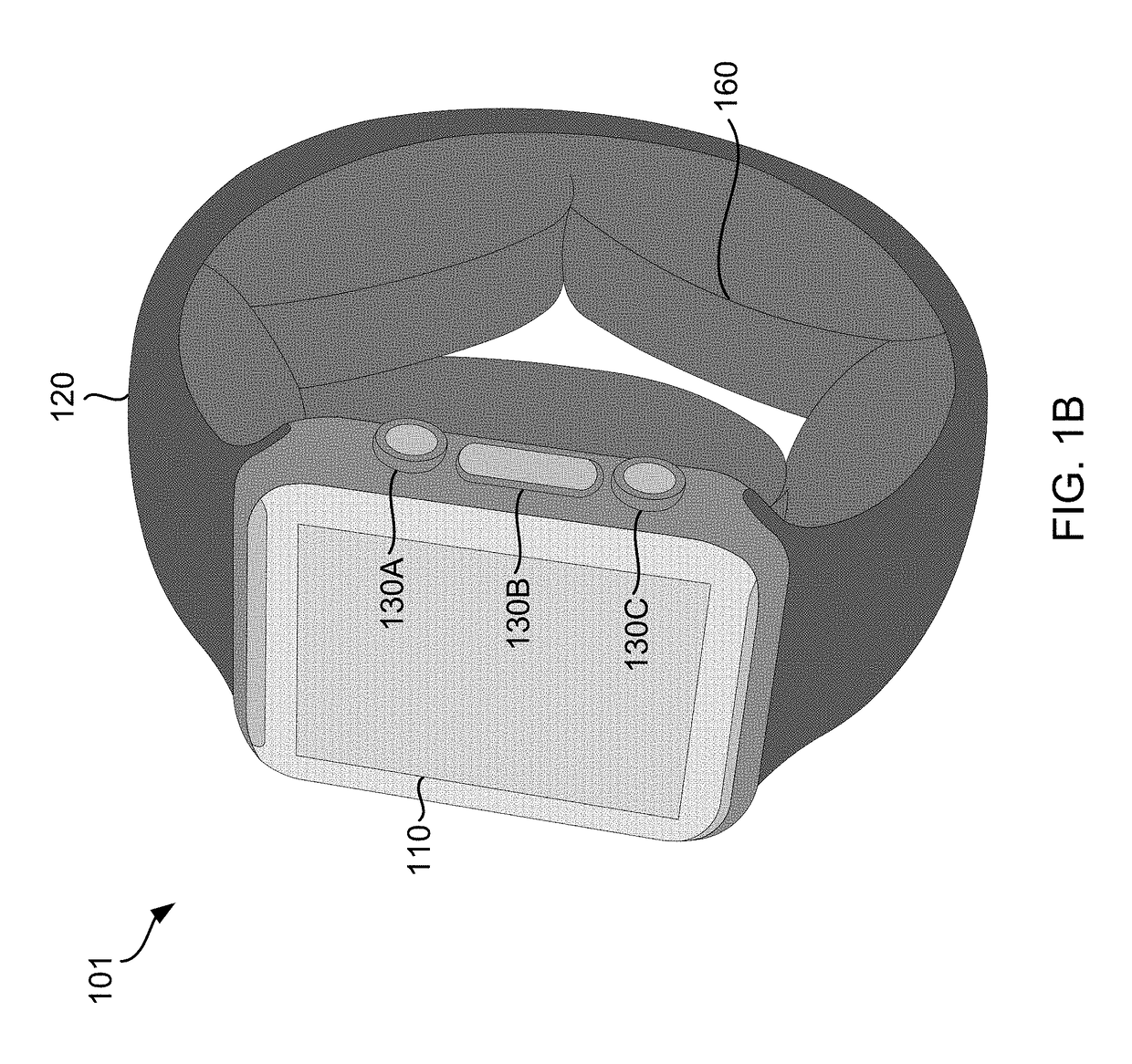
A wearable device comprising a display dial configured to display various health parameters, a wristband assisting the device to wear on wrist, an eject-able tray comprising a micro-chip, a first spring coupled to the eject-able tray, at least one latch provided with a second spring to hold the eject-able tray within the device by compressing the first spring, and a health monitoring unit provided with multiple sensors to determine various health parameters, wherein compression of the second spring results in the latch to release the eject-able tray which in turn relaxes the compressed first spring to eject the micro-chip outside the device for collecting blood samples. The microchip comprises at least one micro-needle and an enzyme test strip for collecting and analyzing the blood samples. The health monitoring unit comprises at least three conductive sense pads which are collectively operable to provide electrocardiograph (ECG) information.
Owner:SENSESEMI TECH PTE LTD
Enzyme test peper for detecting hydrogen peroxide concentration
InactiveCN1821754AEasy to carryEasy to measureMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementHydrogenEnzyme test
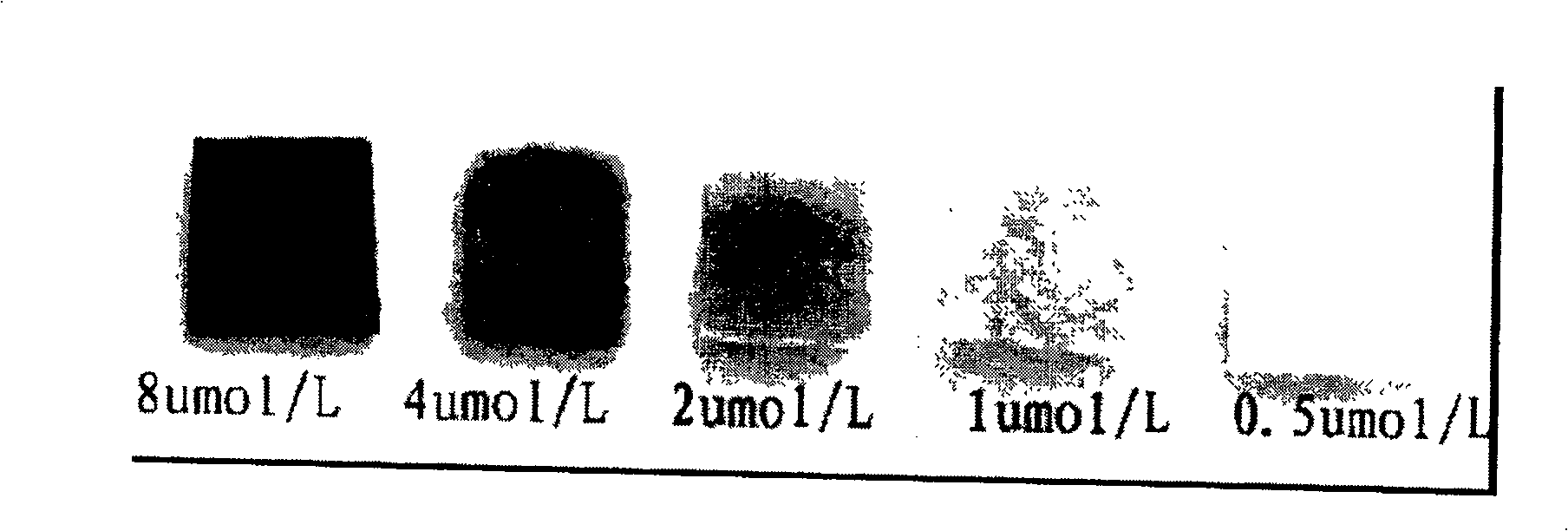
This invention relates to a kind of enzyme test paper capable of testing hydrogen peroxides in a sample quickly prepared by an enzymatic chromogenic reaction, which utilizes the adsorption action with anion carriers to fix the horseradish peroxide enzymes with positive charges and a developer on a carrier at the same time, which can test the existence of hydrogen peroxides with the concentration to 0.5mumol / L in the sample and the response time of the test paper is 1 minute.
Owner:SOUTH CHINA AGRI UNIV
Mimic enzyme test paper for detecting hydrogen peroxide and application thereof
ActiveCN103674939ASimple structureEasy to detectMaterial analysis by observing effect on chemical indicatorChemical industryCellulose
The invention relates to detection on hydrogen peroxide, and specifically discloses mimic enzyme test paper for detecting hydrogen peroxide, and preparation and application thereof. The mimic enzyme test paper is a surface modified cellulosic material loaded with an iron-based compound and a color developing agent, wherein the load amount of the iron-based compound is 1%-30% of the mass of the surface modified cellulosic material; the load amount of the color developing agent is 0.1%-20% of the mass of the surface modified cellulosic material; the color developing agent is 3, 3', 5, 5'-tetramethyl benzidine (TMB). According to the test paper provided by the invention, the color developing agent and the iron-based compound are loaded on the cellulosic material (such as filter paper, cotton and cotton cloth), the test paper simulates enzymatic decomposition of hydrogen peroxide and detects hydrogen peroxide, the mimic enzyme test paper is used in chemical industry, spinning, environment protection, medical treatment and food processing, and the application belongs to detection on hydrogen peroxide in the range of food safety, medical treatment, public health and environmental technology.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Combined detection kit for vaginitis
InactiveCN102253206AIncreased sensitivityImprove featuresMaterial analysis by observing effect on chemical indicatorCandida vaginitisBacterial vaginosis
The invention aims to provide a combined detection kit for vaginitis, capable of evaluating vaginal micro-ecological environment, vaginal cleanliness, and pathogens of bacterial vaginosis, Candida vaginitis, trichomonas vaginitis of the tested person simultaneously. Ihe following technical scheme adopted by the invention, a combined detection kit for vaginitis is provided and comprises a reaction device, diluent and chromogenic liquid, the reaction device is a hole type box-shaped reaction device with six reacting blind holes, in which a hydrogen peroxide concentration detection reagent pad, a leukocyte esterase detection reagent pad, a neuraminidase detection reagent pad, a proline aminopeptidase detection reagent pad, an acetyl glucosaminidase detection reagent pad and pH detection test paper are arranged respectively. Through the combined detection, the vaginitis caused by most of the BV (Bacterial Vaginosis) pathogens, the Candida albicans and the trichomonas can be detected, BV missing detection caused by the simple detection for one kind of enzyme activity is avoided largely, and the detection reliability and accuracy are increased.
Owner:泰普生物科学(中国)有限公司
High-sensitivity polyion-selective electrode and testing method thereof
ActiveCN102313767AReach sensitivityDetection limit reachedMaterial electrochemical variablesCircular discHigh concentration
The invention relates to a sensor, in particular to a high-sensitivity renewable potential sensor for multi-charge ions (polyion-selective electrode). Primary ions (target polyions) of an ion-exchanger-doped polymer film are pre-activated, the electrode is transferred into background electrolyte for activation after pre-activation, and thus the high-sensitivity polyion-selective electrode is obtained. The electrode can be regenerated by the high-concentration primary ions in testing solution during testing or after testing. For high sensitivity, the primary ions to be tested are inhibited in the external surface layer of the film based on the primary ion flow from a sensitive film phase to a film interface. For renewability, the primary ions need not to be completely eliminated from the film. No complex rotary disc electrode device or external current is needed for the sensor, the sensitivity and the renewability of the electrode are significantly improved, and the electrode also has great significance in the direct potential testing of polyions, the titration testing with a polyion electrode as a signal converter, the enzyme testing and the immunoassay.
Owner:YANTAI INST OF COASTAL ZONE RES CHINESE ACAD OF SCI
Stable 5 minute - ribonucleotide hydrolytic enzyme detection kit
InactiveCN104745673AImprove stabilitySolve the problem of long-term storage instabilityMicrobiological testing/measurementMannitolRibose
The invention discloses a 5minute - ribonucleotide hydrolytic enzyme colorimetric method detection kit, which belongs to the technical field of clinic extracorporeal detection reagents. The kit comprises a reagent R1 and a reagent R2. A compound stabilizer formed by five components of 4-FPBA, NaCl, mannitol, Triton100 and BSA is added in the reagent component 1, so that the difficult problem that enzyme cannot be stored stably for a long time is solved, the stabilization time of the enzyme can be increased during the test, the enzyme activity cannot be influenced, the stability of the kit is effectively enhanced, the accuracy of the reagent and the sensitivity for analysis cannot be influenced, and the reagent can be further favorably popularized in market.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Method for quickly determining residual peracetic acid
ActiveCN103439322AHigh sensitivityHigh detection sensitivityMaterial analysis by observing effect on chemical indicatorEnzyme testProcess engineering
The invention discloses a method for quickly determining residual peracetic acid. The method is short in response time, high in sensitivity, good in stability, easy and simple to operate, economical and practical, and can quickly detect the content of residual peracetic acid in a sample on site; the lowest detected content of peracetic acid is 0.5mg / L; a pH modifier in test paper used by the method ensures the accuracy of a test result; an enzyme protecting agent in the test paper ensures the stability of the enzyme test paper; a guarantee period can at least reach 12 months; the used test paper is small in area; a test process is simple; other instruments or accessories are not required; the test cost can be saved greatly; a holding bottom plate of the test paper can be prevented from being polluted by a hand in a taking process; and the method is very safe and reliable.
Owner:GUANGDONG HUANKAI MICROBIAL SCI & TECH
Sialidase detection reagent
InactiveCN1405562AImprove stabilityImprove accuracyMicrobiological testing/measurementBiological testingNeuraminateSialidase
This invention discloses a sialic acid enzyme test reagent for testing sialic acid enzyme activity in vagina secreta outside the body, containing substrate on the carrier named N-acetyl neuraminate and its salt which can be its derivant or thymolphthaleic N-acetyl neuraminate and its salt, 5-Br-4-Cl-3-indolyl-alpha-D-N-acytylneuraminate and its salt. We can diagnose the bacteriogenic vagina deseases quickly and simple by testing sialic acid enzyme to vagina searate with this sialic acid ester reagent which can be used as an independent diagnosis target with good stability, extremely excellent accuracy and simple operation (one step test).
Owner:肖洪武
Kit and method for detecting beta-lactamase
ActiveCN102455357AIncreased sensitivityStrong specificityImmunoglobulinsMaterial analysisFreeze-dryingCarrier protein
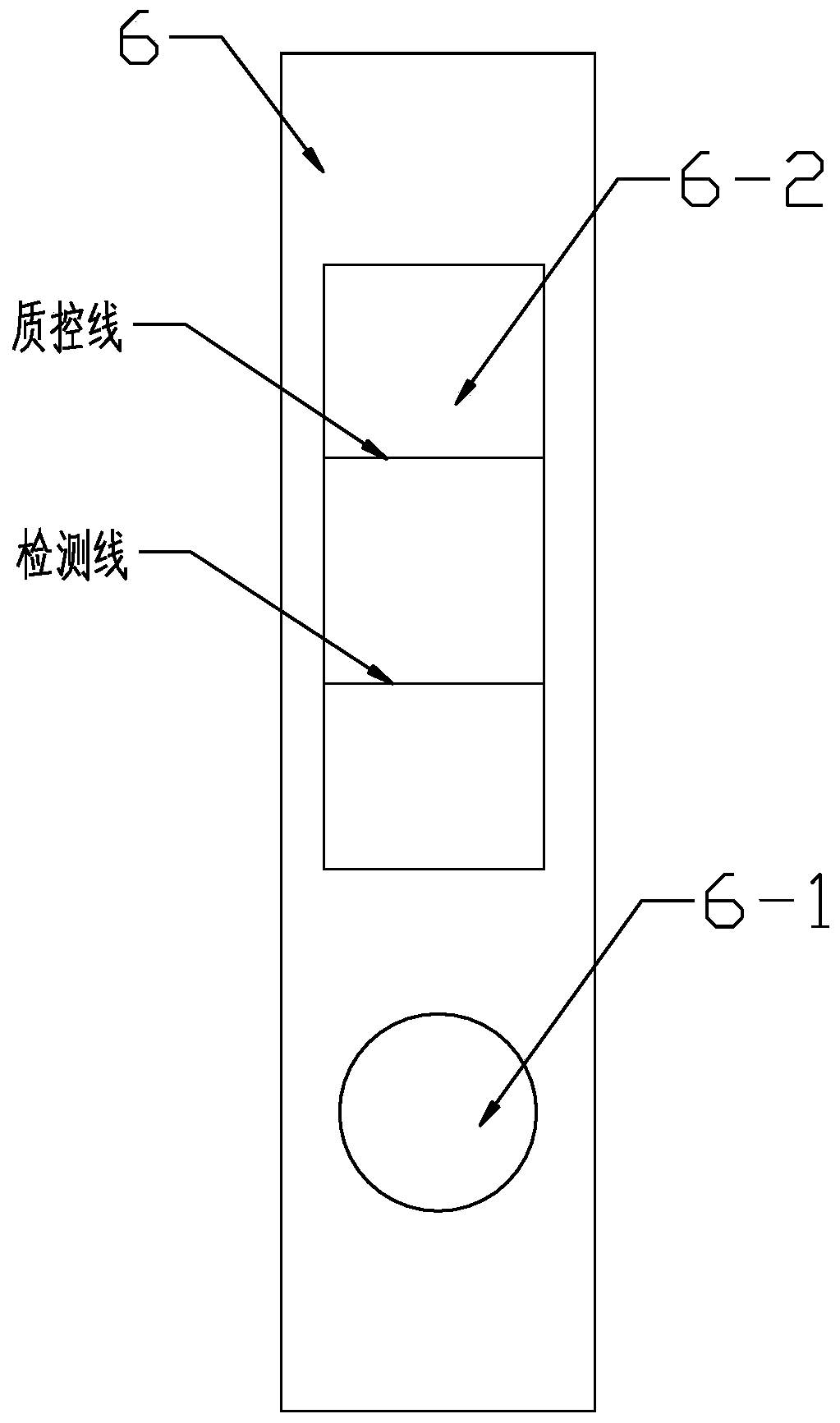
The invention discloses a kit and a method for detecting beta-lactamase. The kit comprises a microporous reagent, an enzyme test reagent and test paper, wherein a cephems medicament specificity-colloidal gold marker is freeze-dried in the microporous reagent; a penicillin medicament is freeze-dried in the enzyme test reagent; a cephems medicament specificity antibody is a cephems medicament monoclonal antibody or a cephems medicament polyclonal antibody; the test paper is formed by connecting a base plate, a sample absorption pad, a reaction film, a water absorption pad and a protection film sequentially; the reaction film comprises a detection region and a quality control region; the detection region is coated with cephems medicament and carrier protein conjugate; and the quality controlregion is coated with an antibody. The method for detecting the beta-lactamase by using the kit is simple, quick, intuitive and accurate, is wide in application range and low in cost, and is easy to popularize and use.
Owner:BEIJING KWINBON BIOTECH
Novel I-type human immunodeficiency virus (HIV-1) infection enzyme test-free reagent kit
The invention provides a novel I-type human immunodeficiency virus (HIV-1) infection enzyme test-free reagent kit. The reagent kit comprises A, HIV-1gp41 protein, B, a blank enzyme linked plate, C, acid eluent, D, goat anti-human immunoglobulin G (IgG) marked with horseradish peroxidase, E, tetramethyl benzidine (TMB) color development solutions and F, stop solutions.
Owner:BEIJING KINGHAWK PHARMA
Salmonella and/or e. coli enzyme test and/or liquid drop test for biological substances, on non-human samples
InactiveUS20140186880A1Assist in cell softeningEasy to useMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementEscherichia coliWater source
A test kit and test apparatus are provided for the detection of enzymes excreted by broken or ruptured bacteria cells in various environments, such as in the field, soil extracts, produce washing, slaughter houses, food preparation area's and other related or non-related areas where a quick test or rapid test is needed to obtain qualitative results. The method utilizes various components which allow for the collection of a sample from suspected contaminated areas. One method is to collect a sample from a pond or other water source possibly contaminated and by using a clean lab sample tube or clean plastic bag, a field hand mixer, a buffer, a pipette and a test strip with a reagent pad.
Owner:LOWENKAMP JR WILLIAM
Two-step enzyme testing method of triglycercide in blood serum
InactiveCN101762577AWill not increase the burdenLow costMaterial analysis by observing effect on chemical indicatorQuinoneGlycerol
The invention discloses a two-step enzyme testing method of triglycercide in blood serum, belonging to a method for testing material according to the color change of the result of test reaction with visible light. The method has the technical scheme as follows: double-color raw materials are in reagent I, and reagent II only has active constituent of lipoprotein lipase. The test method comprises the steps of: bathing the blood serum and the reagent I under the temperature of 37 DEG C for 3-5 minutes; reaching free glycerin in the blood serum with the reagent I to generate quinone imide; bathing under the temperature of 37 DEG C for 4-7 minutes after adding the reagent II; and hydrolyzing the triglycercide and reacting to generate the red quinone imide. An instrument is used for detecting at the position with the wave length of 500-520nm, the quinone imide generated by the reaction of the reagent I is taken as blank, and the content of the triglycercide is computed through the quinone imide generated by the reaction of the reagent II. The method is not influenced by endogenic glycerine, has the same steps and range as the existing two-step method, does not increase the cost of the reagent, and is economic and convenient, thereby being a triglycercide testing method with higher accuracy.
Owner:WHITMAN BIOTECH NANJING
Muramidase detction reagent and its preparing method
InactiveCN1587994AImprove performanceRealize automated detectionMicrobiological testing/measurementColor/spectral properties measurementsThallusAbsorbance
The invention of biochemical analysis field relates to a lysozyme testing agent and its manufacture method. The lysozyme testing agent contains killing Micrococcus and gelatin, which is bacteria suspension with high stability, created by new method of killing Micrococcus and using gelatin as medium and stabilizer of disperse thallus. After more than four hours of standing, if absorbance of bacteria suspension does not change notably, agent abtained is proved to have excellent stability and can satisfy various testing requirement, be used in automatic biochemical analysis and realize automatic and quantitive detection of lysozyme activity.
Owner:SHANGHAI CHEST HOSPITAL
Human pluripotent stem cell derived neurodegenerative disease models on a microfluidic chip
PendingUS20210130774A1Bioreactor/fermenter combinationsBiological substance pretreatmentsNeural cellDisease patient
Described herein is a microphysiological system for models of disease. Specifically, induced pluripotent stem cells (iPSCs) and iPSC-derived cells, including those obtained from disease patients, are seeded onto microfluidic “chip” devices to study cellular development and disease pathogenesis. Herein, neurodegenerative disease modeling, including Parkinson's Disease (PD) is shown to reproduce key PD pathology in a vascularized human model that contains neurons relating to PD pathology. Such compositions and methods are used for research for PD biomarkers, patient screening for PD risk assessment, and therapeutic discovery and testing. A panel of biomarkers are generated through analysis of living PD-chips by neural activity, whole transcriptomic, proteomic, and metabolomic analysis, and functional enzyme tests of media and tissue. Introducing therapeutics through a vasculature channel, coupled with blood brain barrier penetration studies can be assessed for efficacy in the human neural cells present in the PD-Chip.
Owner:CEDARS SINAI MEDICAL CENT
Method for detecting norovirus by using nano-enzyme test strip
PendingCN111007251AIncreased sensitivityImprove reliabilityMaterial analysisEnzyme testBiomedical engineering
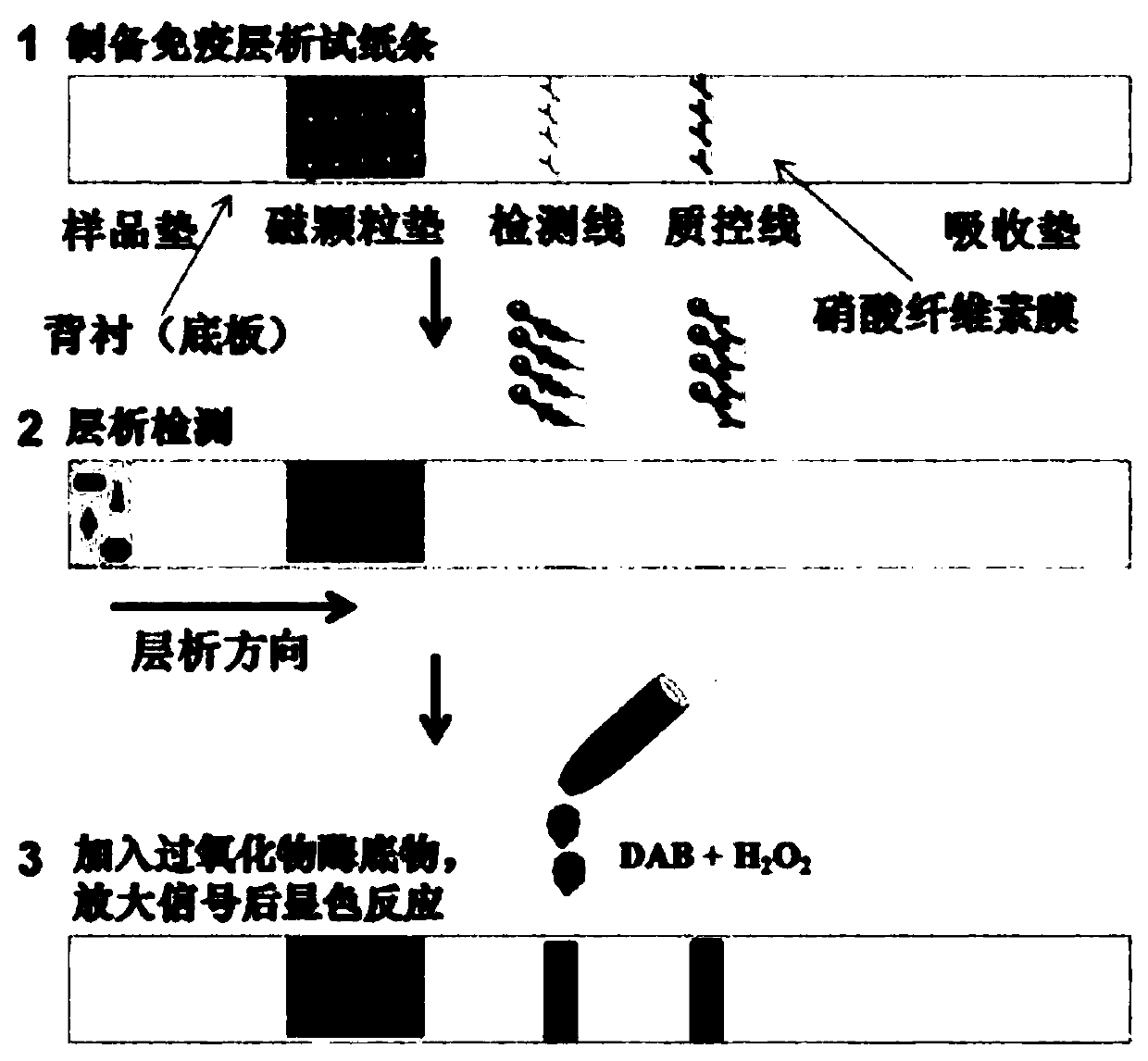
The invention discloses a method for detecting norovirus by using a nano-enzyme test strip. The method comprises the following steps: (1), synthesizing nano-enzyme; (2), preparing a nano enzyme-norovirus antibody probe; (3), assembling a nano-enzyme immunochromatography test strip; and (4), detecting the norovirus by the nano-enzyme immunochromatography test strip. The detection sensitivity of thenano-enzyme immunochromatography test strip can be improved by 10 times by being compared with that of a colloidal gold test strip, and the nano-enzyme immunochromatography test strip is high in sensitivity and reliability to norovirus detection and has specific recognition to norovirus. The method for detecting the norovirus by the nano-enzyme test strip has advantages of high simplicity, greatconvenience, quickness and field application of a colloidal gold technology, has the characteristic of high sensitivity, and has the broad development prospects.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE +1
Smart wearable device for health watch
A wearable device comprising a display dial configured to display various health parameters, a wristband assisting the device to wear on wrist, an eject-able tray comprising a micro-chip, a first spring coupled to the eject-able tray, at least one latch provided with a second spring to hold the eject-able tray within the device by compressing the first spring, and a health monitoring unit provided with multiple sensors to determine various health parameters, wherein compression of the second spring results in the latch to release the eject-able tray which in turn relaxes the compressed first spring to eject the micro-chip outside the device for collecting blood samples. The microchip comprises at least one micro-needle and an enzyme test strip for collecting and analyzing the blood samples. The health monitoring unit comprises at least three conductive sense pads which are collectively operable to provide electrocardiograph (ECG) information.
Owner:SENSESEMI TECH PTE LTD
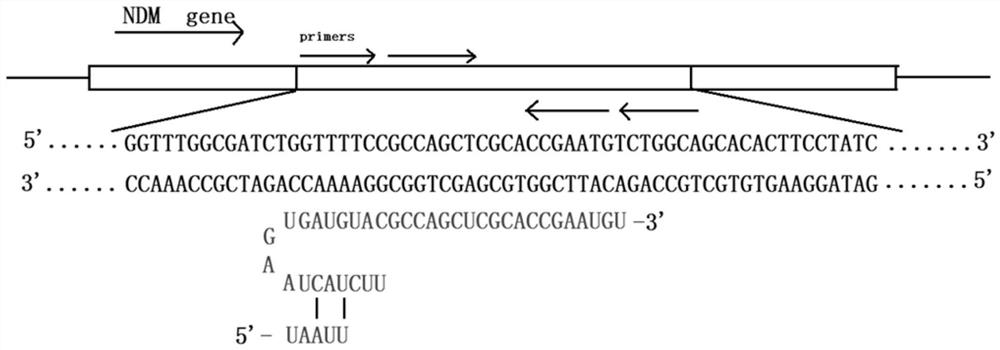
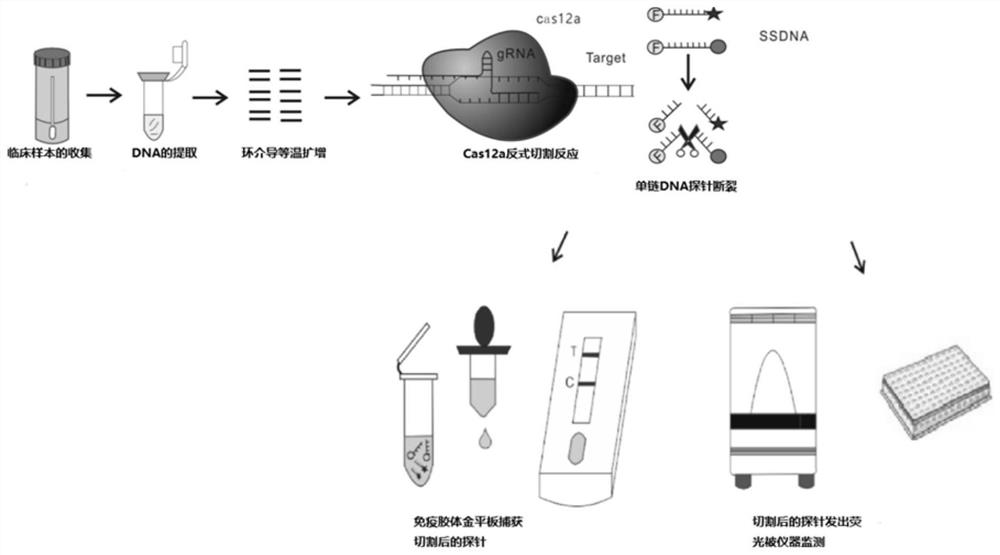
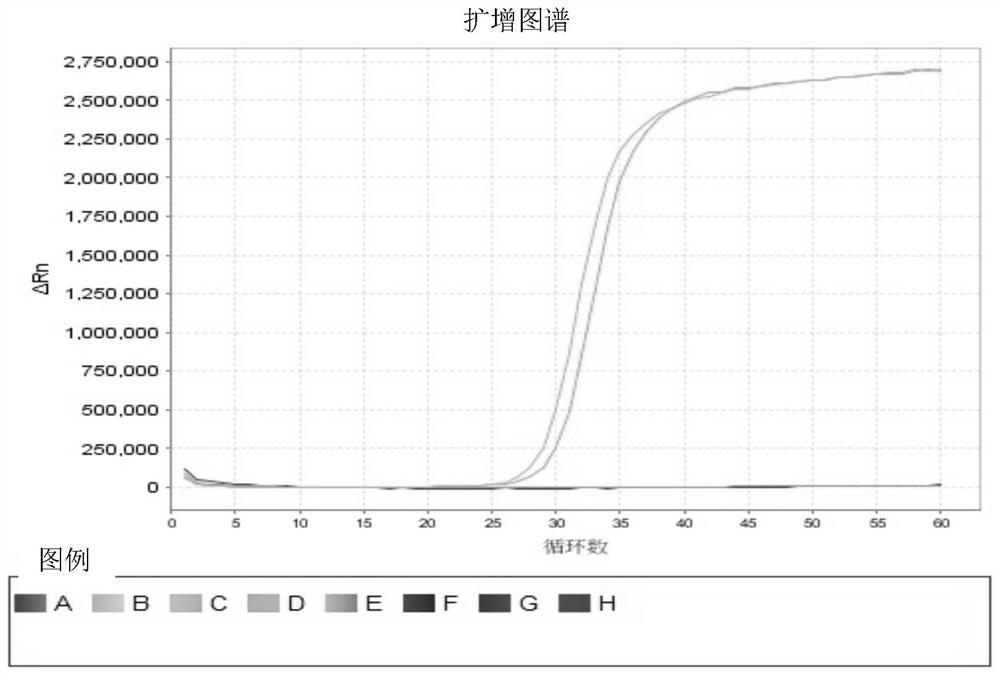
CrRNA and CRISPR-Cas12a system for carbapenemase detection and application
PendingCN113444777AShorten detection timeLow priceHydrolasesMicrobiological testing/measurementBase JSingle strand
The invention relates to the technical field of medical treatment, and provides CrRNA for carbapenemase detection with the base sequence as shown in SEQ ID NO. 1. The CrRNA is used for guiding Cas12a protein identification and combined to a LAMP amplified sequence to cut a target sequence, and meanwhile, the Cas12a protein is used for trans-cleavage of any single-stranded DNA in a reaction system. The invention further provides a CRISPR-Cas12a system and a method for rapidly detecting the carbapenemase based on the CRISPR-Cas12a, as well as application of the CRISPR-Cas12a system in the preparation of a carbapenemase detection kit. The method can be used as a clinical large-scale crowd detection method. Compared with a traditional diagnosis detection mode, the detection mode has the advantages that the detection time is greatly shortened, operators are not needed to be repeatedly trained, and cost is low.
Owner:安徽医科大学第四附属医院
Creatine kinase isoenzyme MB detection kit and preparation method thereof
The invention provides a creatine kinase isoenzyme MB detection kit and a preparation method thereof. Specifically, the kit comprises a creatine kinase isoenzyme MB detection reagent card, and the reagent card comprises a sample pre-adding part and a test strip; wherein the pre-sampling part comprises a sampling container and a creatine kinase isozyme MB monoclonal antibody-fluorescent microsphereconjugate which is pre-added into the sampling container; the test strip comprises a test strip bottom plate, and a sample loading part, a detection part and a liquid absorption part which are arranged on the test strip bottom plate, wherein the detection part comprises a chromatography matrix, a detection band and a quality control band, wherein the detection band and the quality control band are respectively arranged, the detection band is coated with a creatine kinase isozyme CK-MB polyclonal antibody, and the quality control band is coated with an IgG polyclonal antibody. The kit is simple to operate, low in cost, good in repeatability and high in accuracy.
Owner:上海艾瑞德生物科技有限公司
Biological detection device for detecting blood metabolism waste by using enzyme
InactiveCN113444636APrevent leakageAvoid inactivationBioreactor/fermenter combinationsBiological substance pretreatmentsMetabolic wasteHematological test
The invention relates to a detection device, in particular to a biological detection device for detecting blood metabolism waste by using enzyme. The technical problem to be solved is to provide the biological detection device for detecting the blood metabolism waste by using the enzyme, and liquid leakage and enzyme inactivation when a test tube is shaken can be avoided. According to the technical scheme, the biological detection device for detecting the blood metabolism waste by using the enzyme comprises a supporting plate, a first supporting block, a first fixing block and test tubes, wherein the first supporting block is arranged in the middle of the top of the supporting plate; the first fixing block is rotationally arranged on one side of the first supporting block; and a plurality of test tubes are placed in the first fixing block. The test tubes can be sealed through an arranged protection mechanism, so that liquid in the test tubes is prevented from leaking when the test tubes shake.
Owner:JINGGANGSHAN UNIVERSITY
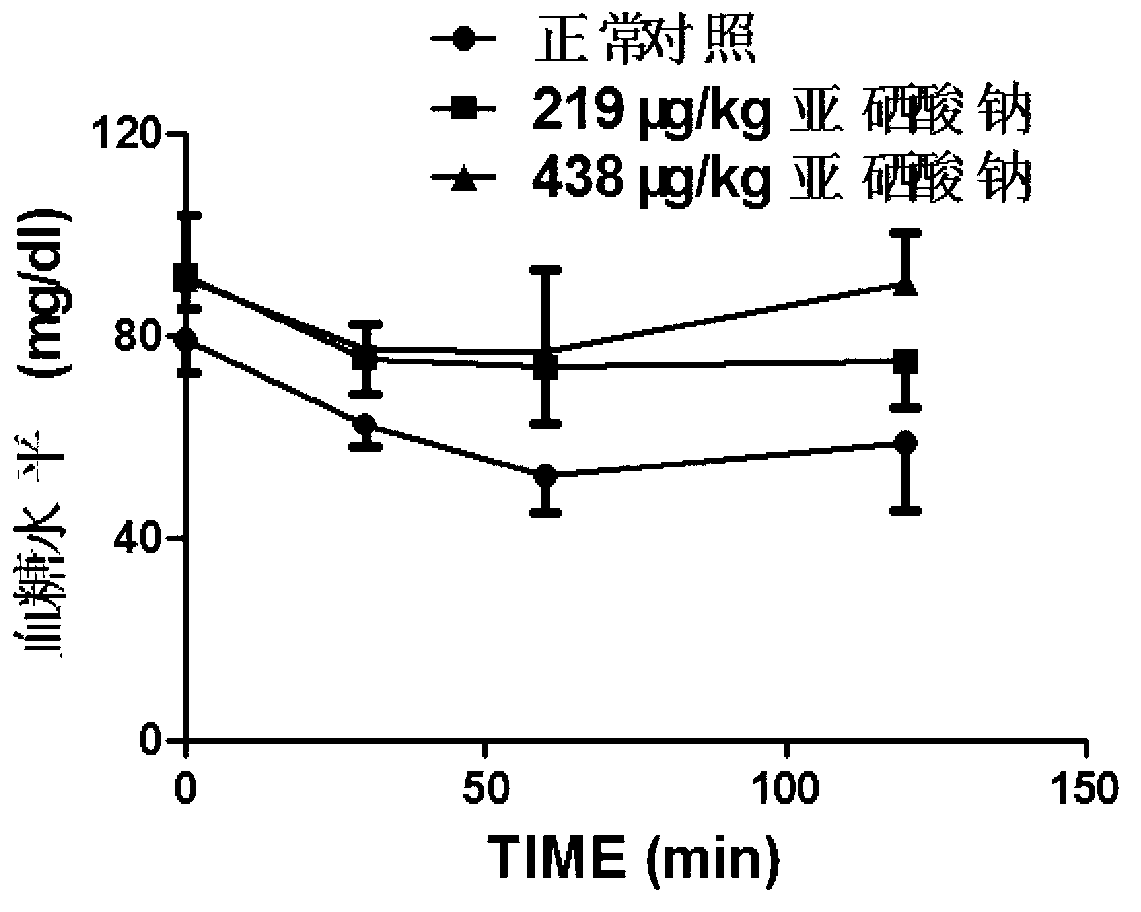
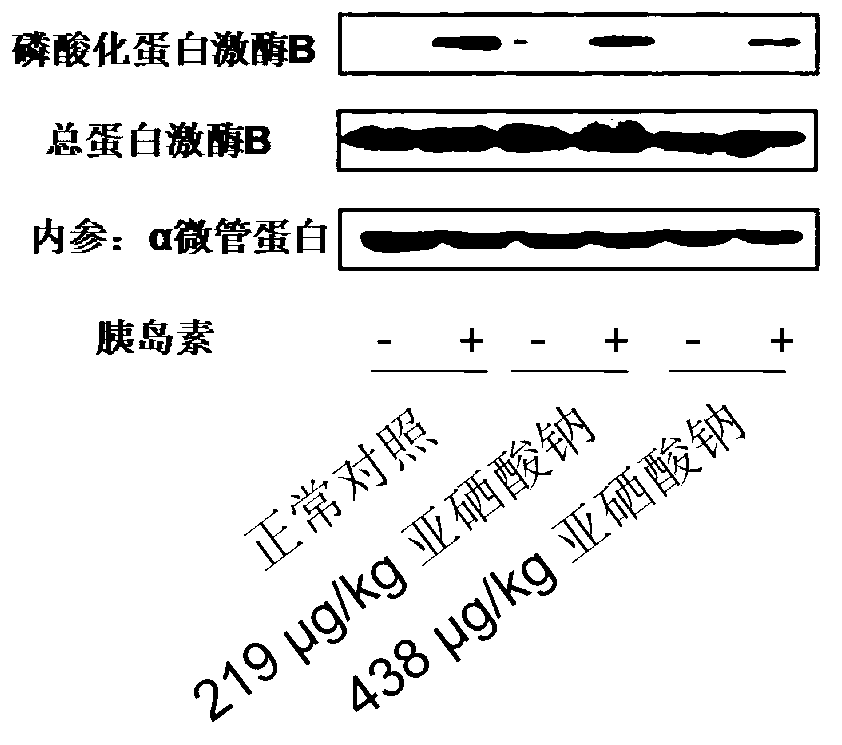
Application and construction method of high-selenium induced insulin resistance animal model
InactiveCN103211834AHigh molding rateModeling method is simpleSulfur/selenium/tellurium inorganic active ingredientsRat modelInsulin humulin
The invention discloses an application and construction method of a high-selenium induced insulin resistance animal model. The application and construction method comprises the following steps of: feeding 100mug / kg-200ug / kg of selenium to the stomach of an SD (Sprague Dawley) rat every day; and six weeks later, obtaining the insulin resistance animal model. According to the statistic analysis of glucose tolerance and insulin tolerance, insulin signal transduction and gluconeogenesis key enzyme test, the construction of the insulin resistance animal model by utilizing sodium selenite is feasible; and the insulin resistance animal model can be used as a high-selenium induced insulin resistance animal model and a pathogenesis and drug action mechanism research model of diabetes mellitus II. The insulin resistance rat model constructed by the application and the construction method disclosed by the invention has the advantages of simpleness in modeling method, high model establishing success rate, low cost, short period, good reproducibility and the like.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Application and construction method of animal model of insulin resistance induced by high selenium
InactiveCN103211834BHigh molding rateModeling method is simpleSulfur/selenium/tellurium inorganic active ingredientsRat modelInsulin humulin
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Two-step enzyme testing method of triglycercide in blood serum
InactiveCN101762577BWill not increase the burdenLow costMaterial analysis by observing effect on chemical indicatorQuinoneA lipoprotein
The invention discloses a two-step enzyme testing method of triglycercide in blood serum, belonging to a method for testing material according to the color change of the result of test reaction with visible light. The method has the technical scheme as follows: double-color raw materials are in reagent I, and reagent II only has active constituent of lipoprotein lipase. The test method comprises the steps of: bathing the blood serum and the reagent I under the temperature of 37 DEG C for 3-5 minutes; reaching free glycerin in the blood serum with the reagent I to generate quinone imide; bathing under the temperature of 37 DEG C for 4-7 minutes after adding the reagent II; and hydrolyzing the triglycercide and reacting to generate the red quinone imide. An instrument is used for detecting at the position with the wave length of 500-520nm, the quinone imide generated by the reaction of the reagent I is taken as blank, and the content of the triglycercide is computed through the quinone imide generated by the reaction of the reagent II. The method is not influenced by endogenic glycerine, has the same steps and range as the existing two-step method, does not increase the cost of the reagent, and is economic and convenient, thereby being a triglycercide testing method with higher accuracy.
Owner:WHITMAN BIOTECH NANJING
Enzyme test peper for detecting hydrogen peroxide concentration
InactiveCN100516843CEasy to carryEasy to measureMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementEnzyme testColor reaction
The invention relates to an enzyme test paper which can rapidly detect the content of hydrogen peroxide in a sample prepared by enzymatic color reaction. The test paper is prepared through the method of immobilizing the positively charged horseradish peroxidase and the color developing agent on the carrier simultaneously by utilizing the adsorption effect of the anion carrier. The test paper can detect the presence of hydrogen peroxide in the sample at a concentration as low as 0.5 μmol / L. The response time of the test paper is 1 minute during detection. It can conveniently and quickly determine the content of hydrogen peroxide in the sample. At the same time, the test paper of the invention is easy to carry and is suitable for mobile operations.
Owner:SOUTH CHINA AGRI UNIV
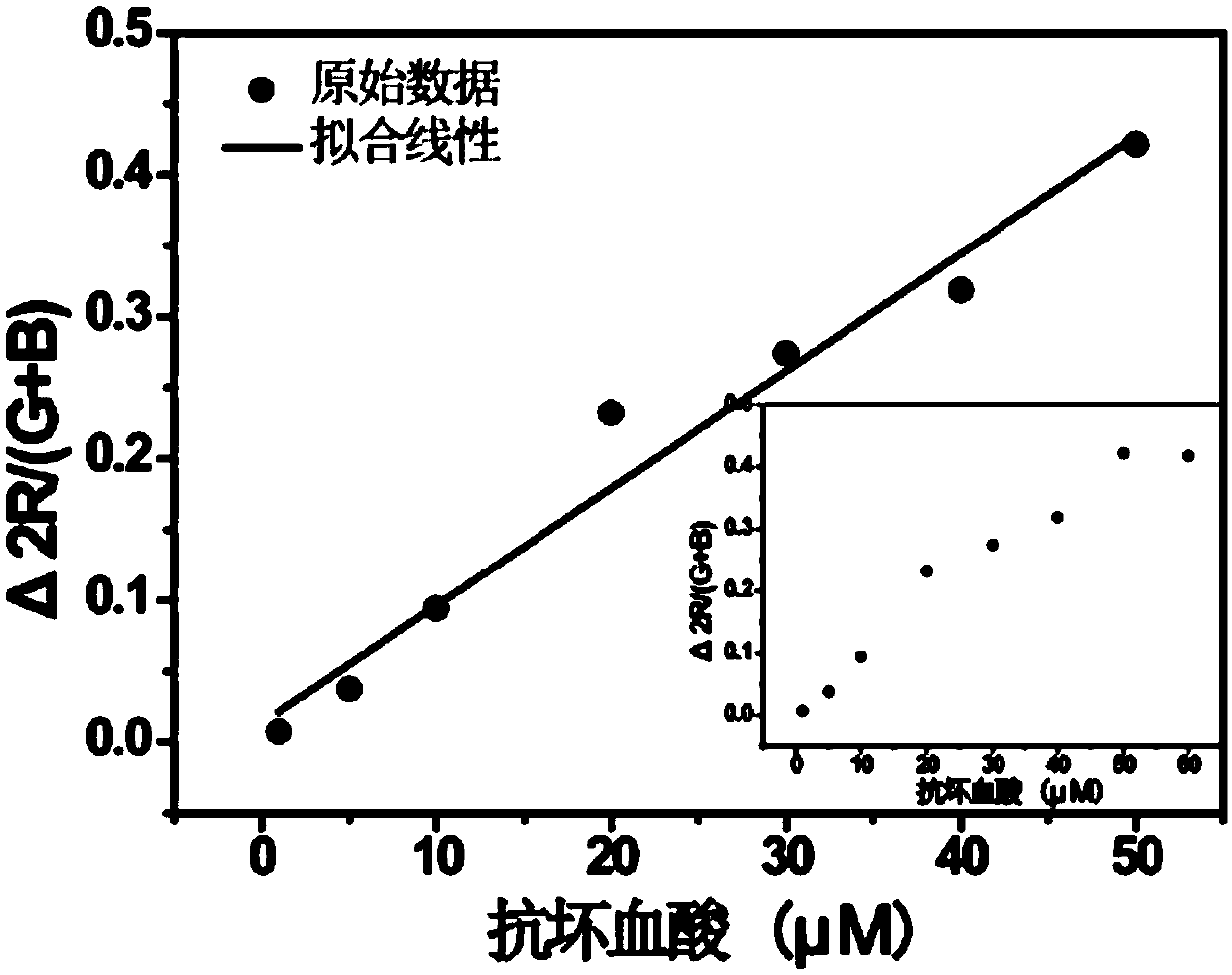
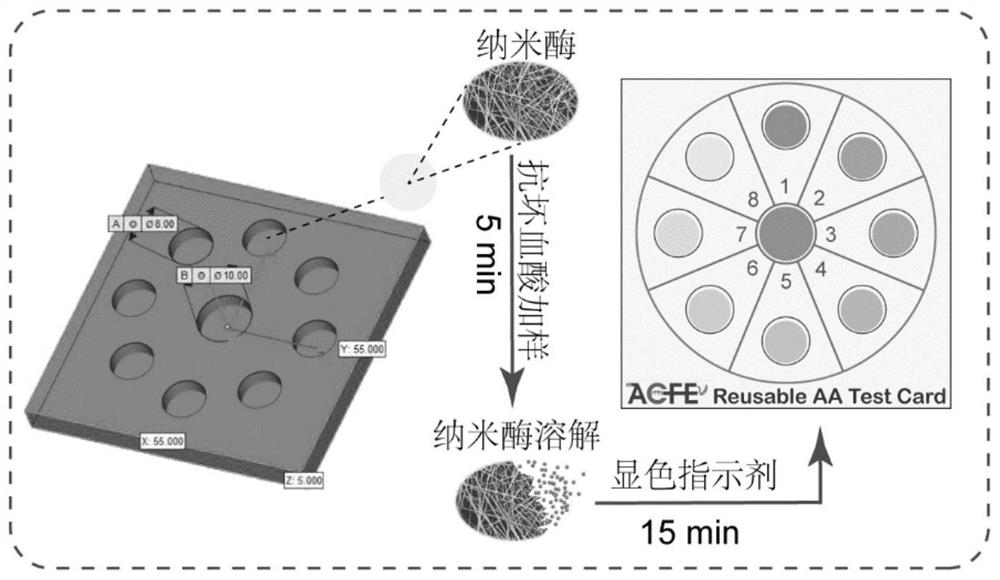
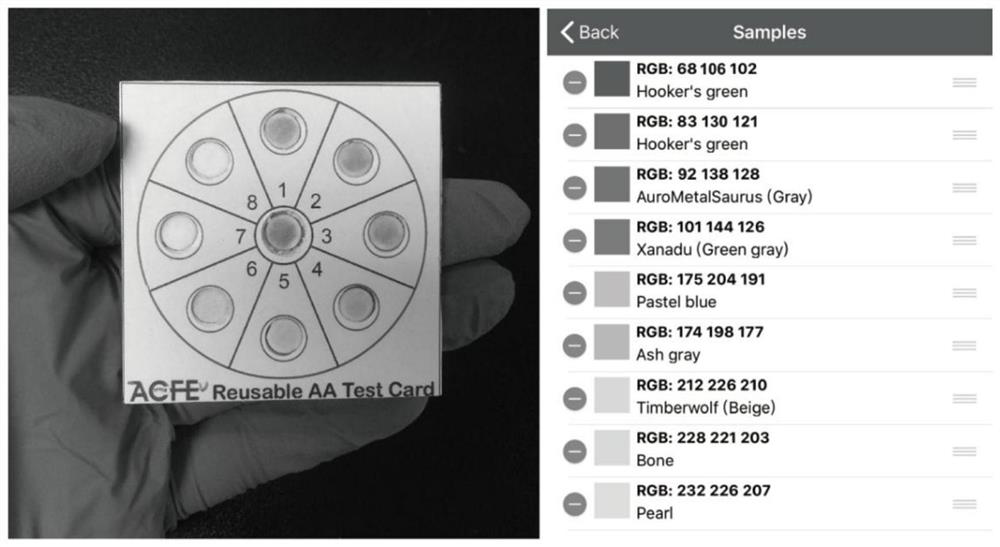
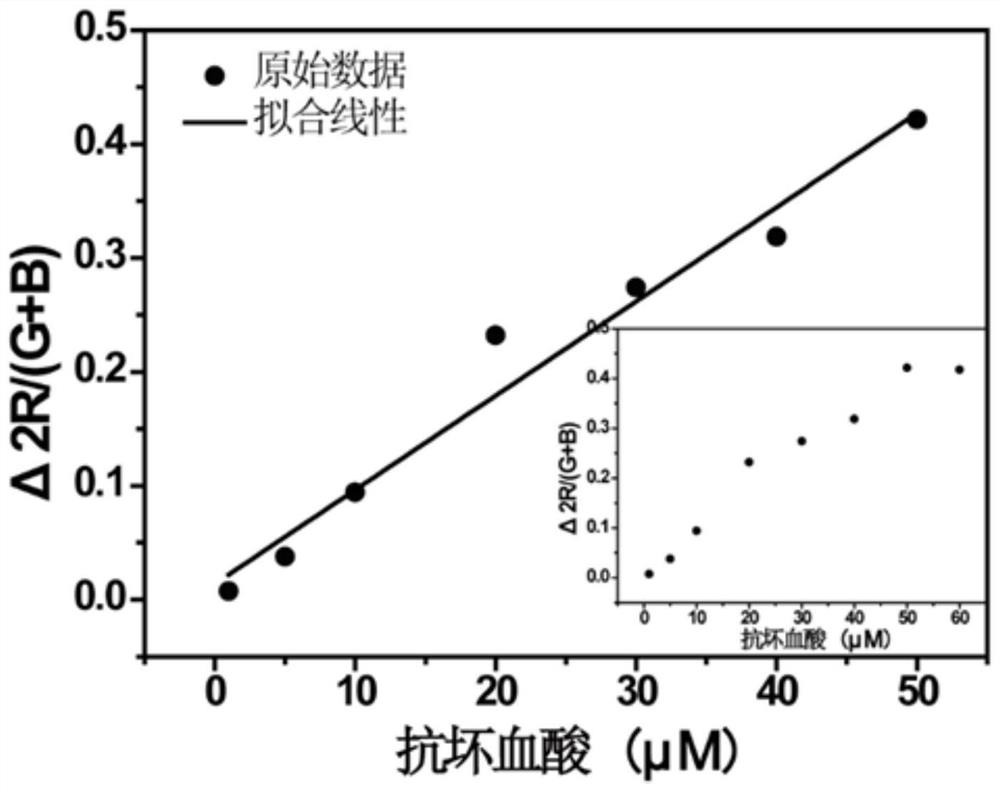
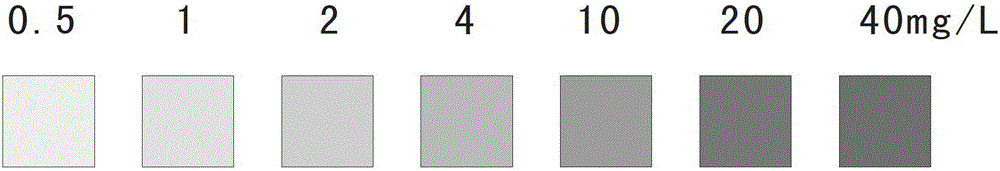
Ascorbic acid colorimetric detection method based on biodegradability nano-enzyme
ActiveCN109596606ARealize the operationRapid determinationMaterial analysis by observing effect on chemical indicatorNanowireColor correction
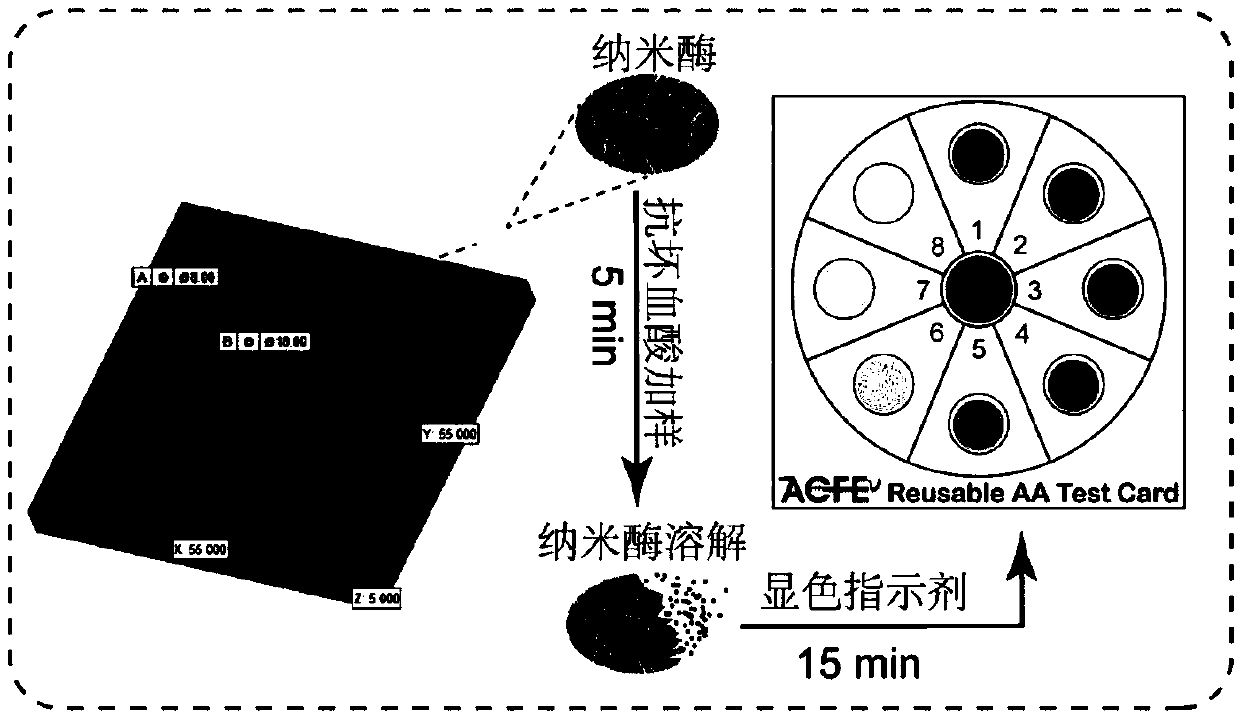
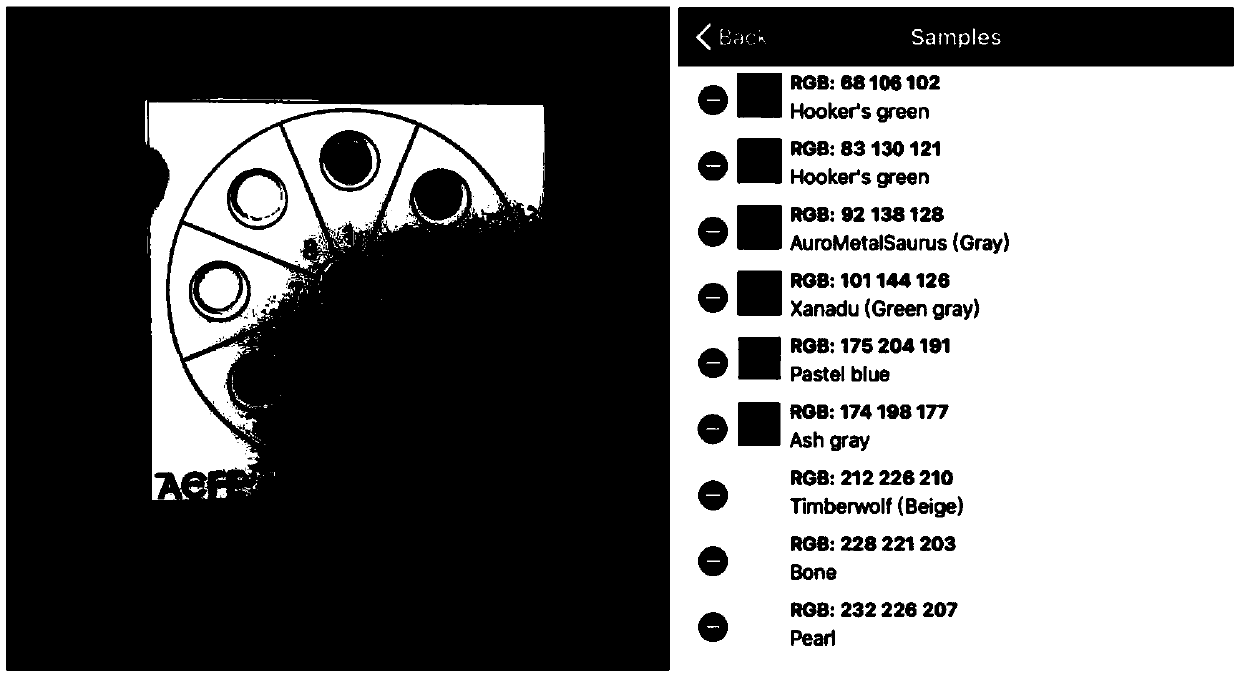
The invention discloses an ascorbic acid colorimetric detection method based on biodegradability nano-enzyme. On the basis of a principle that the degradation function of enzyme catalysis and ascorbicacid for the enzyme is simulated on the basis of a hydroxyl nanowire is simulated, the method comprises the following steps that: preparing degradable nano-enzyme test paper sheets, preparing detection cards, carrying out a sample dripping operation, carrying out the color extraction of the detection cards in sample tanks and a contrast tank, correcting the color evaluation values of the detection cards in the sample tanks, combining the color correction evaluation value with an ascorbic acid fitting model, and detecting a to-be-detected sample which contains ascorbic acid concentration. By use of the method, the degradable nano-enzyme is used for constructing a vision detection card used for measuring the ascorbic acid, and the test paper sheet is replaced and reused to realize the quickmeasurement of the ascorbic acid. Compared with the prior art, the method has a low requirement on detection equipment, and a detection process can be finished only by the detection card and a smartphone.
Owner:SOUTH CHINA UNIV OF TECH
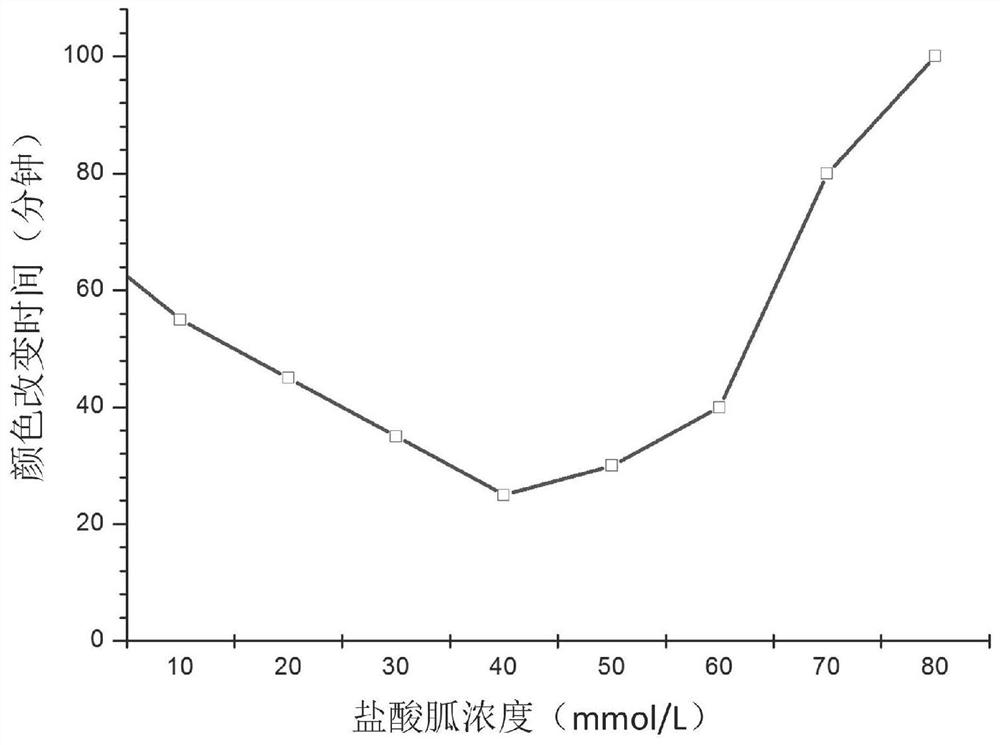
Water bath pot for intermediate-temperature enzyme detection
ActiveCN112934292AImprove insulation effectNot easy to polluteWater/sand/air bathsEngineeringStructural engineering
The invention discloses a water bath pot for medium-temperature enzyme detection. The water bath pot comprises a shell, a placement cylinder, a heat preservation pot body, a blocking ring, a pressing ring, a downpipe, a mounting groove, a water receiving box and a control device, at least one placing cylinder is arranged on the shell; the upper end of the placing cylinder is open, a blocking ring is arranged on the inner wall of the placing cylinder, and a downpipe is connected to the bottom wall of the placing cylinder; a mounting groove is formed in the shell, and a water receiving box capable of being pulled out is arranged in the mounting groove; the water outlet end of the downpipe is communicated with the mounting groove and is positioned above the water receiving box; the heat preservation pot body is inserted into the containing cylinder, a plurality of stopping blocks are arranged on the outer wall of the heat preservation pot body, and the stopping blocks are erected on the stopping ring; a pressing ring is screwed on the inner wall of the containing cylinder and presses the stopping block. According to the invention, the heat preservation pot body is inserted into the containing barrel, and the downpipe is arranged at the bottom of the containing barrel, so that water overflowing or leaking from the interior of the heat preservation pot body can be effectively collected, the environment is not prone to being polluted, and use is safer.
Owner:JIAXING ZHONGXIN MEDICAL INSTR
Preparation process of non-enzyme detection dopamine metal detector
PendingCN114594149AKnow your healthRepetitiveFinal product manufactureMaterial analysis by electric/magnetic meansHuman bodyPotassium hydroxide
The invention belongs to the technical field of biological detection, and particularly relates to a preparation process of a non-enzyme detection dopamine metal detector, which comprises the following steps: step 1, selecting two or more different metals, immersing the metals in highly corrosive liquid, such as concentrated nitric acid, concentrated sulfuric acid, concentrated hydrochloric acid and sodium hydroxide, and stirring for 30-60 minutes; in a single or mixed solution such as potassium hydroxide and the like, the preparation process of the metal detector for non-enzyme detection of dopamine has the beneficial effects that the content of dopamine in human body fluid / blood can be accurately detected, and direct or potential people who need to monitor the content of dopamine in the body fluid are helped to immediately and continuously detect the content of dopamine in the body fluid; the non-invasive in-vitro detection system has the advantages of high accuracy and repeatability, reusability, no pain, continuous monitoring, real-time treatment, simplicity in operation, environmental friendliness and the like, and has a good promotion effect on the field of non-invasive in-vitro detection.
Owner:南京子麒舜生物科技有限公司
A colorimetric assay for ascorbic acid based on degradable nanozymes
ActiveCN109596606BRealize the operationRapid determinationMaterial analysis by observing effect on chemical indicatorColor correctionEngineering
The invention discloses an ascorbic acid colorimetric detection method based on biodegradability nano-enzyme. On the basis of a principle that the degradation function of enzyme catalysis and ascorbicacid for the enzyme is simulated on the basis of a hydroxyl nanowire is simulated, the method comprises the following steps that: preparing degradable nano-enzyme test paper sheets, preparing detection cards, carrying out a sample dripping operation, carrying out the color extraction of the detection cards in sample tanks and a contrast tank, correcting the color evaluation values of the detection cards in the sample tanks, combining the color correction evaluation value with an ascorbic acid fitting model, and detecting a to-be-detected sample which contains ascorbic acid concentration. By use of the method, the degradable nano-enzyme is used for constructing a vision detection card used for measuring the ascorbic acid, and the test paper sheet is replaced and reused to realize the quickmeasurement of the ascorbic acid. Compared with the prior art, the method has a low requirement on detection equipment, and a detection process can be finished only by the detection card and a smartphone.
Owner:SOUTH CHINA UNIV OF TECH
A kind of rapid determination method of peracetic acid residual
ActiveCN103439322BHigh sensitivityHigh detection sensitivityMaterial analysis by observing effect on chemical indicatorBiochemical engineeringEnzyme test
The invention discloses a rapid determination method for residual peracetic acid, which is a fast response time, high sensitivity, good stability, simple operation, economical and practical method capable of rapidly detecting the content of residual peracetic acid in a sample on site , the minimum detectable peracetic acid content is 0.5mg / L. The pH regulator in the test paper used in the method of the present invention ensures the accuracy of the test results; the enzyme protective agent in the test paper ensures the stability of the enzyme test paper, and the shelf life can reach at least 12 months; the area of the test paper used is small, and the test process is simple, There is no need for other instruments and accessories, which can greatly save the cost of testing; the bottom plate of the test paper can prevent contamination by human hands during the process of taking it, which is very safe and reliable.
Owner:GUANGDONG HUANKAI MICROBIAL SCI & TECH
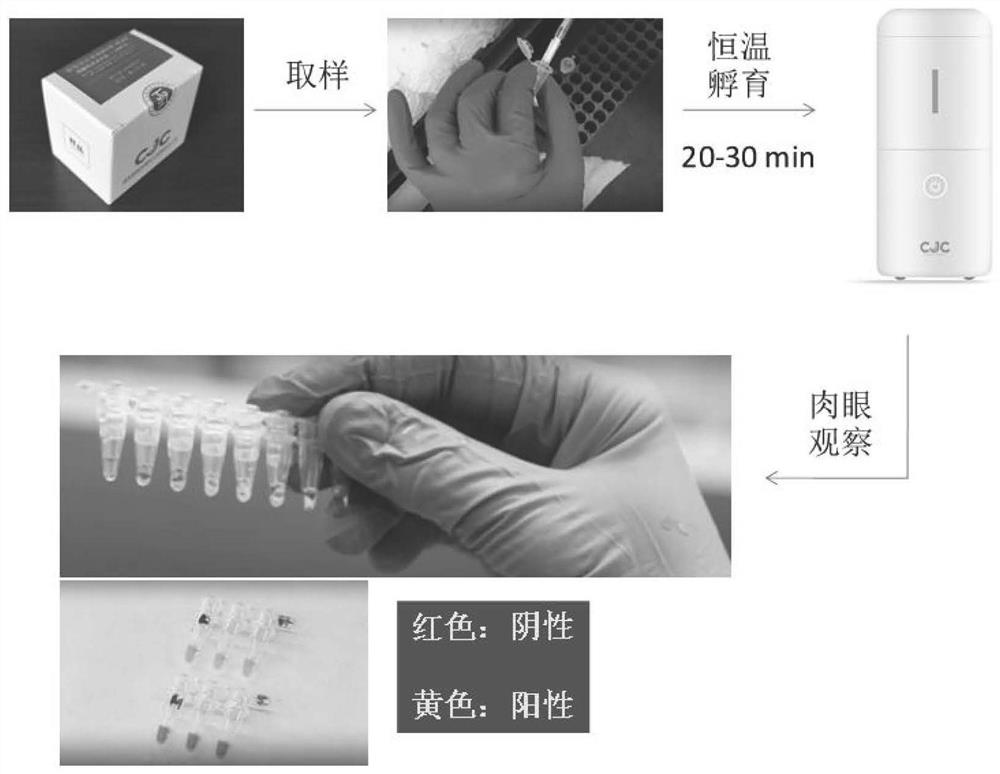
Primer group for one-step visual detection of novel coronavirus nucleic acid and kit thereof
ActiveCN112646932AEasy to operateAvoid pollutionMicrobiological testing/measurementAgainst vector-borne diseasesNucleotidePharyngeal swab
The invention provides a primer group for one-step visual detection of novel coronavirus and a kit thereof, and belongs to the technical field of novel coronavirus detection, the primer group comprises F3, B3, FIP-1, BIP-1, FL-1 and BL-1; wherein the nucleotide sequences of the F3, the B3, the FIP-1, the BIP-1, the FL-1 and the BL-1 are respectively as shown in SEQ ID No. 1 to SEQ ID No. 6; the kit comprises the primer group and a detection reagent, and the detection reagent comprises an enzyme detection mixed solution, guanidine hydrochloride and a pH indicator; wherein the enzyme detection mixed solution comprises Taq SSB. In the detection process of the primer group and the kit, nucleic acid extraction is not needed, and detection can be performed by adopting a nasal / pharyngeal swab sample soaked in an inactivated virus preservation solution; after virus RNA amplification, the color of the reaction solution is changed, and the result can be judged through naked eye observation.
Owner:HUNAN TARGETING DETECTION TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com