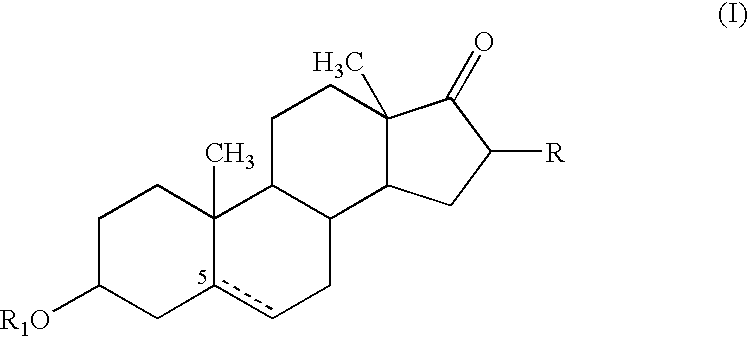
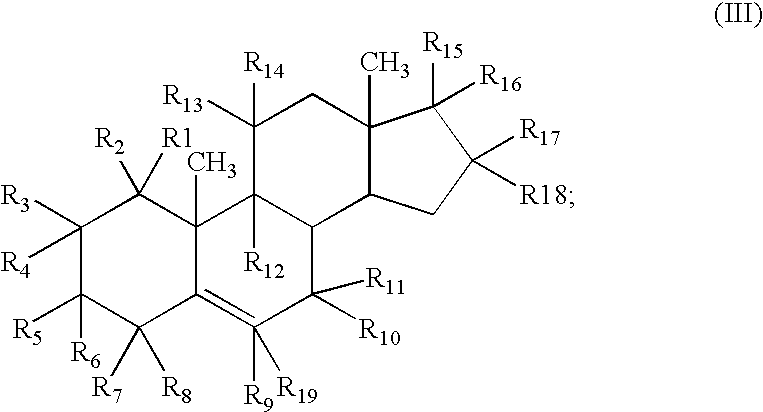
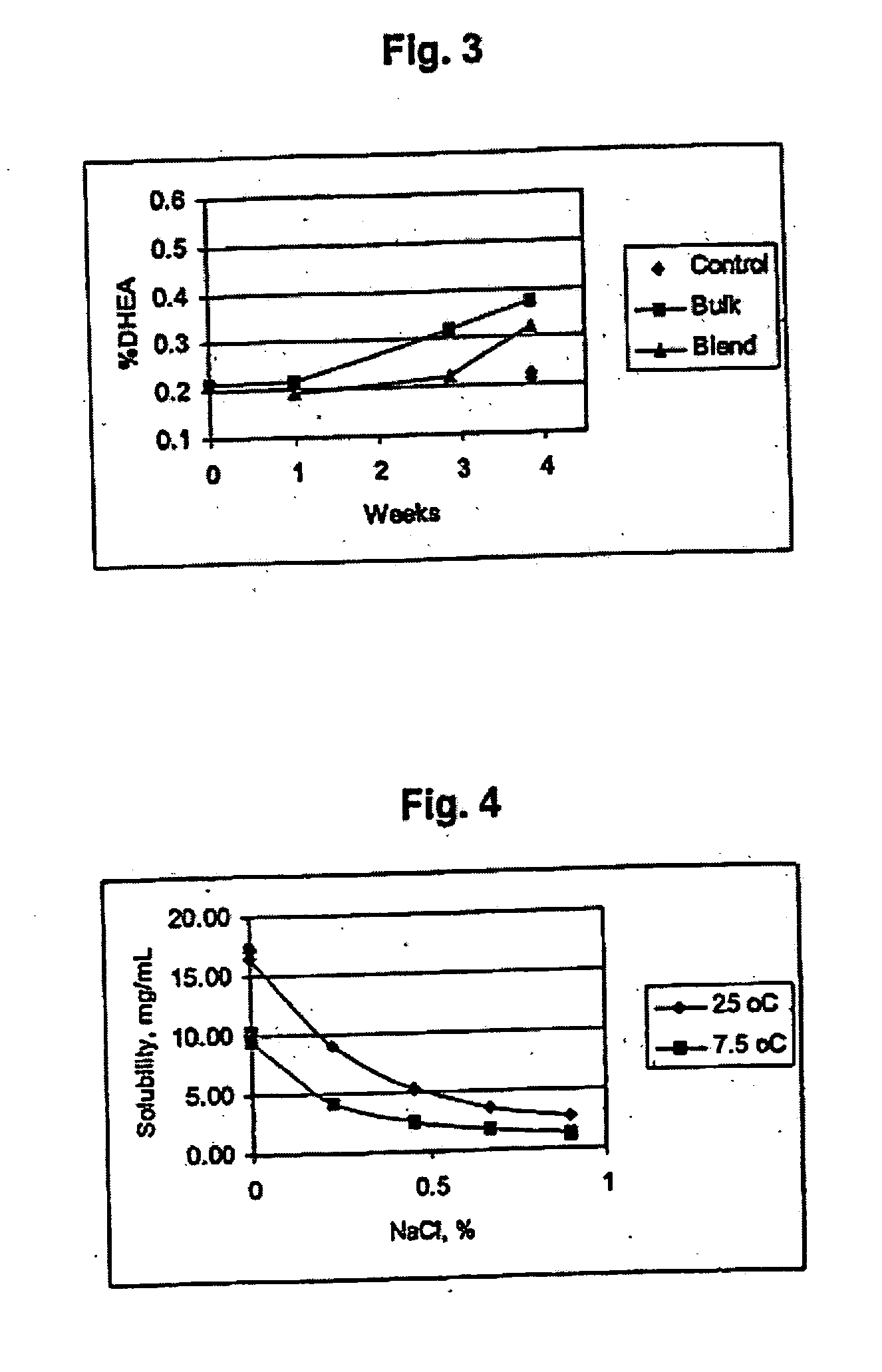
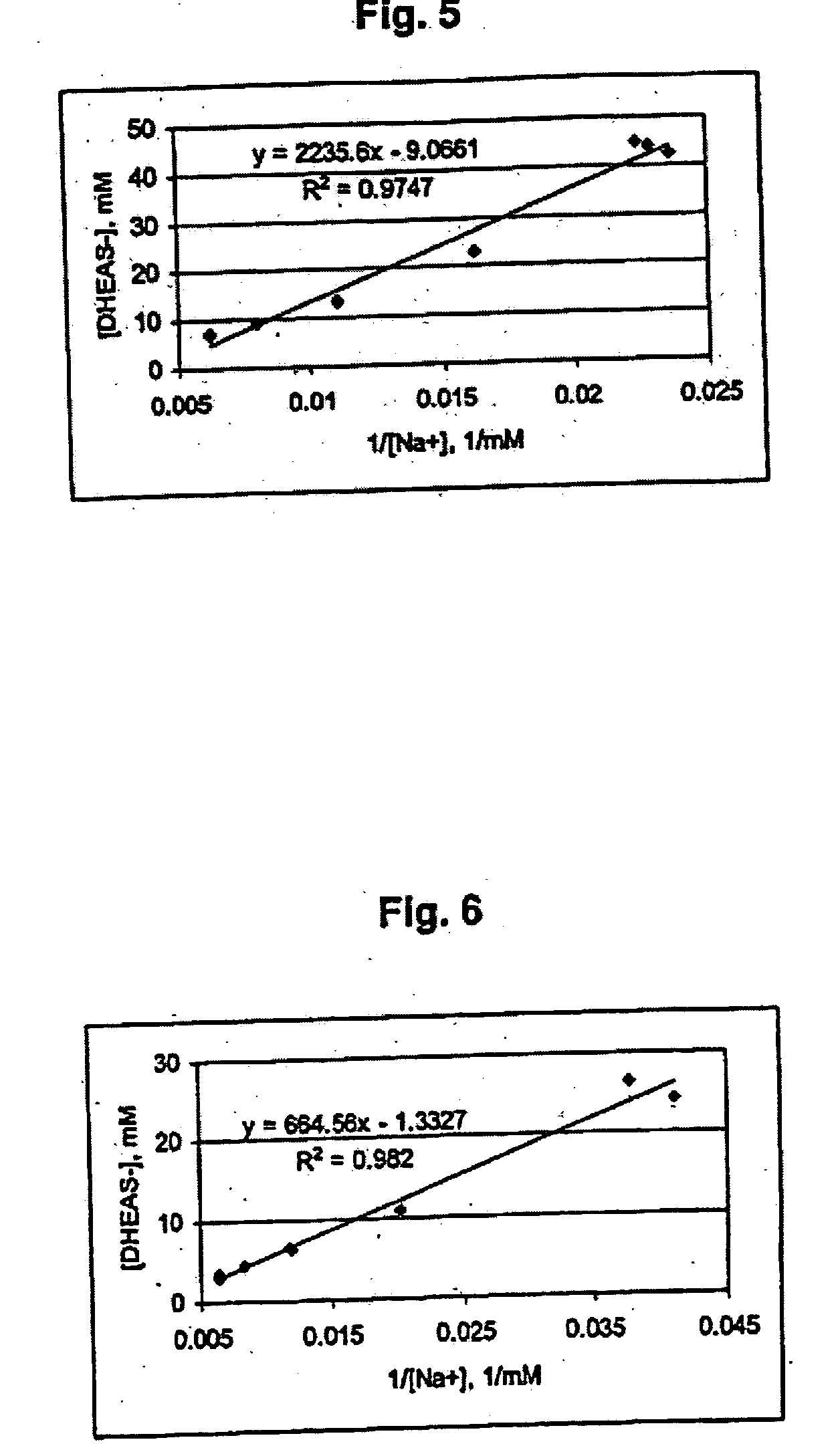
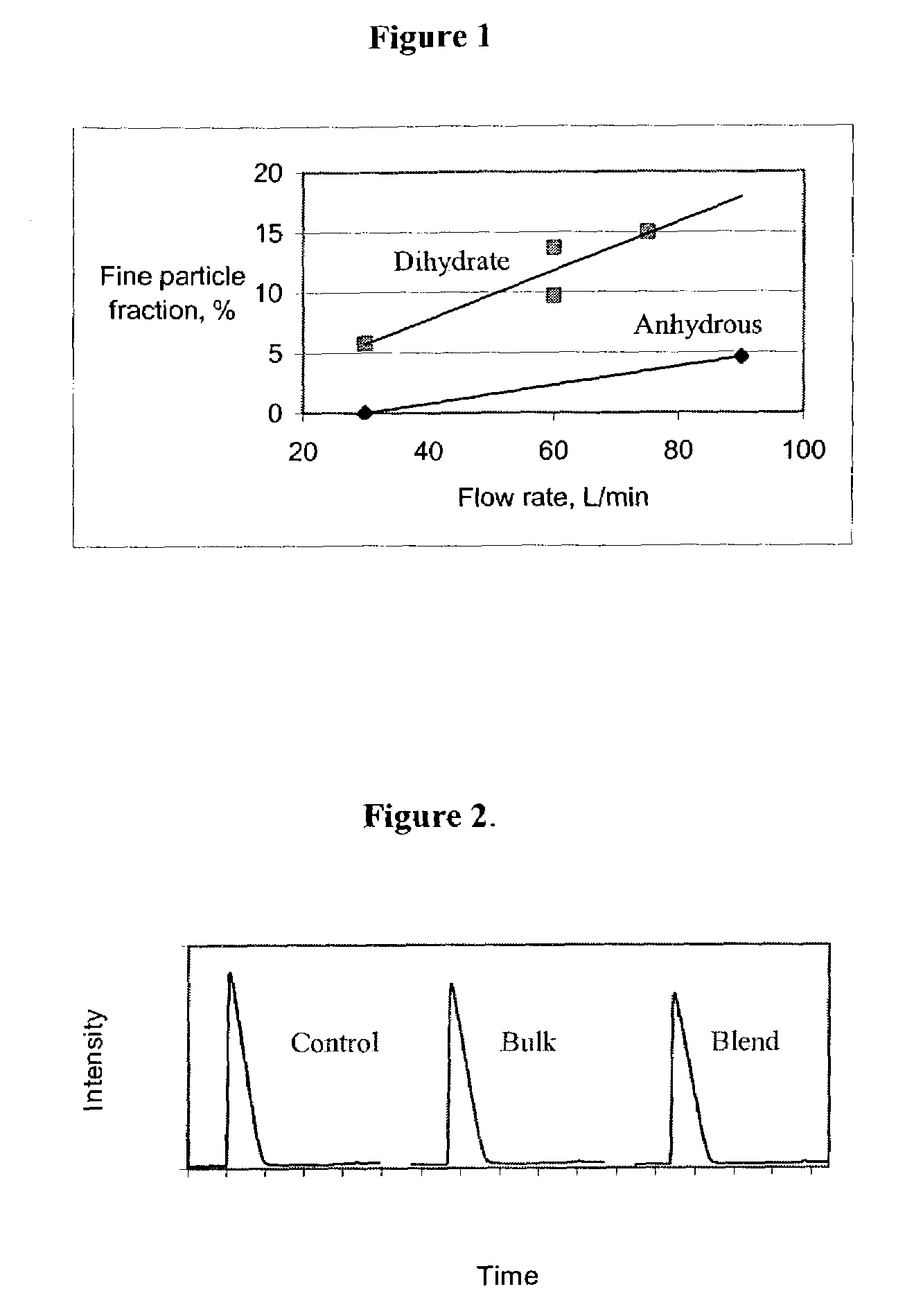
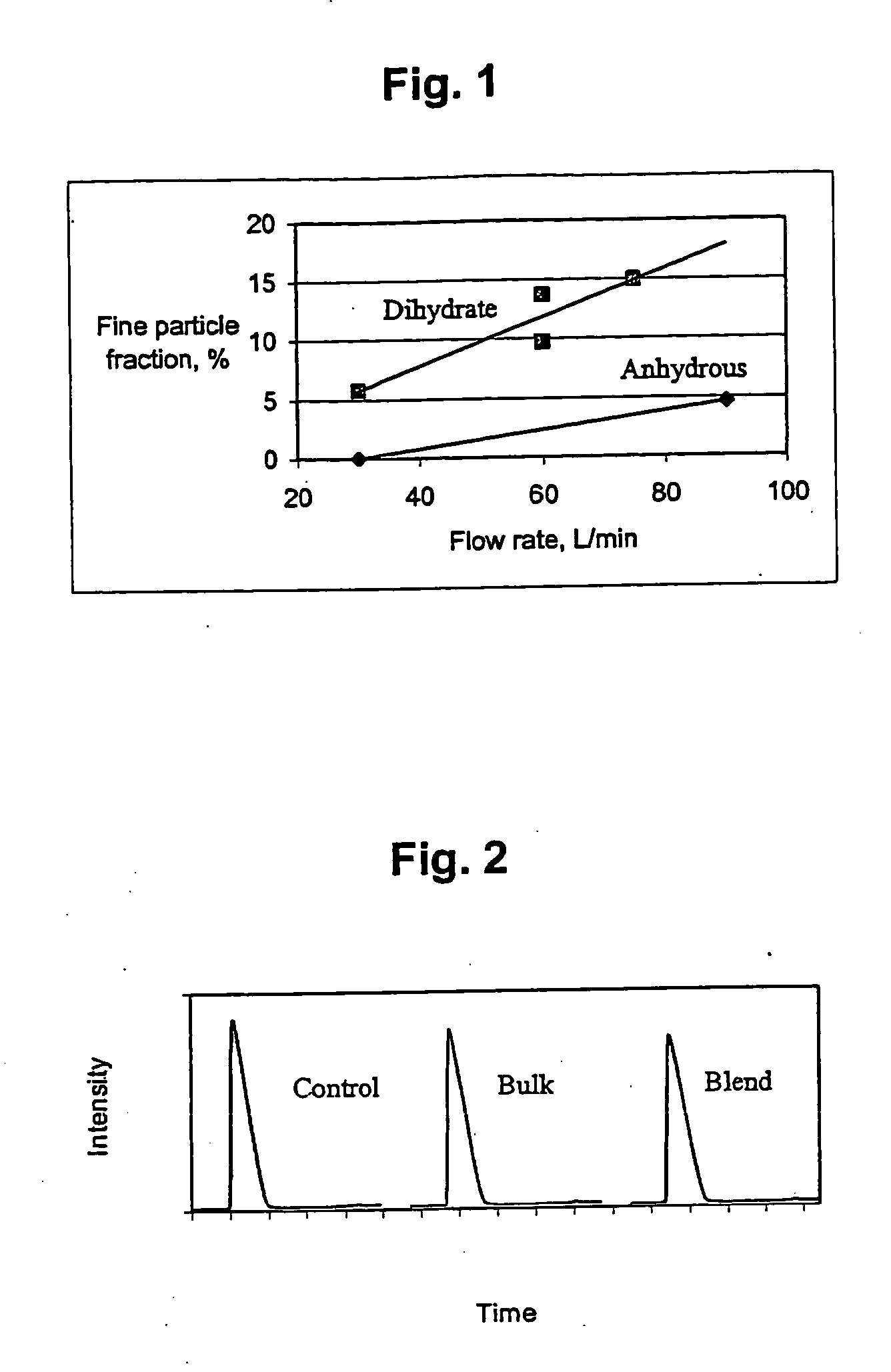
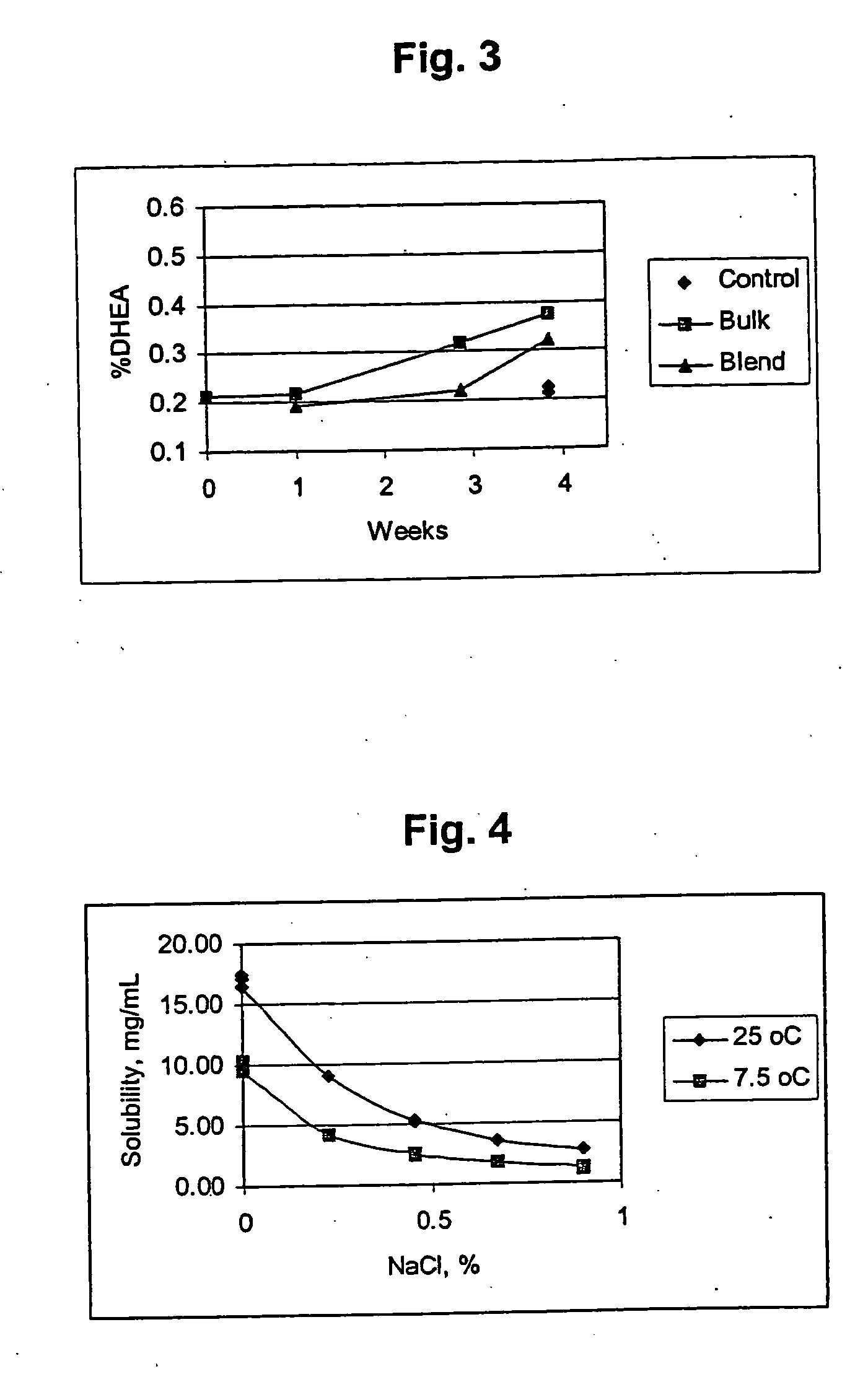
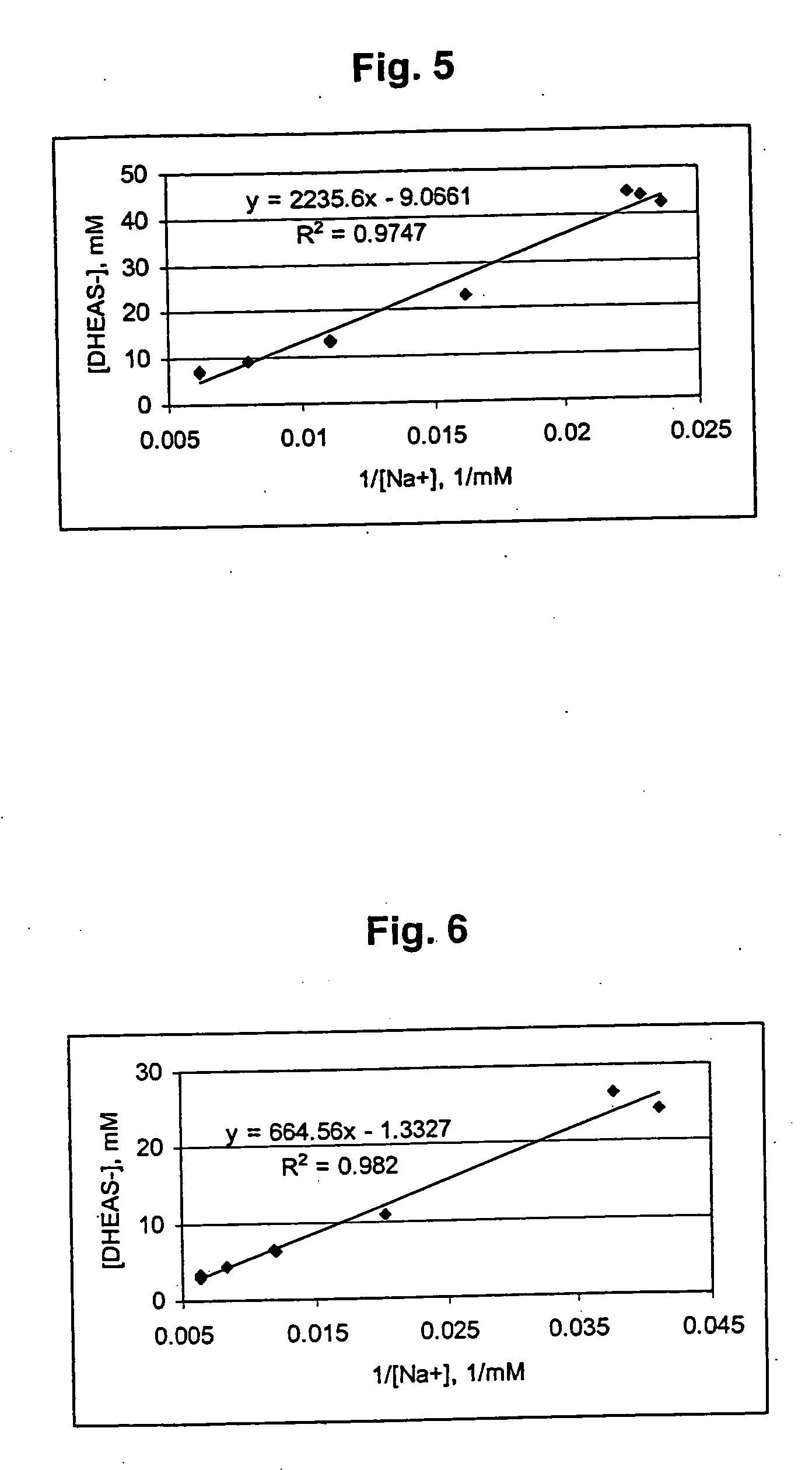
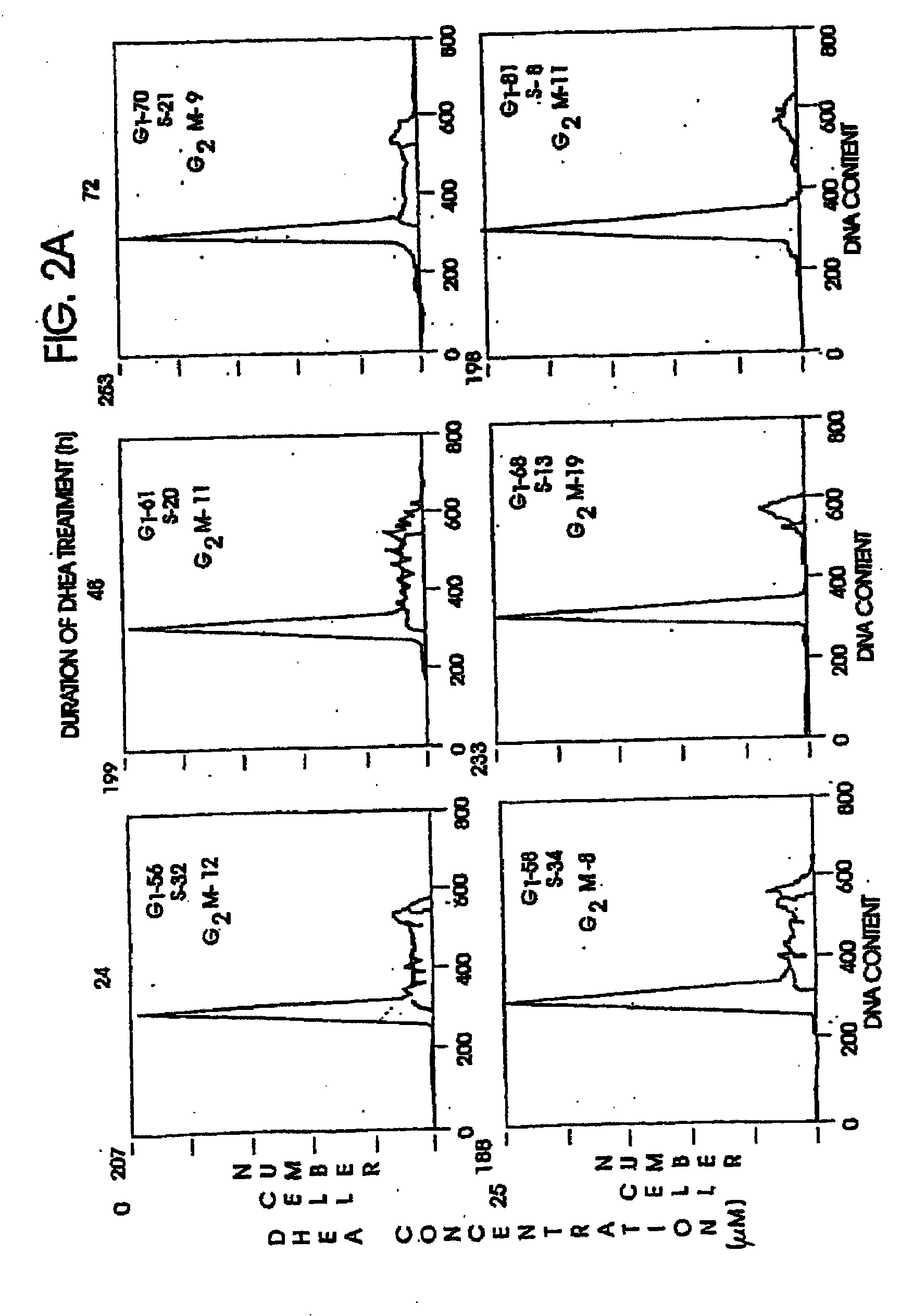
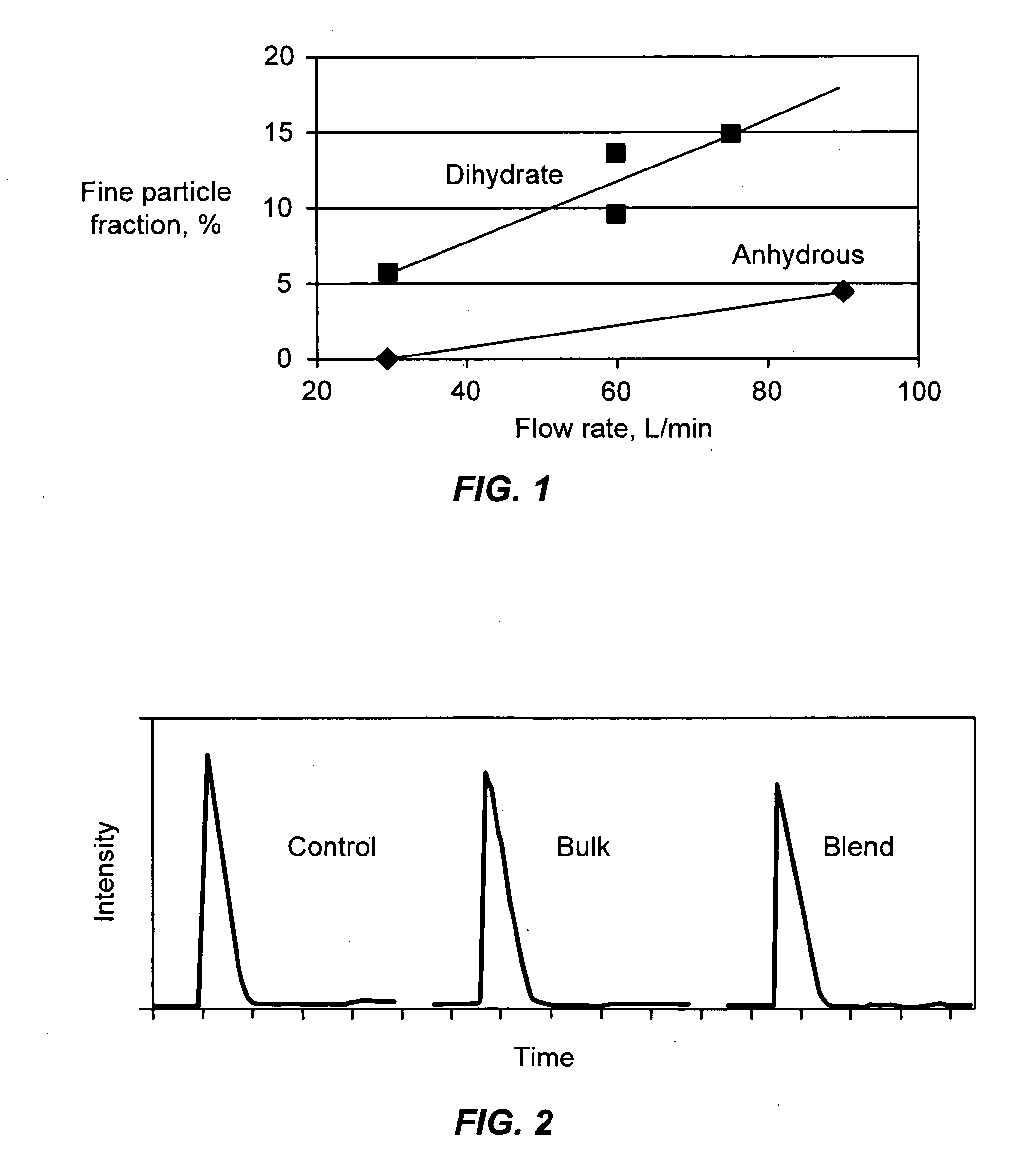
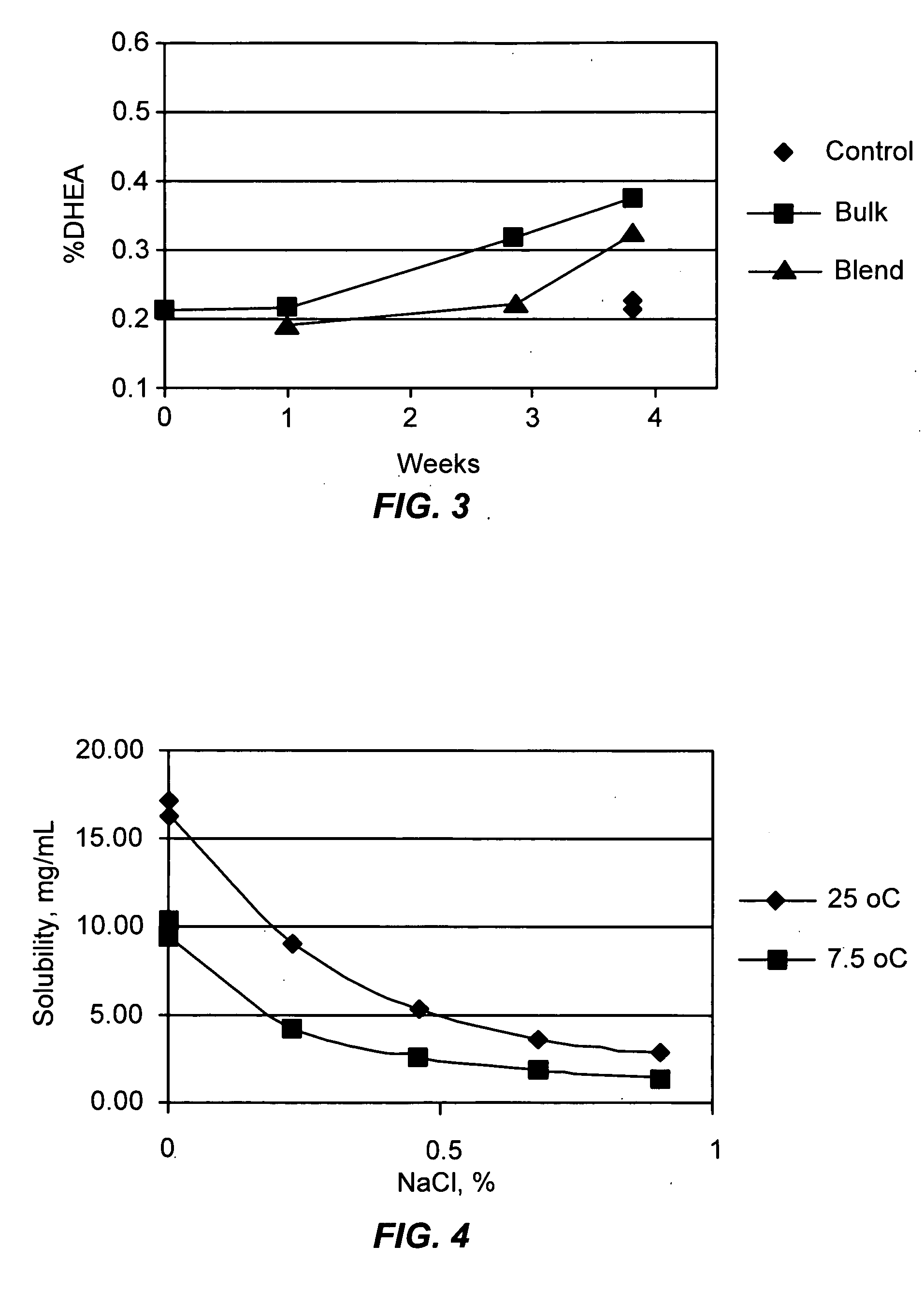
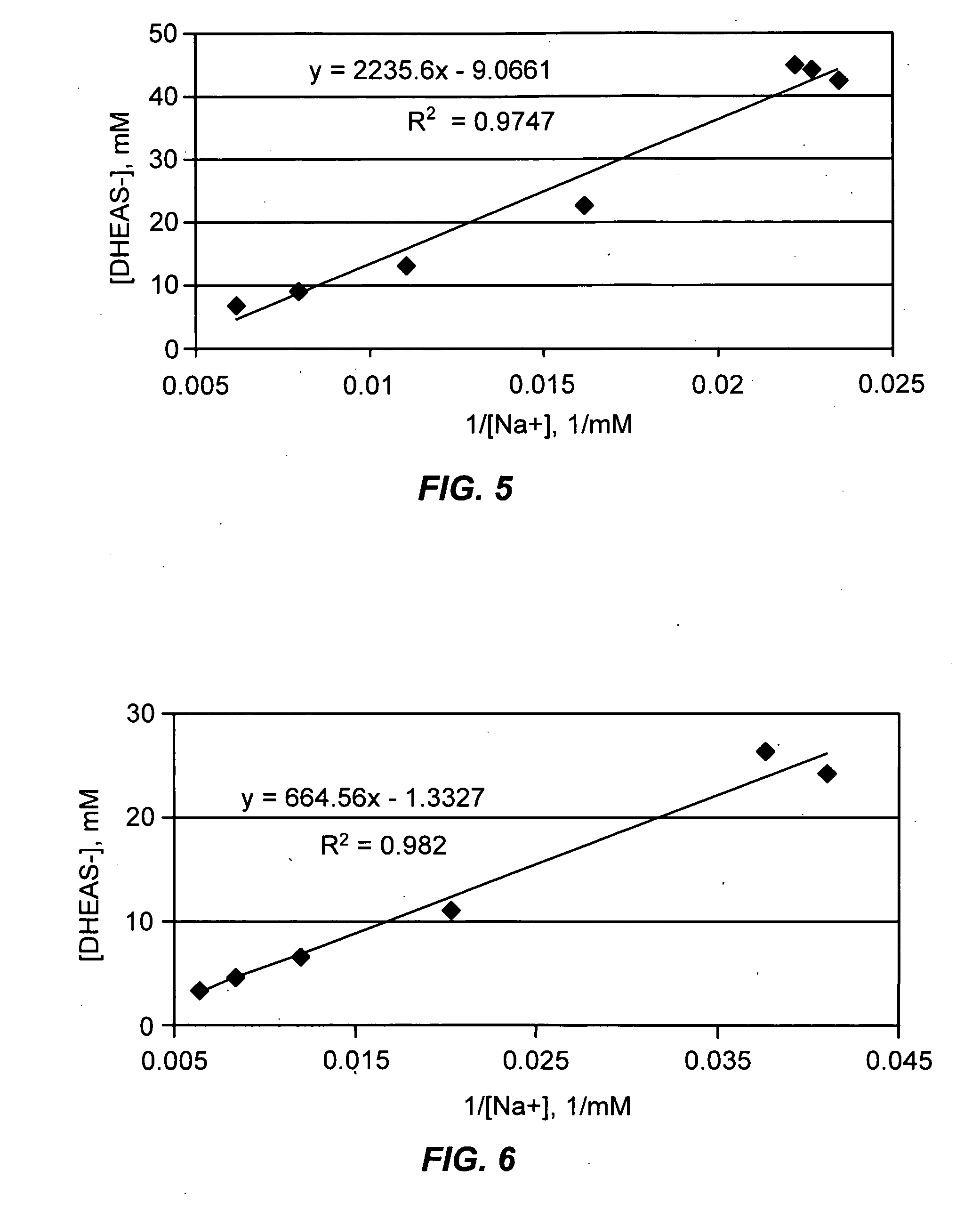
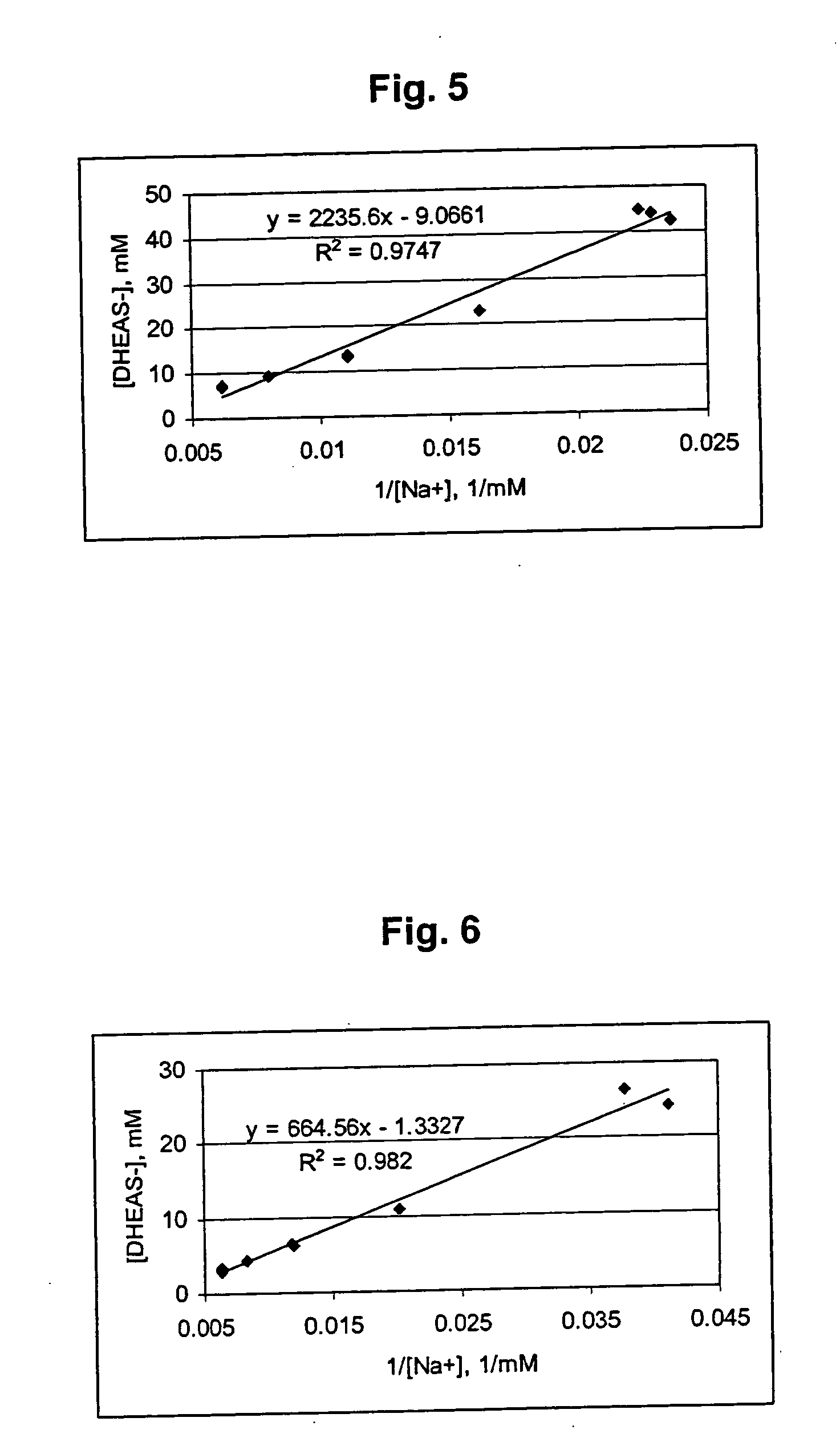
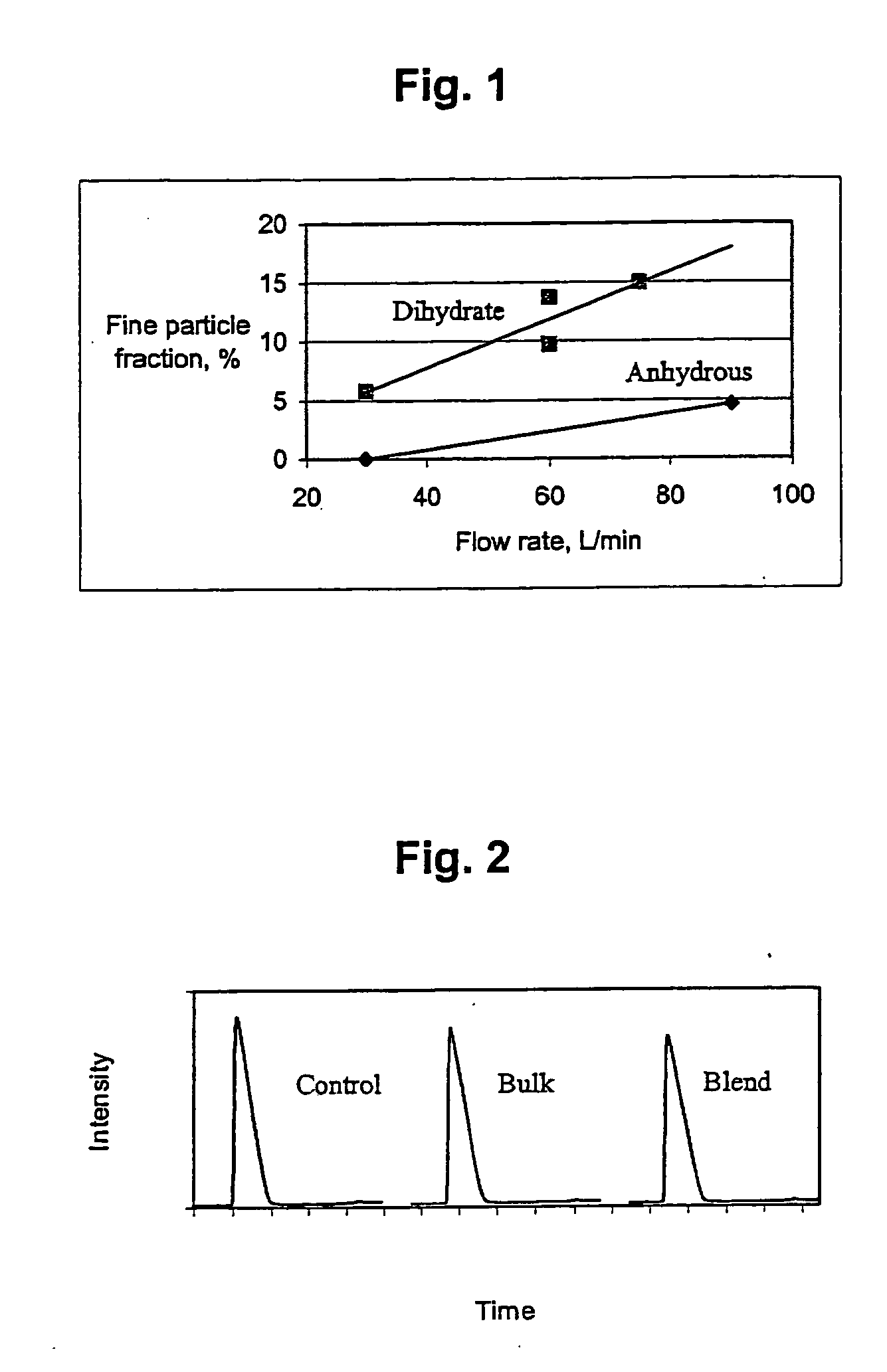
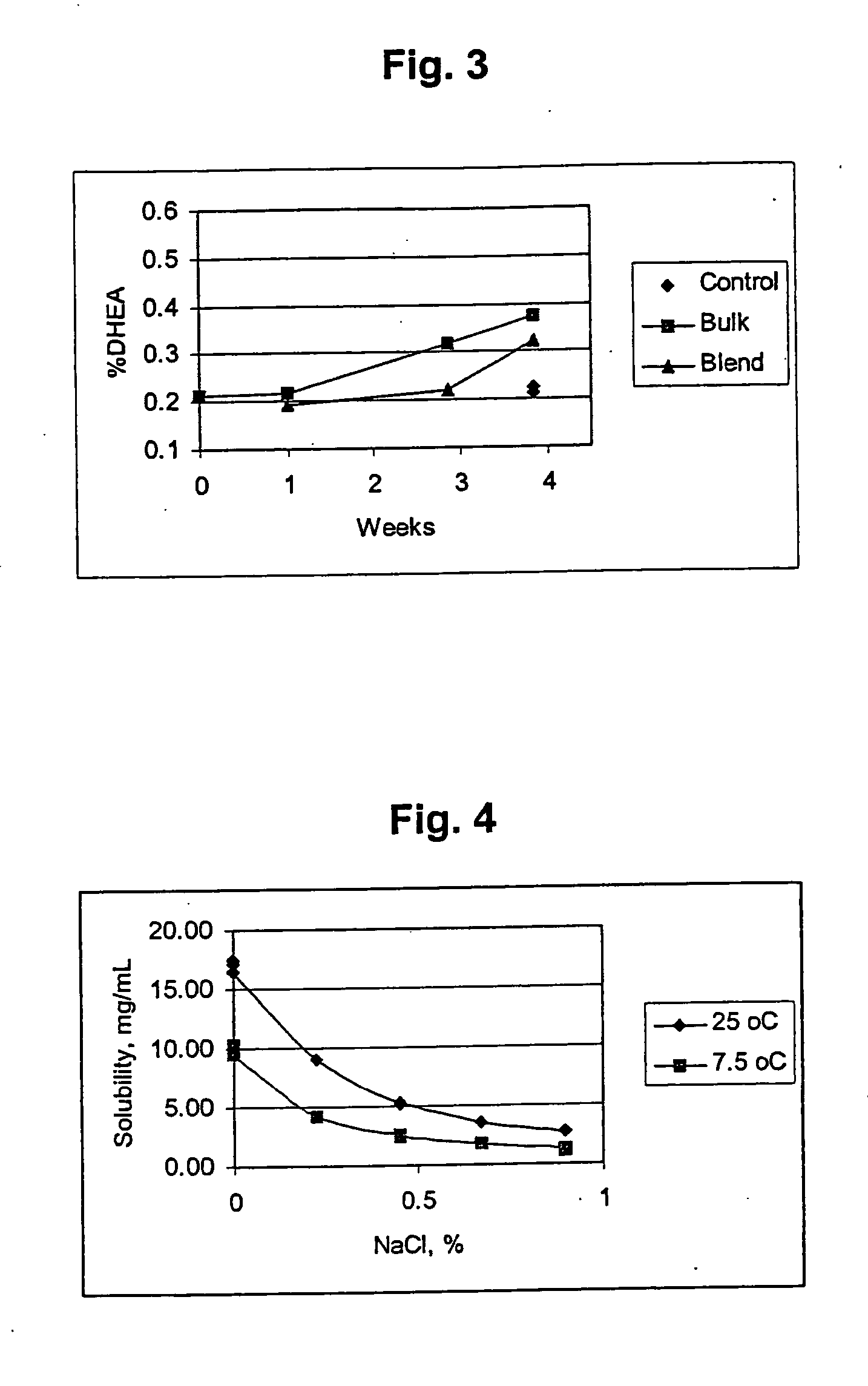
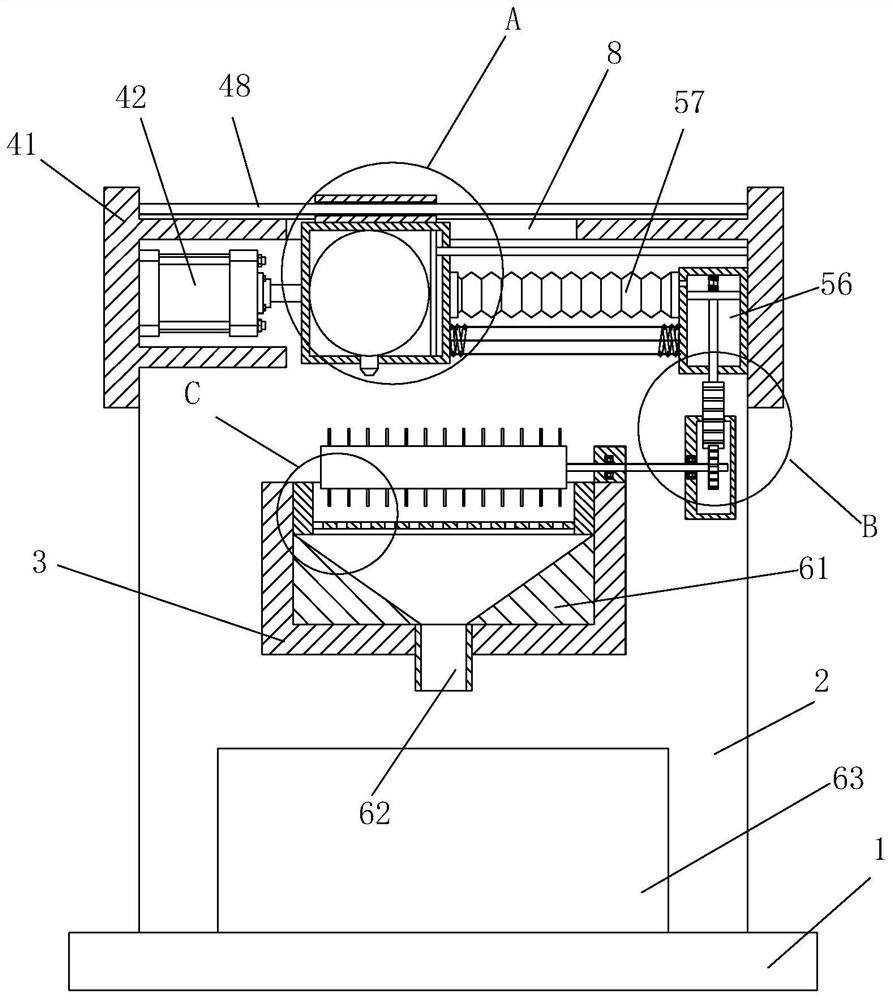
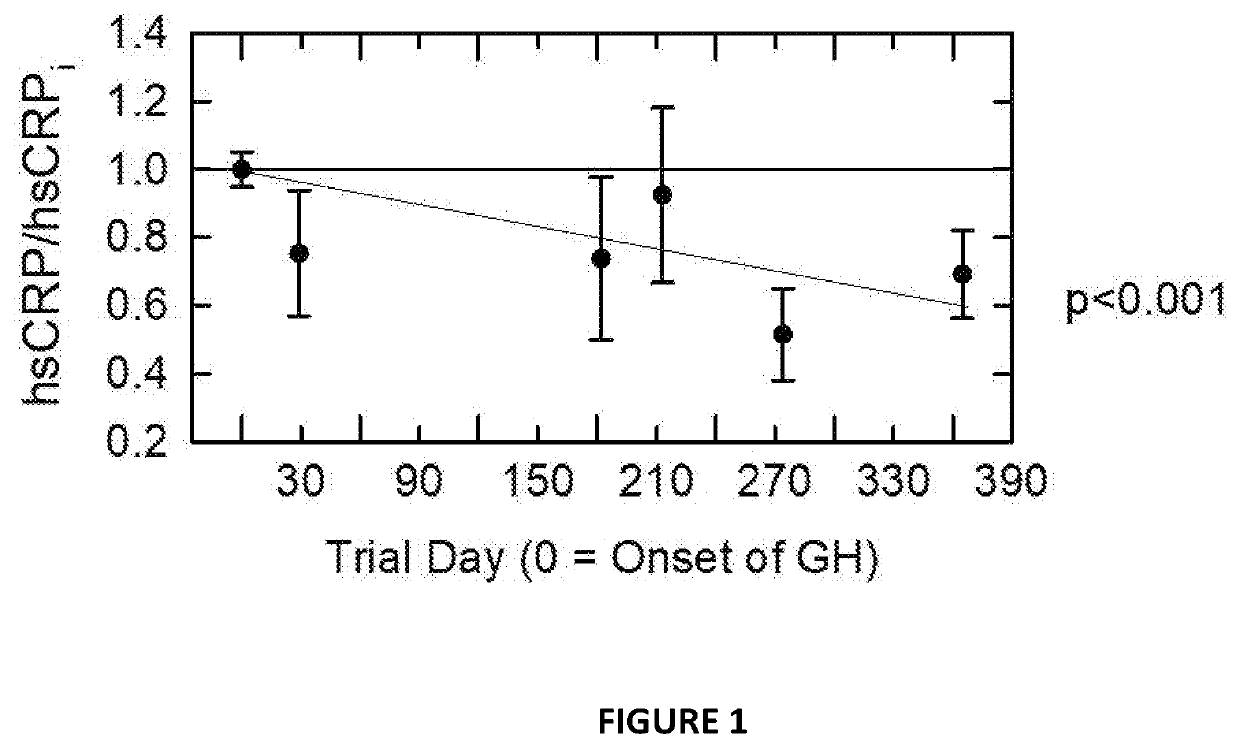
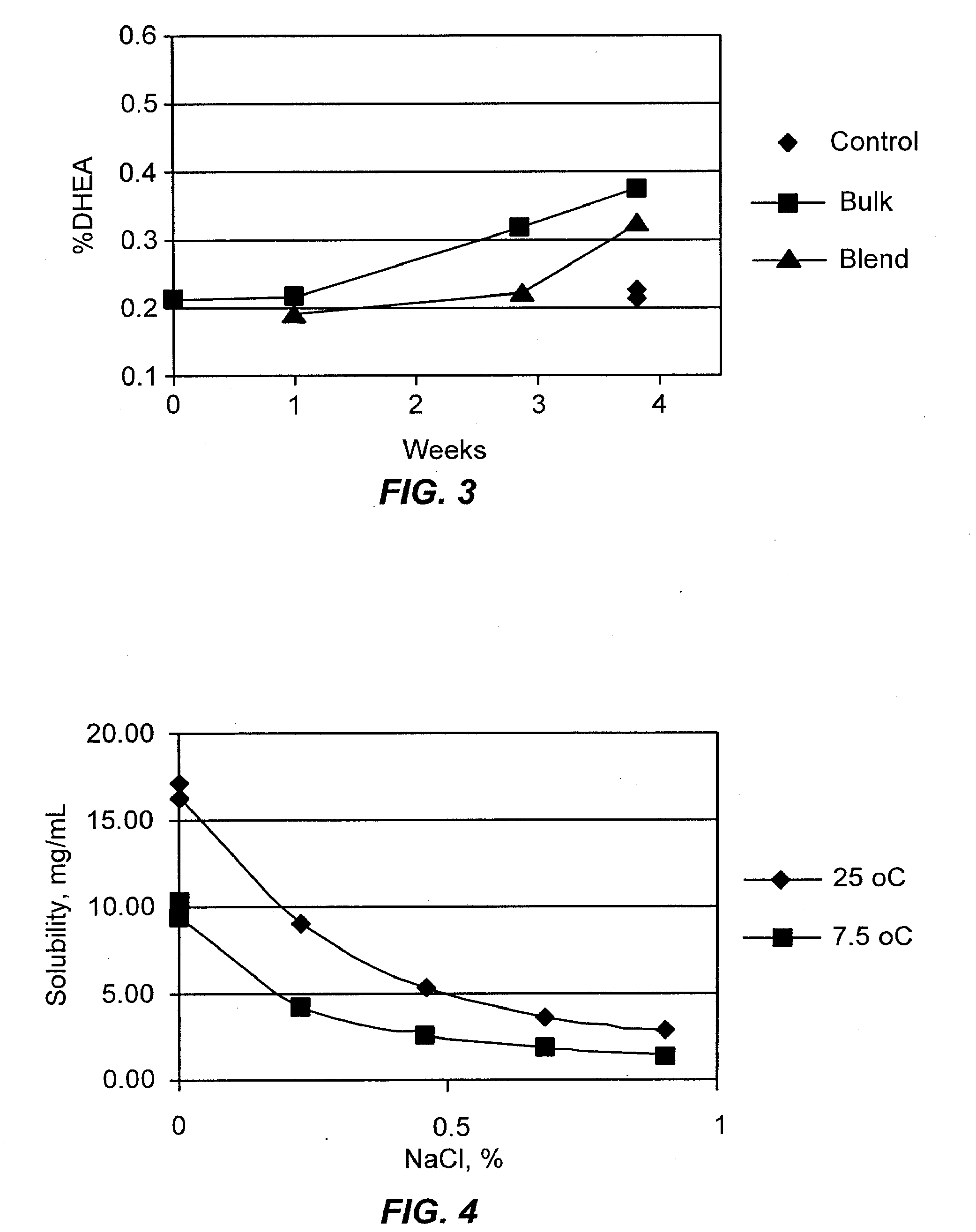
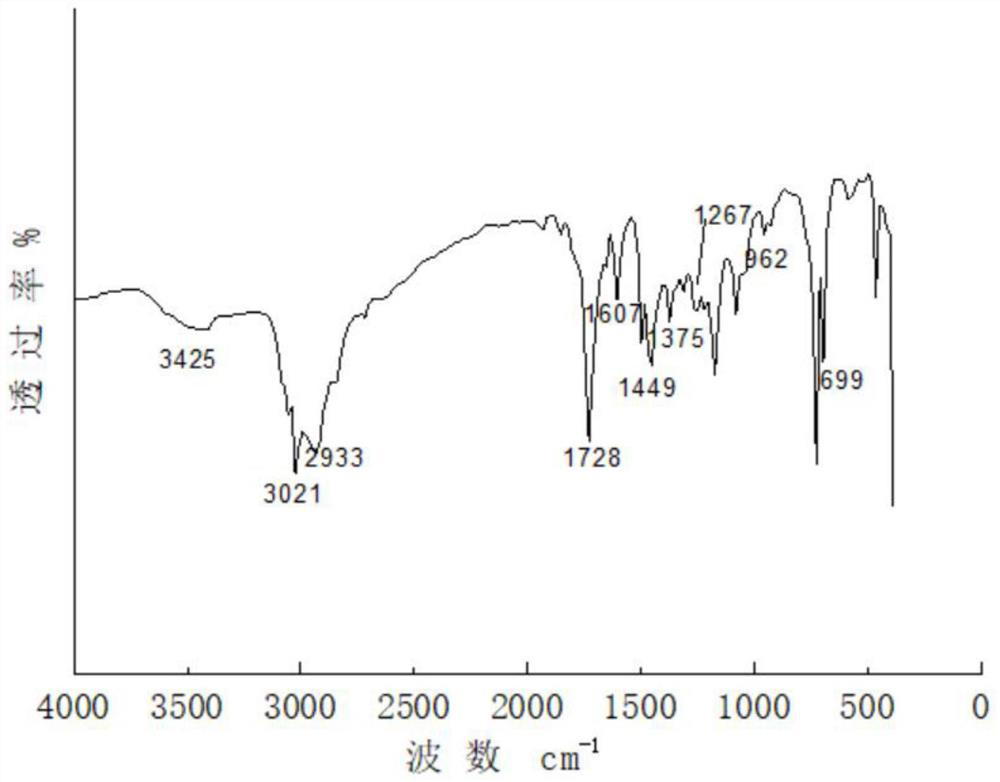
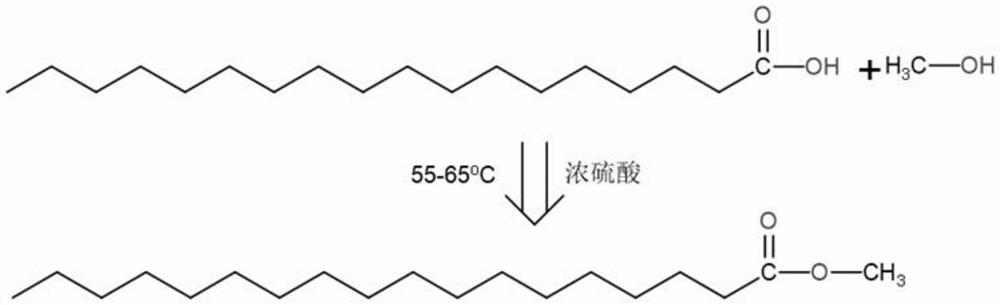
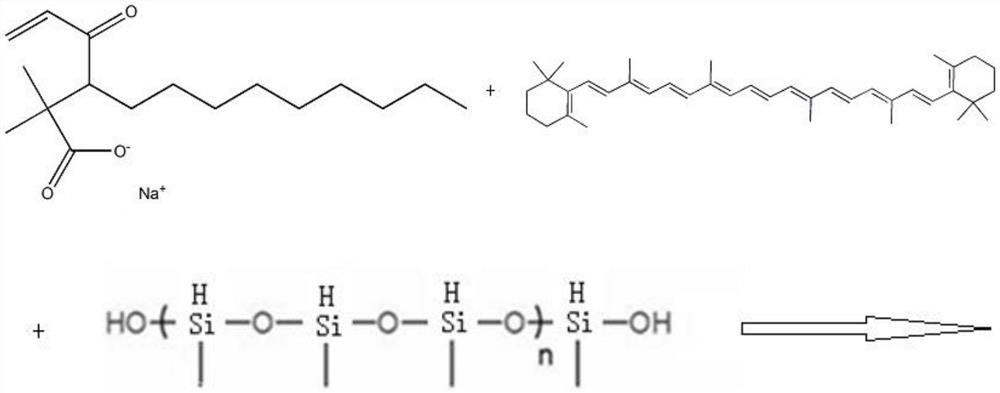
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "DHEA - Dehydroepiandrosterone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
DHEA (dehydroepiandrosterone) is a hormone produced by your body's adrenal glands. These are glands just above your kidneys . DHEA supplements can be made from wild yam or soy.
Combination of dehydroepiandrosterone or dehydroepiandrosterone-sulfate with an anti-IgE antibody for treatment of asthma or chronic obstructive pulmonary disease
A pharmaceutical or veterinary composition, comprises a first active agent selected from a dehydroepiandrosterone and / or dehydroepiandrosterone-sulfate, or a salt thereof, and a second active agent comprising an anti-IgE antibody for the treatment of asthma, chronic obstructive pulmonary disease, or any other respiratory disease. The composition is provided in various formulations and in the form of a kit. The products of this patent are applied to the prophylaxis and treatment of asthma, chronic obstructive pulmonary disease, or any other respiratory disease.
Owner:EPIGENESIS PHARMA LLC
Combination of dehydroepiandrosterone or dehydroepiandrosterone-sulfate with a tyrosine kinase inhibitor, delta opioid receptor antagonist, neurokinin receptor antagonist, or VCAM inhibitor for treatment of asthma or chronic obstructive pulmonary disease
InactiveUS20050026850A1Alleviate different aspectConvenient treatmentBiocideOrganic active ingredientsDiseaseActive agent
A pharmaceutical or veterinary composition, comprises a first active agent selected from a dehydroepiandrosterone and / or dehydroepiandrosterone-sulfate, or a salt thereof, and a second active agent comprising a tyrosine kinase inhibitor, delta opioid receptor antagonist, neurokinin receptor antagonist, or VCAM inhibitor for the treatment of asthma, chronic obstructive pulmonary disease, or other respiratory diseases. The composition is provided in various formulations and in the form of a kit. The products of this patent are applied to the prophylaxis and treatment of asthma, chronic obstructive pulmonary disease, or other respiratory diseases.
Owner:EPIGENESIS PHARMA LLC
Nebulizer formulations of dehydroepiandrosterone and methods of treating asthma or chronic obstructive pulmonary disease using compositions thereof
InactiveUS7405207B2Reduce and deplete adenosine levelReducing and depleting adenosineOrganic active ingredientsPowder deliveryDiseaseNebulizer
This invention relates to a sealed container containing a powder formulation comprising a dehydroepiandrosterone, its analogue(s) or salt(s) by itself or with a pharmaceutically or veterinarily acceptable carrier or diluent, and having a particle size of about 0.1 μm to about 100 μm. The formulation can be used to treat or prevent asthma, chronic obstructive pulmonary disease, lung inflammation, and other respiratory diseases or conditions. The formulation may be prepared by jet milling, and may be delivered through the respiratory tract or other routes using a nebulizer. The sealed container is provided in a device and / or a therapeutic kit.
Owner:EPIGENESIS PHARMA LLC
Combination of dehydroepiandrosterone or dehydroepiandrosterone-sulfate with an antihistamine for treatment of asthma or chronic obstructive pulmonary disease
InactiveUS20050026890A1Alleviate different aspectConvenient treatmentBiocideOrganic active ingredientsDiseaseActive agent
A pharmaceutical or veterinary composition, comprises a first active agent selected from a dehydroepiandrosterone and / or dehydroepiandrosterone-sulfate, or a salt thereof, and a second active agent comprising an antihistamine for the treatment of asthma, chronic obstructive pulmonary disease, or any other respiratory disease. The composition is provided in various formulations and in the form of a kit. The products of this patent are applied to the prophylaxis and treatment of asthma, chronic obstructive pulmonary disease, or any other respiratory disease.
Owner:EPIGENESIS PHARMA LLC
Composition and preparation for resisting ageing and improving male energy, preparation method of preparation and application of composition
InactiveCN109045059AReasonable compositionAppropriate compatibilityOrganic active ingredientsInorganic active ingredientsSexual functionProtopanaxadiol
The invention discloses a composition for resisting ageing and improving male energy. The composition is prepared from a raw material and an auxiliary material; the raw material is prepared from the following components in parts by weight: 1-15 parts of nicotinamide mononucleotide, 1-10 parts of protopanoxadiol, 1-9 parts of icarisid I, 2-8 parts of baohuoside I, 3-7 parts of dehydroepiandrosterone and 2-10 parts of ursodesoxycholic acid. The composition is reasonable in composing prescription and proper in compatibility; in the composition, the four of the nicotinamide mononucleotide (NMN), the icarisid I, the baohuoside I and protopanoxadiol are used as monarch drugs together, the composition is capable of comprehensively regulating gonad axis and invigorating kidney-yang and liver, candelay ageing period of the gonad axis and improve the sexual function after being taken for a long time; through intercoordination and synergistic effect of various components, the composition is comprehensive in nutrition, has very good palatability, is particularly suitable for males to take and thus relieving the symptoms of male ageing syndrome and improving the male energy. The invention alsoprovides a preparation containing the composition and a preparation method of the preparation. The preparation method is simple, is moderate in condition and is suitable for industrial batch production.
Owner:HOBOOMLIFE BIO TECH SHENZHEN CO LTD
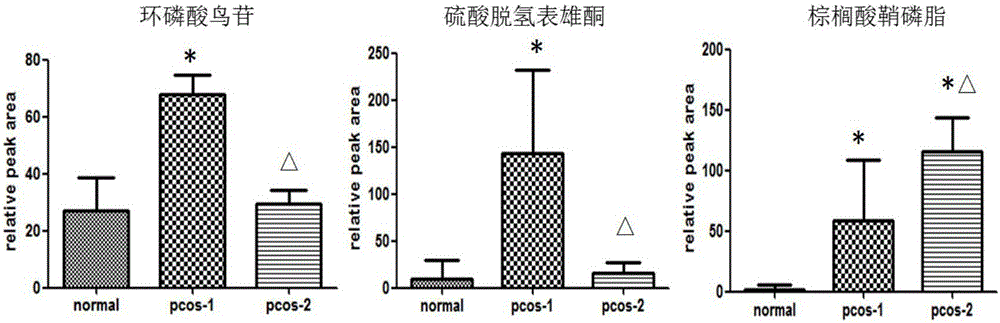
Diagnosis and/or typing marker for PCOS (polycystic ovarian syndrome) and application of preparation reagent
ActiveCN106442764AEasy diagnosisSolve complexityComponent separationDehydroepiandrosterone sulfateIndex system
The invention discloses a diagnosis and / or typing marker for the PCOS (polycystic ovarian syndrome) and an application of the preparation reagent and particularly relates to an application of cyclic guanosine monophosphate, dehydroepiandrosterone sulfate, palm sphingomyelin combined HDL-C (high-density lipoprotein cholesterol) and the left follicle number as the diagnosis and / or typing marker for the PCOS. Compared with existing PCOS diagnosis clinical indexes, the marker can realize the effect of distinguishing a PCOS subgroup I and a PCOS subgroup II through combined diagnosis of cyclic guanosine monophosphate, dehydroepiandrosterone sulfate and palm sphingomyelin. In combination of combined diagnosis of HDL-C (high-density lipoprotein cholesterol) and the left follicle number, the very high diagnosis accuracy is realized for a normal group, the PCOS subgroup I and the PCOS subgroup II, and accurate and effective index systems are provided for clinical disease diagnosis.
Owner:王义明
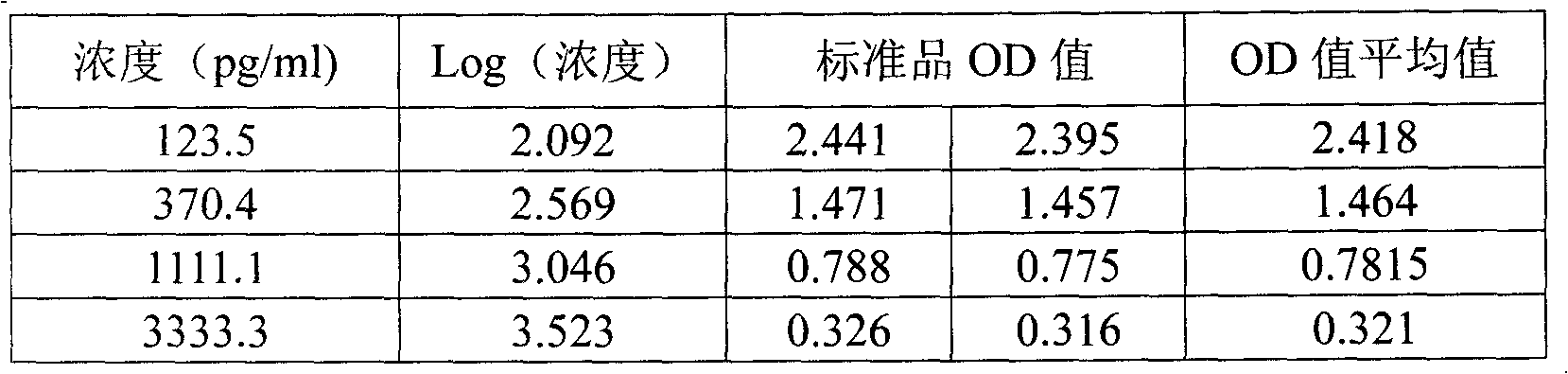
Dehydroepiandrosterone enzyme-linked immunosorbent assay kit development method
InactiveCN102998464ASimplify operation stepsImprove stabilityBiological testingElisa methodDehydroepiandrosterone
The present invention discloses a dehydroepiandrosterone (DHEA) enzyme-linked immunosorbent assay kit development method, which comprises: adopting dehydroepiandrosterone and succinic anhydride as raw materials, and adopting an anhydride mixing method to respectively prepare dehydroepiandrosterone artificial antigen (DHEA-BSA and DHEA-OVA) and anti-dehydroepiandrosterone (DHEA) monoclonal antibody so as to prepare an enzyme-linked immunosorbent assay (ELISA) kit. Compared with the high performance liquid chromatography (HPLC) method, the method of the present invention has the following advantages that: operation steps are simple, a plurality of samples can be detected in one time, expensive instrument is not required, and detection sensitivity, the required time and other aspects are better than the HPLC method. Compared to the HPLC method, the ELISA method has the following characteristics that: uses of organic reagents such as n-hexane, acetonitrile, methanol and the like are not required, poison on experimenters and environment can be reduced, and an environmental protection characteristic is provided.
Owner:WUHAN CLOUD CLONE CORP
Combination of dehydroepiandrosterone or dehydroepiandrosterone-sulfate with a leukotriene receptor antagonist for treatment of asthma or chronic obstructive pulmonary disease
InactiveUS20050101544A1Improve patient complianceSimplify therapyBiocidePowder deliveryActive agentAsthma
A pharmaceutical or veterinary composition, comprises a first active agent selected from a dehydroepiandrosterone and / or dehydroepiandrosterone-sulfate, or a salt thereof, and a second active agent comprising a leukotriene receptor antagonist for the treatment of asthma, chronic obstructive pulmonary disease, or other respiratory diseases. The composition is provided in various formulations and in the form of a kit. The products of this patent are applied to the prophylaxis and treatment of asthma, chronic obstructive pulmonary disease, or other respiratory diseases.
Owner:EPIGENESIS PHARMA LLC
Combination of dehydroepiandrosterone or dehydroepiandrosterone-sulfate with a beta-agonist bronchodilator for treatment of asthma or chronic obstructive pulmonary disease
InactiveUS20050113318A1Alleviate different aspectConvenient treatmentBiocideOrganic active ingredientsDiseaseActive agent
A pharmaceutical or veterinary composition, comprises a first active agent selected from a dehydroepiandrosterone and / or dehydroepiandrosterone-sulfate, or a salt thereof, and a second active agent comprising a β2-agonist bronchodilator for the treatment of asthma, chronic obstructive pulmonary disease, or other respiratory diseases. The composition is provided in various formulations and in the form of a kit. The products of this patent are applied to the prophylaxis and treatment of asthma, chronic obstructive pulmonary disease, or other respiratory diseases.
Owner:EPIGENESIS PHARMA LLC
Kits for dhea and dhea-sulfate for the treatment of chronic obstructive pulmonary disease
Kits for treating or preventing chronic obstructive pulmonary disease (COPD) by using as active agent a non-glucorticoid steroid, analogue thereof, such as dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEA-S), or their salts, in an amount effective for preventing or treating COPD.
Owner:EAST CAROLINA UNIVERISTY
Combination of dehydroepiandrosterone or dehydroepiandrosterone-sulfate with an anticholinergic bronchodilator for treatment of asthma or chronic obstructive pulmonary disease
InactiveUS20050101545A1Alleviate different aspectConvenient treatmentPowder deliveryBiocideActive agentObstructive Pulmonary Diseases
A pharmaceutical or veterinary composition, comprises a first active agent selected from a dehydroepiandrosterone and / or dehydroepiandrosterone-sulfate, or a salt thereof, and a second active agent comprising an anticholinergic bronchodilator for the treatment of asthma, chronic obstructive pulmonary disease, or other respiratory diseases. The composition is provided in various formulations and in the form of a kit. The products of this patent are applied to the prophylaxis and treatment of asthma, chronic obstructive pulmonary disease, or other respiratory diseases.
Owner:EPIGENESIS PHARMA LLC
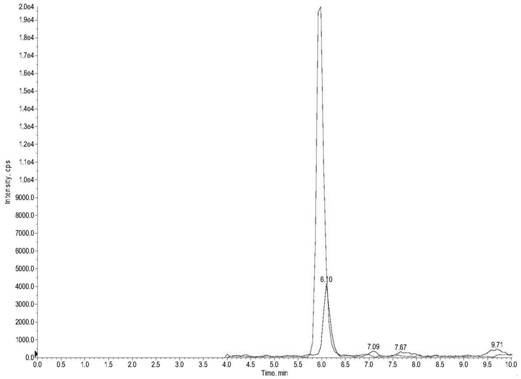
Method for detecting content of DHEA in human body fluid
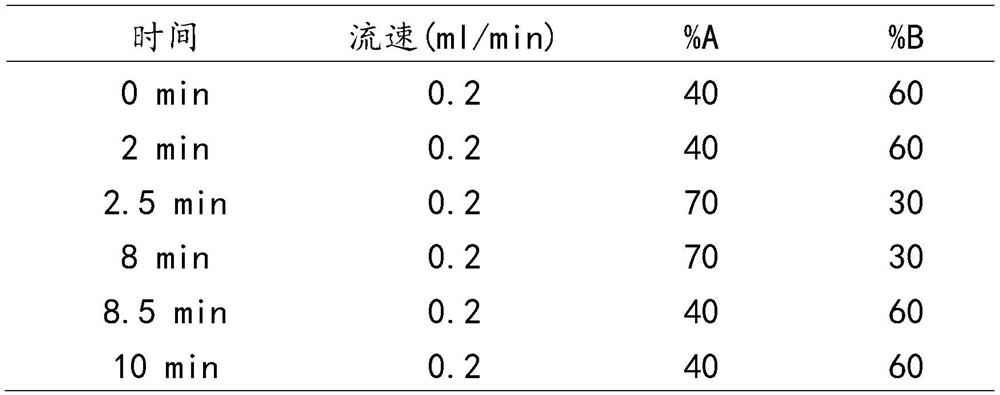
The invention discloses a method for detecting the content of DHEA in human body fluid. The method comprises the steps of detecting dehydroepiandrosterone in saliva by using a high performance liquid chromatography-tandem mass spectrometry technology, specifically, S1, adding the following reagents into a kit: a mobile phase I, a mobile phase II, a standard substance mother solution, an internal standard solution, a diluent, an extracting solution, a quality control substance and a redissolving solvent; S2, detecting the dehydroepiandrosterone in the pretreated saliva by adopting the high performance liquid chromatography-tandem mass spectrometry technology; S3, separating the dehydroepiandrosterone from the interferent by using high performance liquid chromatography; S4, quantifying by using an isotope internal standard method; S5, taking the concentration ratio of the standard substance to the internal standard substance as an X axis and the peak area ratio of the standard substance to the internal standard substance as a Y axis; and S6, establishing a standard curve, and calculating the content of the dehydroepiandrosterone in the sample to be detected. The method is high in sensitivity and good in specificity, saliva sample treatment is simple, methodology verification meets requirements, and it is indicated that the method is reliable and capable of being applied to quantitative detection of dehydroepiandrosterone in human saliva.
Owner:艾可泰科(浙江)控股有限公司
Combination of dehydroepiandrosterone or dehydroepiandrosterone-sulfate with a PDE-4 inhibitor for treatment of asthma or chronic obstructive pulmonary disease
InactiveUS20050026883A1Alleviate different aspectConvenient treatmentBiocidePowder deliveryPhosphodiesteraseDisease
A pharmaceutical or veterinary composition, comprises a first active agent selected from a dehydroepiandrosterone and / or dehydroepiandrosterone-sulfate, or a salt thereof, and a second active agent comprising a phosphodiesterase-4 inhibitor for the treatment of asthma, chronic obstructive pulmonary disease, or any other respiratory disease. The composition is provided in various formulations and in the form of a kit. The products of this patent are applied to the prophylaxis and treatment of asthma, chronic obstructive pulmonary disease, or any other respiratory disease.
Owner:EPIGENESIS PHARMA LLC
Weight loss formulation
A weight loss product comprises a mixture of Alchemilla vulgaris, Olea europaea, Cuminum cyminum, and Mentha longiflora (in the weight proportion 12:10:5:4), to which additional ingredients have been added to increase weight loss efficacy. The additional ingredients may include, but are not limited to, Gamma-oryzanol, Caffeine, Cirsimarin, Fucoxanthin, Guggulsterones, Evodiamine, Forskolin, Sclareolide, Chromium, Hydroxycitric acid, Pinolenic acid, Conjugated linoleic acid, Potato protein, Raspberry ketone, Capsaicin, Synephrine, Dehydroepiandrosterone, Phenylethylamine, Orlistat, Glucomannan, or Vitamin C, or sources thereof, as well as herbal ingredients such as Guarana, Yerba Mate, Damiana, Green tea, Green coffee bean, Cinnamon, White kidney bean, Garcinia Cambogia, Nopal Cactus, Hoodia, Yohimbine, Malpighia glabra, Eurycoma Longifolia, Caralluma Fimbriata, Citrus aurantium, Gymnema Sylvestre, Lyceum barbarurn, Aloe Vera, Cayenne, Pomegranate, Blueberry, Billberry, or extracts of such herbal ingredients, and plant sources of antioxidants. A preferred additional ingredient is caffeine provided by caffeine anhydrous, Coffea Arabica, Coffea canephora, Camellia sinensis, Ilex paraguariensis, Guarana, Theobroma cacao, and kola nut. Other additional ingredients may be used as well in accordance with specific disclosed formulations.
Owner:NORTHERN INNOVATIONS HLDG
Combination of dehydroepiandrosterone or dehydroepiandrosterone-sulfate with a cromone for treatment of asthma or chronic obstructive pulmonary disease
InactiveUS20050026880A1Alleviate different aspectConvenient treatmentOrganic active ingredientsBiocideDiseaseActive agent
A pharmaceutical or veterinary composition, comprises a first active agent selected from a dehydroepiandrosterone and / or dehydroepiandrosterone-sulfate, or a salt thereof, and a second active agent comprising a cromone for the treatment of asthma, chronic obstructive pulmonary disease, or any other respiratory disease. The composition is provided in various formulations and in the form of a kit. The products of this patent are applied to the prophylaxis and treatment of asthma, chronic obstructive pulmonary disease, or any other respiratory disease.
Owner:EPIGENESIS PHARMA LLC
Chinese yam and radix angelicae soup formula and preparation method thereof
InactiveCN112516281AAdjust the tastePromote secretionNervous disorderDispersion deliveryNutritionSpleen
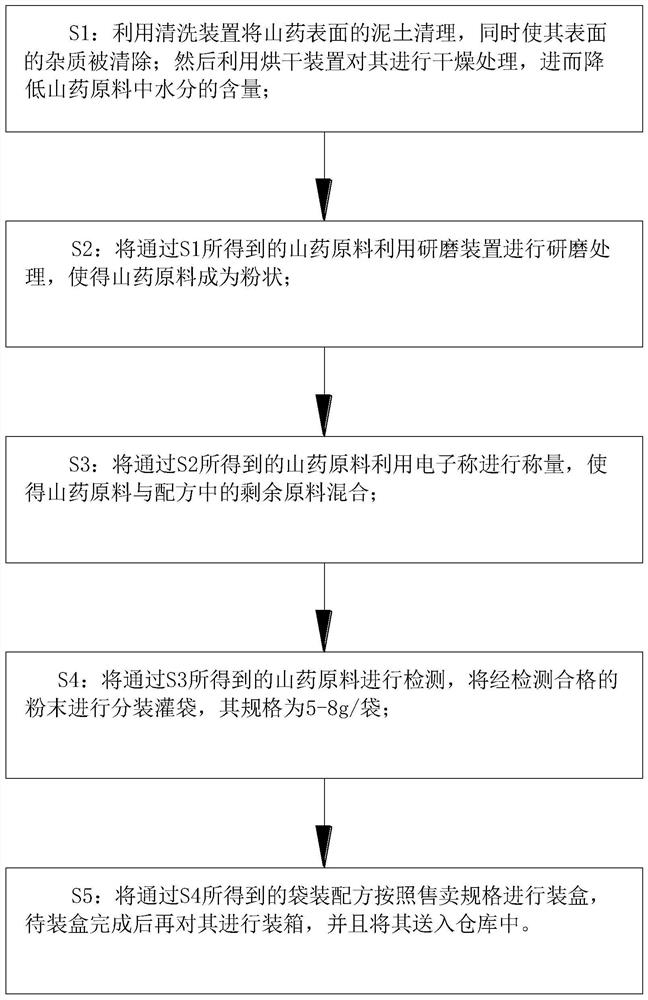
The invention belongs to the technical field of formula production, and particularly relates to a Chinese yam and radix angelicae soup formula and a preparation method thereof. Chinese yam has the effects of tonifying spleen and stomach, promoting salivation and tonifying lung, and tonifying kidney and arresting seminal emission, and can promote brain to secrete dehydroepiandrosterone so as to improve sleep, nourish blood vessels and nerves, preserve a good mood and enhance body immunity; radix angelicae can relieve exterior syndrome, dispel cold, dispel wind, relieve pain, eliminate dampness,arreseting leucorrhea, diminish swelling and expel pus, and also has the effects of exciting central nerves, increasing hypertension, resisting inflammation, easing pain, resisting cancer and relieving spasm; honeysuckle has the effects of clearing away heat and toxic materials and diminishing inflammation and swelling; liquorice is added into the formula, so that the taste of Chinese yam and radix angelicae soup can be adjusted, and the Chinese yam and radix angelicae soup has a sweet taste, is convenient for a drinker to drink, and also can eliminate phlegm to arrest coughing and tonify qito moisten the lung; and through cooperative use of a cleaning unit and a soil brushing mechanism, soil on the surfaces of the Chinese yam can be conveniently flushed, and the cleaning effect is good.
Owner:HARBIN LING CHUN WEI DAO FOOD DEV
Pharmaceuticals and dosing means for human aging reversal
ActiveUS20200254066A1Organic active ingredientsPeptide/protein ingredientsSomatotropic hormoneMetforminum
A combination of medications and medication doses is disclosed whereby age-related changes in systemic inflammation, cancer risk, heart disease risk, CD38 expression, hair color, thymotrophic hormones, immune cell populations, the CD4 / CD8 cell ratio, bone marrow density, thymus structure, kidney function, and epigenetic age can be reversed in humans. Surprisingly, agents that accelerate the growth of cells reduce cancer risk, agents that intensify immune responses attenuate age-related inflammation, agents with no prior connection to hair color reverse age-related hair whitening, and a combination of agents that induces IGF-1, a hormone previously thought to drive systemic aging, results in a reversal of systemic aging as documented by an epigenetic clock. Medication combinations useful in the present invention include human growth hormone (GH) or GH releasers, dehydroepiandrosterone (DHEA), and metformin.
Owner:INTERVENE IMMUNE INC
Method and kit for detecting content of dehydroepiandrosterone in saliva
PendingCN113009006AGuaranteed specificityEasy to excludeComponent separationSaliva sampleMethoxyamine
The invention relates to a method and a kit for detecting the content of dehydroepiandrosterone in saliva, and the method comprises the following steps: preparing a standard substance, preparing a quality control substance, treating the standard substance, analyzing and detecting by adopting a high performance liquid chromatography-tandem mass spectrometry technology, pre-treating a saliva sample, analyzing and detecting by adopting the high performance liquid chromatography-tandem mass spectrometry technology, drawing a calibration curve: establishing a standard curve by adopting an isotope internal standard quantification method by taking the concentration ratio of a standard substance to an internal standard substance as an X axis and the peak area ratio of the standard substance to the internal standard substance as a Y axis, and calculating the content of the dehydroepiandrosterone in the to-be-detected sample. According to the method, dehydroepiandrosterone and interferents are separated by adopting the high performance liquid chromatography-tandem mass spectrometry technology, then the content of dehydroepiandrosterone in saliva is measured by combining an isotope internal standard quantification method, and the dehydroepiandrosterone reacts with methoxyamine to generate oxime ester, so that the detection sensitivity is remarkably improved, and the detection time is shortened. The specificity of the detection method is ensured by adopting a reaction detection mode (MRM).
Owner:泊迈生物医学检测(苏州)有限公司
Composition with health-care function as well as preparation method and application of composition
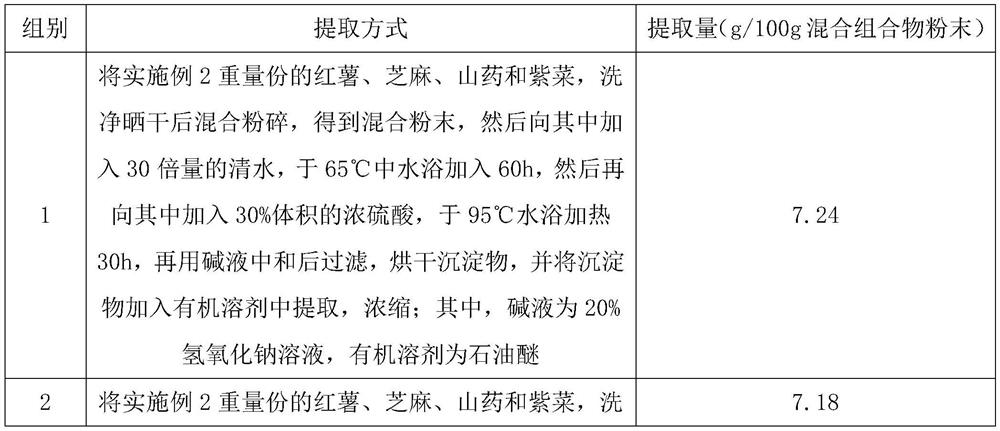
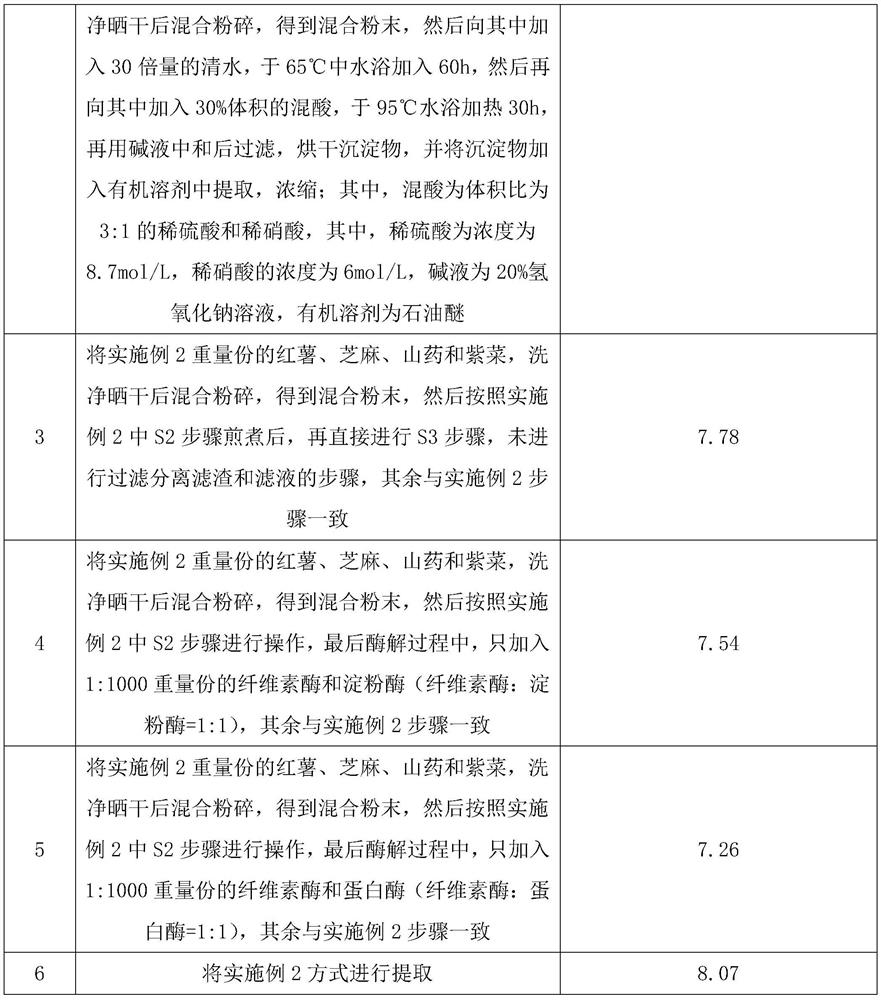
PendingCN113855755ALittle side effectsReduce cholesterolMetabolism disorderAlgae medical ingredientsForest yamTraditional medicine
The invention discloses a composition with a health-care function, and relates to the technical field of medicines. The composition is prepared from the following components in parts by weight: 30-40 parts of sweet potatoes, 10-18 parts of sesame seeds, 16-24 parts of Chinese yams, 5-8 parts of laver and 12-18 parts of a herba epimedii extract. Most of the composition disclosed by the invention is common in life and can be directly eaten, so that the composition has small side effects. Because the composition contains a large amount of dehydroepiandrosterone, the composition can be used for treating the symptoms of insufficiency of kidney yang, dizziness and tinnitus, soreness and weakness of waist and knees, intolerance of cold and cold limbs, fatigue and weakness and the like, is very favorable for enhancing immunity, can eliminate fatigue, and has a great health-care value.
Owner:GUIZHOU SHENQI PHARMA RES INST
A composition for improving ovarian reserve function and preventing premature ovarian failure and its application
ActiveCN110101707BImprove reserve functionPrevent premature agingOrganic active ingredientsSexual disorderDehydroepiandrosterone sulfatePhysiology
Owner:北京华睿鼎信科技有限公司
Combination Of Dehydroepiandrosterone Or Dehydroepiandrosterone-Sulfate With An Anticholinergic Bronchodilator For Treatment Of Asthma Or Chronic Obstructive Pulmonary Disease
A pharmaceutical or veterinary composition, comprises a first active agent selected from a dehydroepiandrosterone and / or dehydroepiandrosterone-sulfate, or a salt thereof, and a second active agent comprising an anticholinergic bronchodilator for the treatment of asthma, chronic obstructive pulmonary disease, or other respiratory diseases. The composition is provided in various formulations and in the form of a kit. The products of this patent are applied to the prophylaxis and treatment of asthma, chronic obstructive pulmonary disease, or other respiratory diseases.
Owner:ROBINSON CYNTHIA B +1
Preparation method of skin-moistening and anti-wrinkle cosmetic
InactiveCN112220709AImprove the efficiency of scavenging free radicalsImprove wrinkle resistanceCosmetic preparationsToilet preparationsDimethyl siloxaneSodium hyaluronate
The invention discloses a preparation method of a skin-moistening and anti-wrinkle cosmetic. The preparation method comprises the following steps: step I, adding squalane, silyl carotene, cetyl, beewax, propyl p-methoxycinnamate and dimethyl siloxane into a container A, performing heating to 75-85 DEG C, and performing stirring at 100-200r / min for 20-40min to obtain a mixed solution A; step II, adding a humectant, polyoxyethylene hydrogenated castor oil, fumed silica, sodium hyaluronate and deionized water into a container B, performing heating to 85-95 DEG C, performing stirring at the speedof 100-200 r / min for 20-40 min, and performing cooling to 75-85 DEG C to obtain a mixed solution B; step III, adding the mixed solution B into a vacuum homogenizing tank, then slowly adding the mixedsolution A into the mixed solution B which is stirred at the speed of 50-100 r / min, controlling the temperature to be 70-80 DEG C, then performing homogenizing and emulsifying for 3-10 minutes at therotating speed of 500-1000 r / min, performing stirring at the rotating speed of 50-100 r / min, and performing cooling to 35-45 DEG C to obtain a pre-emulsified solution; and step IV, adding a natural anti-aging agent, dehydroepiandrosterone, a free radical scavenger, a preservative and essence into the pre-emulsified solution in parts by mass, performing stirring at the speed of 50-100 r / min and thetemperature of 35-45 DEG C for 30-60 minutes to obtain emulsified solution, and performing ageing for 1-3 days to obtain the skin-moistening and anti-wrinkle cosmetic. The cosmetic with excellent skin moistening and anti-wrinkle performance is prepared through the preparation method.
Owner:广州美莱洁化妆品开发有限公司
Dehydroepiandrosterone (DHEA) supplementation on female sexual desire and function in pre-menopausal women
PendingUS20200030342A1Improve sexual functionOrganic active ingredientsHealth-index calculationObstetricsSerum androgens
A method of improving sexual function in premenopausal women, who have low Female Sexual Function Index (FSFI) baseline of less than or equal to 25.7. The method includes providing information to premenopausal women to provide input for calculating the FSFI score and, if the score is low, providing dehydroepiandrosterone (DHEA) supplementation to be taken in 25 mg dosages daily once, twice, thrice or four times over a period of time of four to six weeks. By the end of the period of time, the FSFI baselines of the premenopausal women improves by at least seven percent, serum androgen levels of the premenopausal women increase and follicle-stimulating hormone (FSH) levels of the premenopausal women decrease.
Owner:AMERICAN INFERTILITY OF NEW YORK P C +3
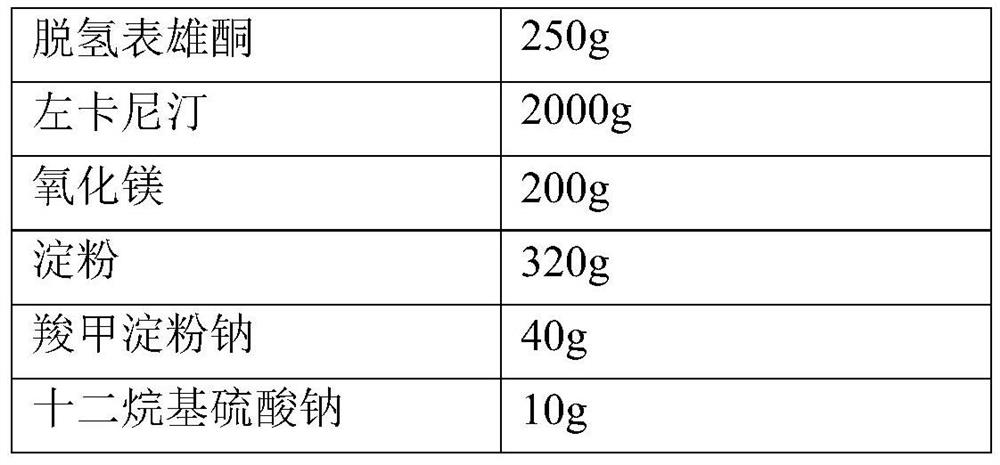
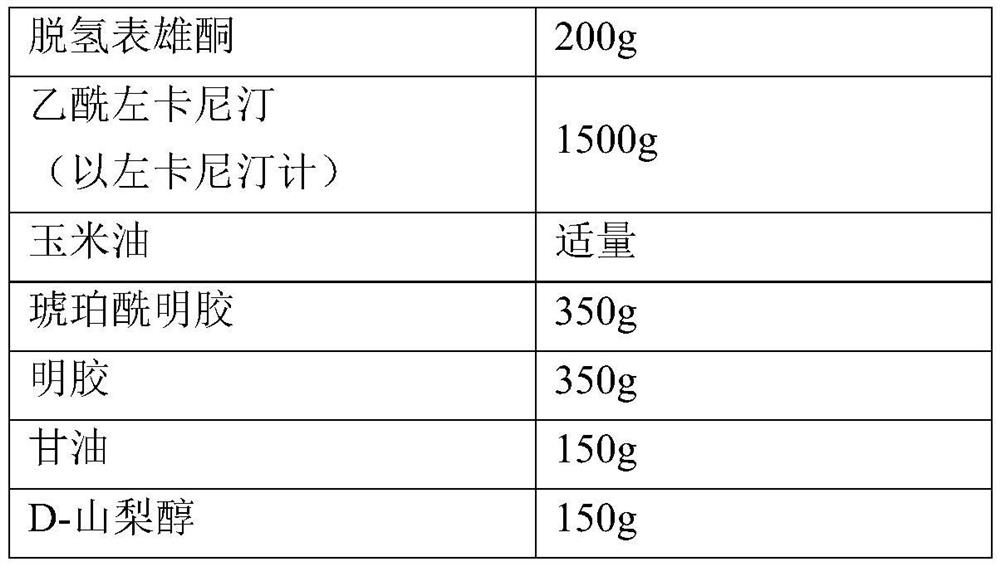
Compositions comprising dehydroepiandrosterone or its sulfate ester, levocarnitine, acetyl levocarnitine or their pharmaceutically acceptable salts and applications thereof
ActiveCN109999042BIncrease concentrationIncrease vitalityOrganic active ingredientsSexual disorderPhysiologyDietary supplement
The present invention describes a synergistic composition and its use in the preparation of medicines or dietary supplements for improving male oligospermia, weak sperm, and reducing sperm deformity. The composition comprises dehydroepiandrosterone (DHEA) or dehydroepiandrosterone sulfate (DHEAS), levocarnitine or a pharmaceutically acceptable salt thereof, acetyl levocarnitine or a pharmaceutically acceptable salt thereof, compared to The existing technology is safer and more effective, can significantly improve semen concentration and sperm motility, and reduce sperm deformity.
Owner:北京华睿鼎信科技有限公司
Composition and method to aid in hormone replacement therapy
ActiveUS20200297738A1Less agitationOrganic active ingredientsEmulsion deliveryJojoba oilEpiandrosterone
A pharmaceutical two-phase admixture for topical application, transdermal or transmucosal, characterized by components in two phases, a liquid and a solid, adapted for topical application, transdermal or transmucosal, to various skin and / or mucosal surface areas of the body is disclosed. The solid phase is comprised of one or more bio-identical hormones and the liquid phase is comprised of one or more excipient carrier oils. The bio-identical hormone component is comprised of one or more of Bi-Est, testosterone, progesterone, and dehydroepiandrosterone. The excipient carrier oil component is comprised of one or more of a wide range of common and rare pharmacological oils including specific formulations of jojoba oil, evening primrose oil, and borage seed oil. The solid phase bio-identical hormone component is comprised of either a standard coarse formulation or a formulation comprised of nanoparticles. The pharmaceutical admixture is especially useful in a regime of hormone replacement therapy.
Owner:THE MENOPAUSE METHOD INC
Methods for Preparing Bile Acids
PendingUS20220009957A1Antibacterial agentsOrganic active ingredientsChenodeoxycholic acidDHEA - Dehydroepiandrosterone
The present disclosure provides methods of preparing cholic acid, deoxycholic acid, or chenodeoxycholic acid, an ester thereof, or a pharmaceutically or cosmetically acceptable salt thereof, and novel and useful synthetic intermediates, for example, as described for methods 1, 1A, 1B, 2, 3, 3A, and 4. The method can start with a plant derived steroid as a starting material, such as dehydroepiandrosterone (DHEA) or Acetyl-dehydroepiandrosterone (Ac-DHEA). Also provided are pharmaceutical or cosmetic compositions and uses thereof, which comprise one or more of cholic acid, deoxycholic acid, or chenodeoxycholic acid, an ester thereof, or a pharmaceutically or cosmetically acceptable salt thereof, which is of high purity, for example, free of animal derived impurities.
Owner:MEDY TOX INC
Preparation method of maxacalcitol
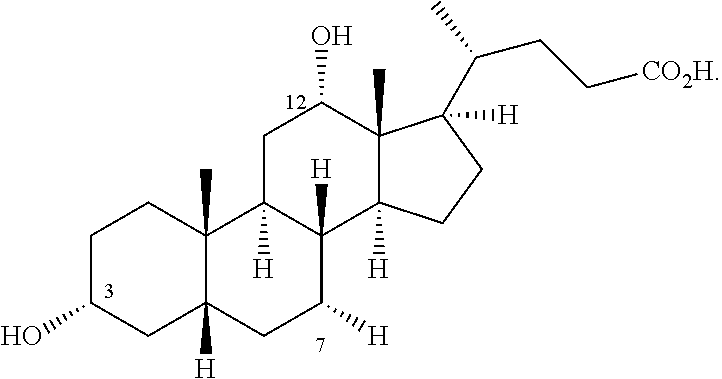
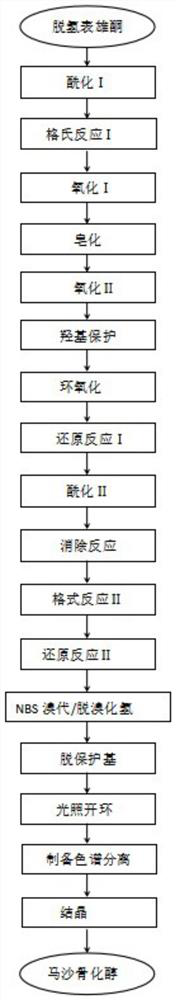
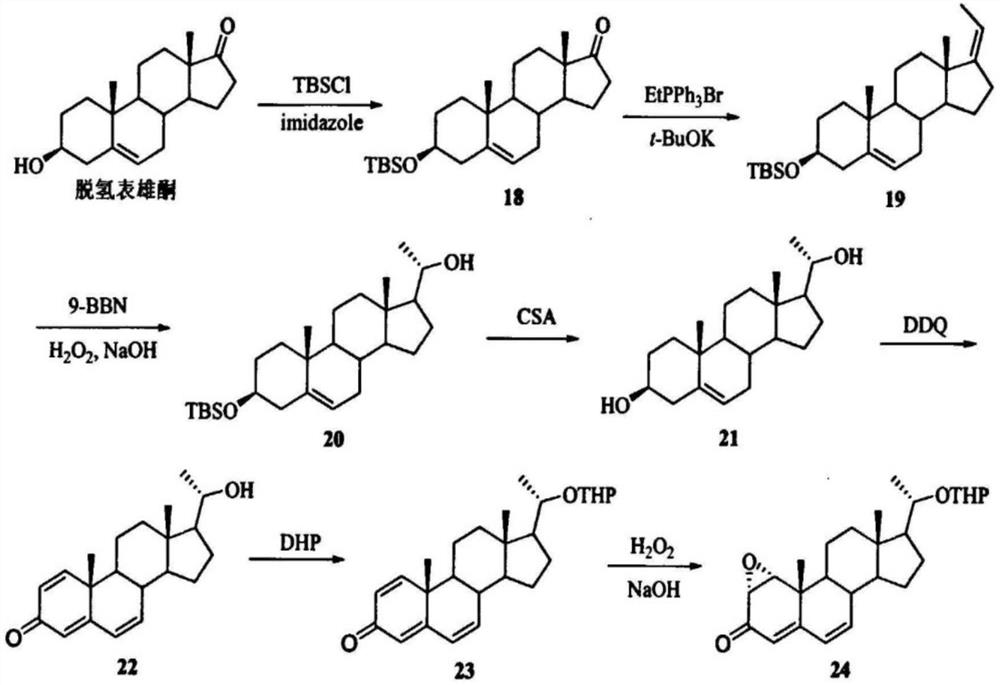
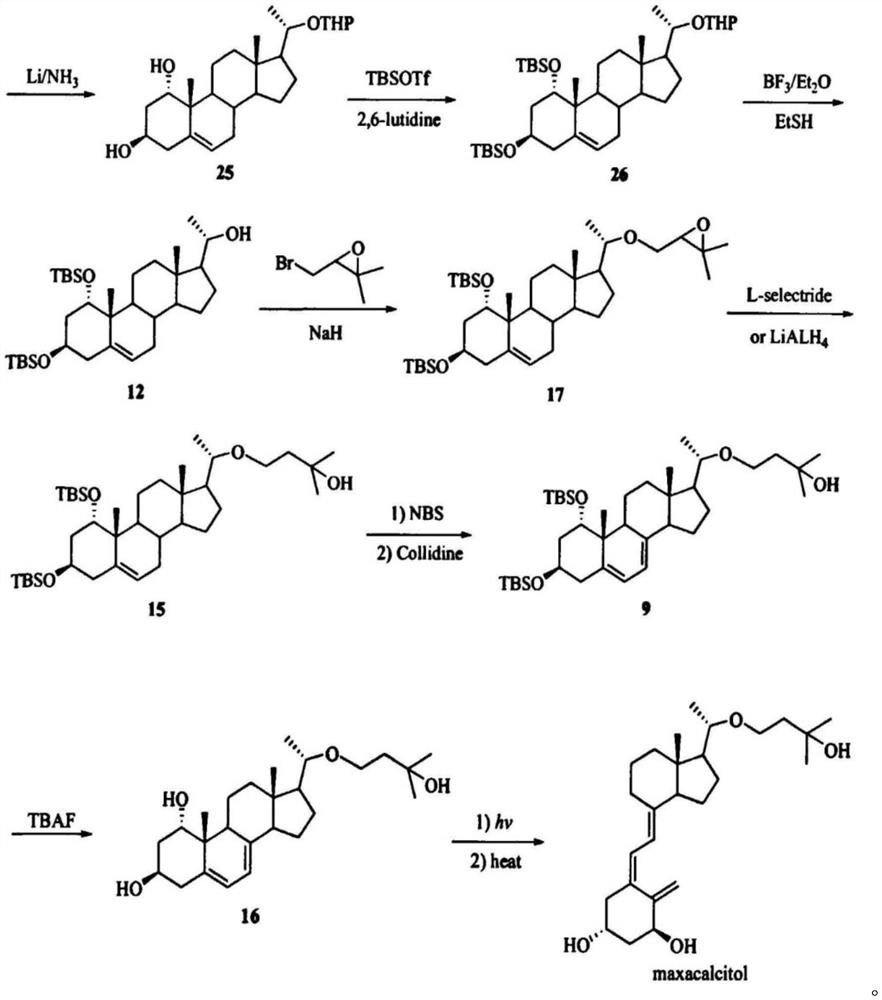
PendingCN114656385AReduce usageLow yieldOrganic chemistryGrignard reactionDHEA - Dehydroepiandrosterone
The invention relates to a synthesis method of a medicine, in particular to a preparation method of maxacalcitol. The maxacalcitol is prepared by taking dehydroepiandrosterone as an initial raw material through acylation, Grignard reaction, oxidation, saponification, hydroxyl protection, epoxidation, reduction, elimination, NBS bromination / dehydrobromination, deprotection and illumination ring opening, the operation is simple, the yield is high, and the maxacalcitol is suitable for large-scale industrial production.
Owner:HANGZHOU XIASHA BIOCHEM TECH
A kind of method extracting dehydroepiandrosterone from sweet potato
The invention discloses a method for extracting dehydroepiandrosterone from sweet potatoes, which comprises the steps of pre-conversion treatment, acid hydrolysis, extraction, purification and the like. The present invention aims at the defect that the combined diosgenin and cellulose cannot be completely separated by traditional acid hydrolysis, resulting in a very low yield of the product. By stirring the sweet potato pulp with a treatment liquid, the cellulose is fully swollen and processed by mechanical grinding. Break the hydrogen bond and part of the glycosidic bond combining sweet potato cellulose and dioscin, promote the separation of dioscin, thereby improving the rate of subsequent acid hydrolysis and the extraction rate of DHEA, and achieve the purpose of rapid and efficient extraction of DHEA in sweet potato.
Owner:HUAZHONG AGRI UNIV
Dehydroepiandrosterone health-care food and making method thereof
The invention discloses a dehydroepiandrosterone health-care food and a making method thereof, and relates to the technical field of health-care foods. The problem that vitamins cannot be supplementedto women in the gestation period is solved. The dehydroepiandrosterone health-care food comprises the following components by weight: 50mg of DHEA and 400mg of folic acid. The making method of the dehydroepiandrosterone health-care food comprises the following steps: respectively weighing the DHEA, the folic acid, vitamin A and vitamin B6 in parts by weight. The health-care food is used for supplementing vitamins, minerals and trace elements for pregnant women and lactating women, helps normal development of brain and nervous systems of fetuses, promotes erythrocyte formation, improves body immunity of the pregnant women, is suitable for iron deficiency and folic acid in the pregnant period, and can meet special requirements of females in the gestation period and the lactation period forother nutritional ingredients every day. Various vitamins and other trace elements daily required by such crowds are comprehensively supplemented, so that the purpose of improving anemia is achieved.
Owner:武汉林宝莱生物科技有限公司
Organic anti-aging beverage and preparation method thereof
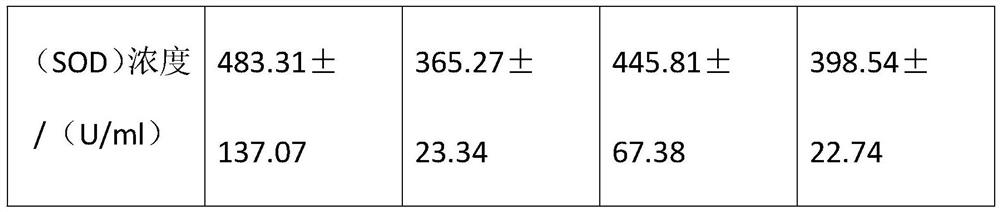
PendingCN112795457AGood water solubilitySlow down the degree of agingOrganic chemistryOrganic compound preparationBiotechnologyEthylic acid
The invention discloses an organic anti-aging beverage and a preparation method thereof. The organic anti-aging beverage comprises the following raw materials: dehydropregnenolone acetate, ethanol, pyridine, 1-aminoanthraquinone, epichlorohydrin, ethyl acetate, tetrahydrofuran, edible essence, a preservative, vitamins, a sweetening agent, purified water and the like. According to the invention, dehydroepiandrosterone reacts with free radicals under the catalysis of acetic acid so as to block a peroxidation reaction, so the aging degree of an organism is relieved; the side amine chain of amine chain-substituted anthraquinone reacts with cell telomerase, so the content of D-galactose is increased, the atrophy and degeneration of thymus are delayed, and the aging phenomenon of an organism is relieved; an amine N-oxyalkyl side chain is reduced and activated into amine molecules with positive charges by hormone enzyme in a body, so anthraquinone can be diffused to the whole body to play a role; and after a traditional process is conducted, a crude 1-aminoanthraquinone product is dissolved in tetrahydrofuran, chloroform is used for packing a column for column chromatography separation, and a hydroxyl group is not easy to reduce after being immobilized by the tetrahydrofuran, so the quality and yield of the product are improved.
Owner:陈广会
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com