A composition for improving ovarian reserve function and preventing premature ovarian failure and its application
A technology for ovarian reserve and hypofunction, applied in application, drug combination, function of food ingredients, etc., can solve problems such as low ovarian reserve function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
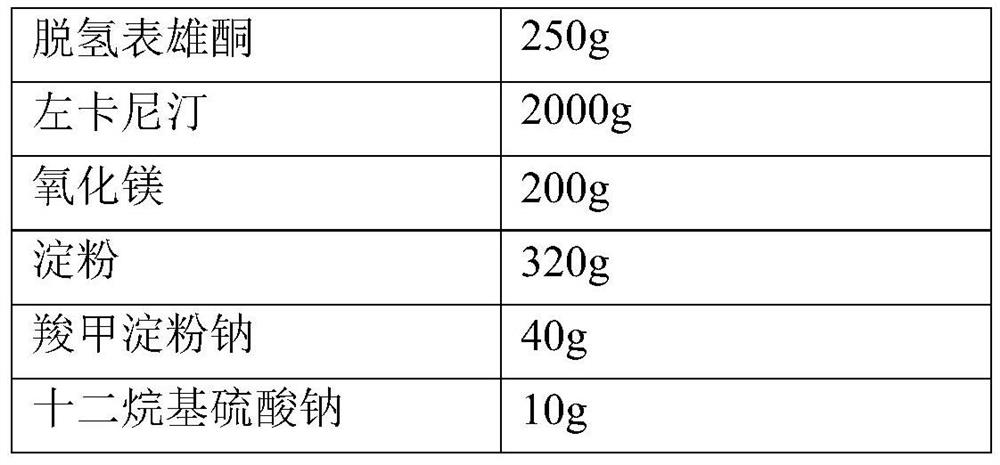
[0026] The preparation of embodiment 1 dehydroepiandrosterone and levocarnitine composition hard capsule (10000 prescriptions)
[0027]
[0028]
[0029] Weigh the raw materials of the prescription amount, pass through an 80-mesh sieve for subsequent use, and the auxiliary materials pass through a 60-mesh sieve for subsequent use. Prepare 80% ethanol solution for later use. Thoroughly mix DHEA, levocarnitine, magnesium oxide, starch, sodium starch glycolate and sodium lauryl sulfate evenly. The mixed powder is made into a soft material with the above-mentioned 80% ethanol solution, and granulated through a 16-mesh sieve. The prepared wet granules are dried at 50°C to 60°C. The dry granules are sized with a 16-mesh sieve. Add the prescribed amount of magnesium stearate to the granulated granules and mix well. Fill capsules, each containing 25mg of DHEA and 200mg of L-carnitine.
Embodiment 2
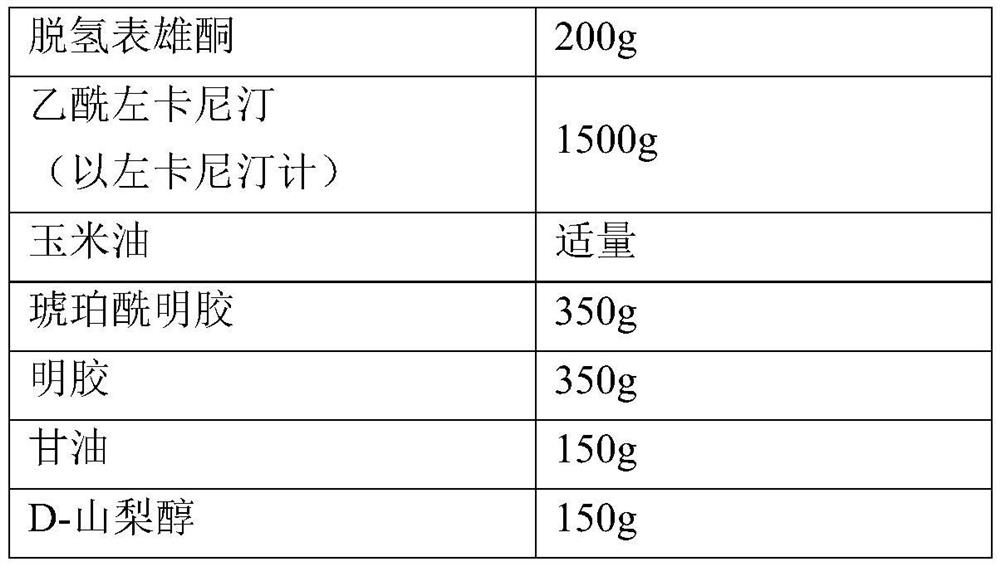
[0030] The preparation of embodiment 2 dehydroepiandrosterone and acetyl-levocarnitine soft capsules (10000 prescriptions)
[0031]
[0032] Weigh the prescribed amount of dehydroepiandrosterone and acetyl-levocarnitine and add them to the corn oil and stir evenly. Take a specified amount of succinylated gelatin, gelatin, glycerin, and D-sorbitol, disperse them in an appropriate amount of purified water, stir at 60°C to dissolve, and prepare soft capsules by using a soft capsule forming machine punching method. The content of each capsule contains dehydrogenated form Androsterone 20mg, acetyl L-carnitine (calculated as L-carnitine) 150mg.
Embodiment 3
[0033] The preparation of embodiment 3 dehydroepiandrosterone sulfate and levocarnitine tablet (10000 prescriptions)
[0034]
[0035] Weigh the raw and auxiliary materials of the prescription amount, the raw materials are passed through an 80 mesh sieve, and the microcrystalline cellulose and cross-linked sodium carboxymethyl cellulose are passed through a 60 mesh sieve for subsequent use. Add dehydroepiandrosterone sulfate, levocarnitine, croscarmellose sodium, and microcrystalline cellulose into the tank mixer, and mix for 20 minutes. After fully mixing the aforementioned raw and auxiliary materials, add purified water, mix for 10 minutes, pass through a 16-mesh sieve to granulate, and dry the prepared wet granules at 40-50°C. The dry granules are sized with a 16-mesh sieve. Add the prescribed amount of magnesium stearate and silicon dioxide to the granulated granules, and mix well. Regulate tablet press and carry out tabletting, each tablet contains dehydroepiandroste...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com