Compositions comprising dehydroepiandrosterone or its sulfate ester, levocarnitine, acetyl levocarnitine or their pharmaceutically acceptable salts and applications thereof
A technology of acetyl-L-carnitine meter and dehydroepiandrosterone, which is applied in the field of improving men
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation of embodiment 1 levocarnitine, acetyl levocarnitine and dehydroepiandrosterone dry suspension (1000 bags of prescriptions)
[0025] L-carnitine 1000g Acetyl L-carnitine 500g DHEA 50g sucrose 2000g Sucralose 100g sodium benzoate 50g Strawberry Spice 10g xanthan gum 50g colloidal silica 20g
[0026] Take the raw and auxiliary materials, pulverize them, pass through a 200-mesh sieve respectively, dry them in an appropriate way, and set aside; weigh the raw and auxiliary materials according to the prescription amount, and use physical mixing method to directly mix them evenly. The process should be as fast as possible to minimize the entry of water; mix evenly The final powder is evenly put into aluminized film bag, and it is ready.
Embodiment 2
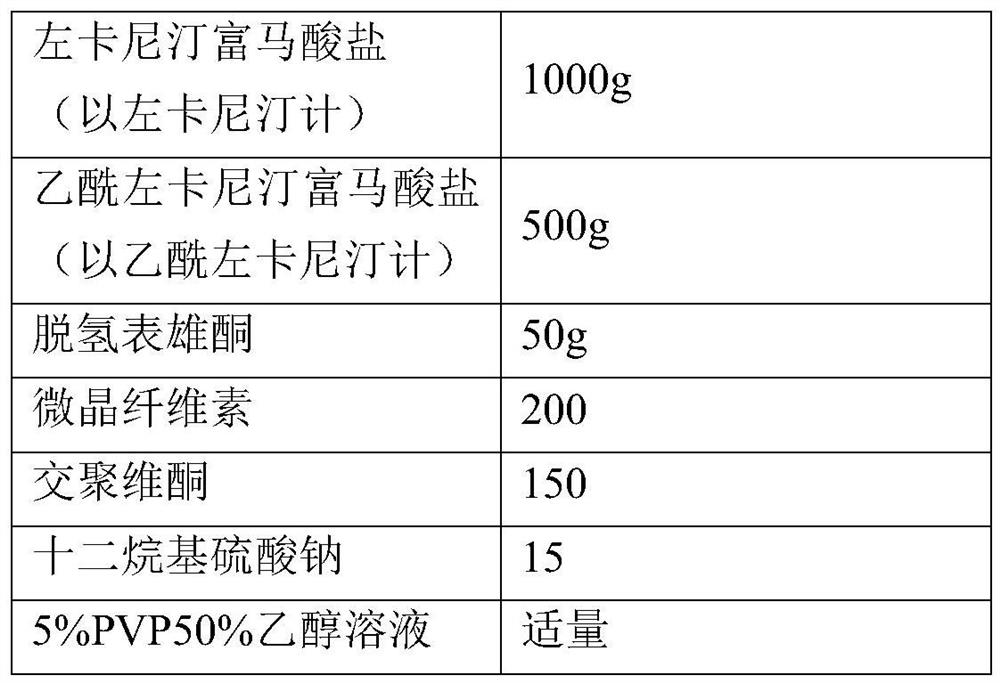
[0027] Preparation of Example 2 Levocarnitine Fumarate, Acetyl Levocarnitine and DHEA Granules (1000 Bags of Prescription)
[0028]
[0029] Pass the bulk drug through an 80-mesh sieve and set aside. The sucrose and gum arabic are pulverized in a high-speed multifunctional pulverizer, and passed through a 60-mesh sieve for later use, and other auxiliary materials are respectively passed through a 60-mesh sieve for later use. Weigh the prescribed amount of hydroxypropyl cellulose, add an appropriate amount of purified water to dissolve it, stir evenly, and set aside. Weigh sucrose, dextrin, gum tragacanth, gum arabic, levocarnitine fumarate, acetyl levocarnitine and DHEA in the wet granulator and stir evenly, then add the above The prepared adhesive is stirred evenly to make a soft material. The above-mentioned soft material is granulated in a oscillating granulator (40 mesh sieve). The above granules were dried in a fluidized bed at 60°C until the water content was less ...
Embodiment 3
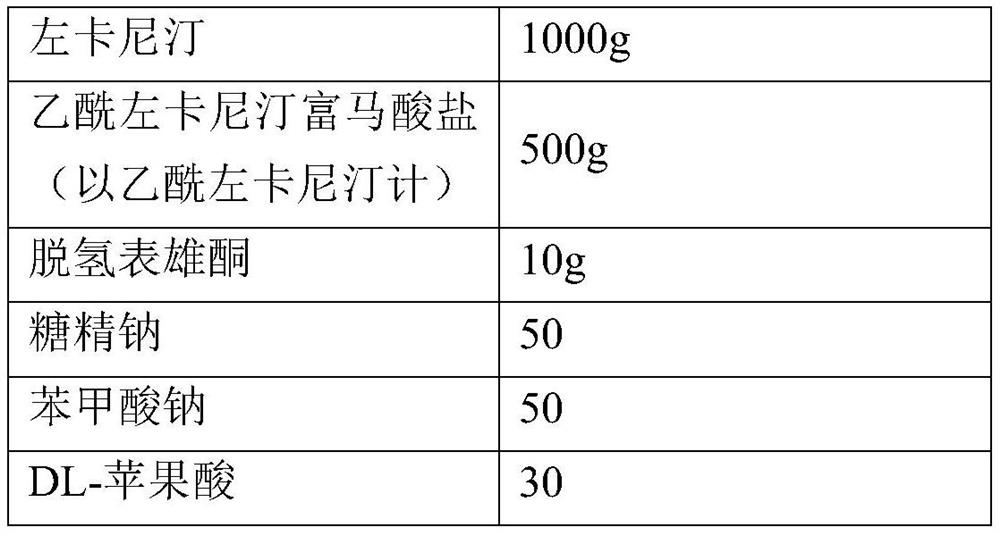
[0030] The preparation of embodiment 3 levocarnitine, acetyl levocarnitine fumarate and dehydroepiandrosterone oral liquid (1000 prescriptions)
[0031]
[0032] The sodium saccharin, sodium benzoate, DL-malic acid, levocarnitine, acetyl levocarnitine fumarate and dehydroepiandrosterone were added to 80% of the total volume of purified water and stirred to dissolve. Add purified water to full volume. Stir evenly and filter, fill the obtained filtrate into soda-lime glass control oral liquid bottles, cap it, and sterilize it by autoclaving at 105°C for 30 minutes to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com