Preparation method of maxacalcitol
A technology of maxacalcitol and methanol, applied in the direction of organic chemistry, can solve the problems of cumbersome chemical synthesis steps, lengthy chemical reaction steps, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
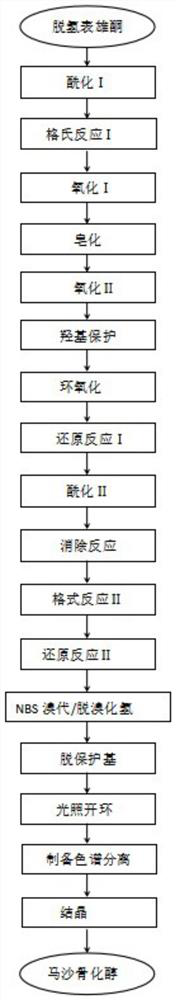
[0035] A kind of preparation method of maxacalcidol, concrete process is such as figure 1 As shown, the specific process is:
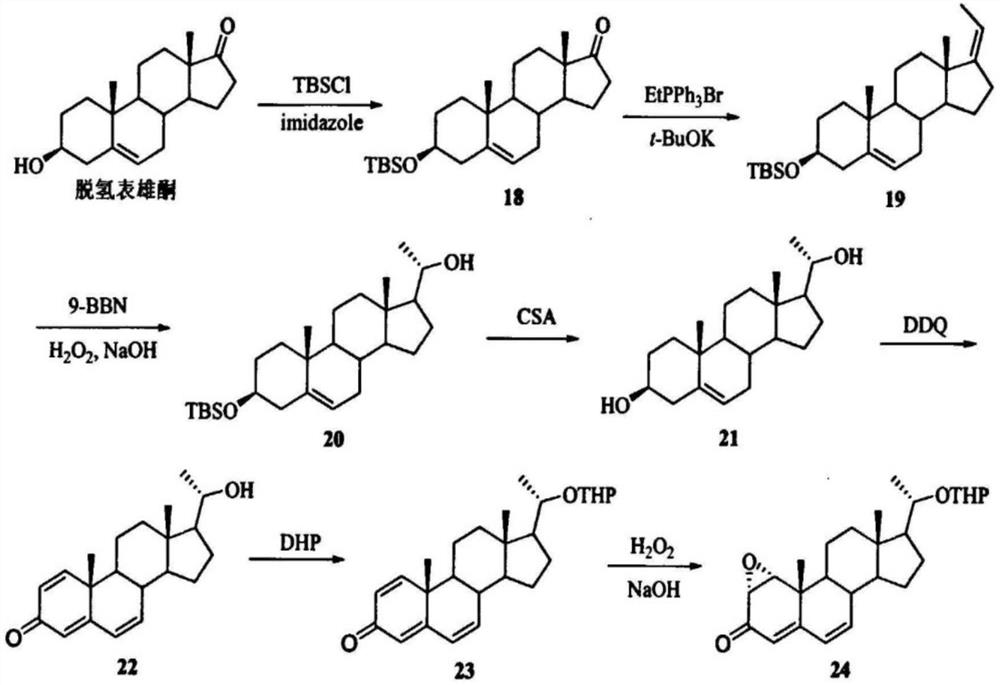
[0036] S1. Preparation of (3R)-tert-butyldimethylsiloxane-androst-5en-17 ketone (acylation I): 20 g of dehydroepiandrosterone was dissolved in 200 mL of tetrahydrofuran, at 37 ° C, and 9.5g of imidazole and 15.8g of tert-butyldimethylsilyl chloride were added for reflux reaction, and after 3 hours of reaction, the solvent was distilled off under reduced pressure, extracted with ethyl acetate, washed with saturated sodium chloride solution, dehydrated, and evaporated to obtain 28.05g of white solid. That is (3R)-tert-butyldimethylsiloxane-androst-5-ene-17 ketone, denoted as compound 18, and the reaction process is as follows:
[0037] C 19 H 28 O 2 +C 6 H 15 ClSi+C 3 H 4 N 2 →C 25 H 42 O 2 Si+C 3 H 5 N 2 Cl;
[0038] Preparation of S2, (17Z,3R)-tert-butyldimethylsiloxane-androst-5,17-diene (Grignard reaction I): keep the temperature at 5...
Embodiment 2
[0086] A preparation method of maxacalcitol, the concrete technological process is:
[0087] Preparation of S1, (3R)-tert-butyldimethylsiloxane-androst-5en-17 ketone: dissolve 20 g of dehydroepiandrosterone in 200 mL of tetrahydrofuran, and add 15.0 g of imidazole and 21.9 g of tert-butyl 28.0g of white solid was obtained, which was (3R)-tert-butyldimethylsiloxane after refluxing reaction with dimethylsilyl chloride, distillation under reduced pressure, extraction with ethyl acetate, washing with saturated sodium chloride water, dehydration, and evaporation of the solvent. -Androst-5en-17one, denoted as compound 18.
[0088] Preparation of S2, (17Z,3R)-tert-butyldimethylsiloxane-androst-5,17-diene: add 28.0 g of compound 18 and 77.19 g of ethyltriphenylphosphonium bromide to 200 mL of tetrahydrofuran solvent , 98.0g potassium tert-butoxide refluxed for reaction, extracted with petroleum ether, washed with saturated sodium chloride, dried with anhydrous sodium sulfate, concent...
Embodiment 3
[0104] A preparation method of maxacalcitol, the concrete technological process is:
[0105] Preparation of S1, (3R)-tert-butyldimethylsiloxane-androst-5en-17 ketone: 20.0 g of dehydroepiandrosterone was dissolved in 400 mL of tetrahydrofuran, and 7.03 g of imidazole and 11.42 g of tert-butyl were added 26.2g of white solid was obtained, which was (3R)-tert-butyldimethylsilicon Oxy-androst-5en-17one, denoted as compound 18.
[0106] Preparation of S2, (17Z,3R)-tert-butyldimethylsiloxane-androst-5,17-diene: add 26.0g compound 18 and 35.78g ethyltriphenylphosphonium bromide to 260mL tetrahydrofuran solvent , 10.81g potassium tert-butoxide was refluxed for reaction, extracted with petroleum ether, washed with saturated sodium chloride, dried with anhydrous sodium sulfate, concentrated in vacuo to obtain a yellowish solid, and purified by recrystallization to obtain 22.1g (17Z,3R)-tertiary Butyldimethylsiloxane-androsta-5,17-diene, denoted as compound 19.
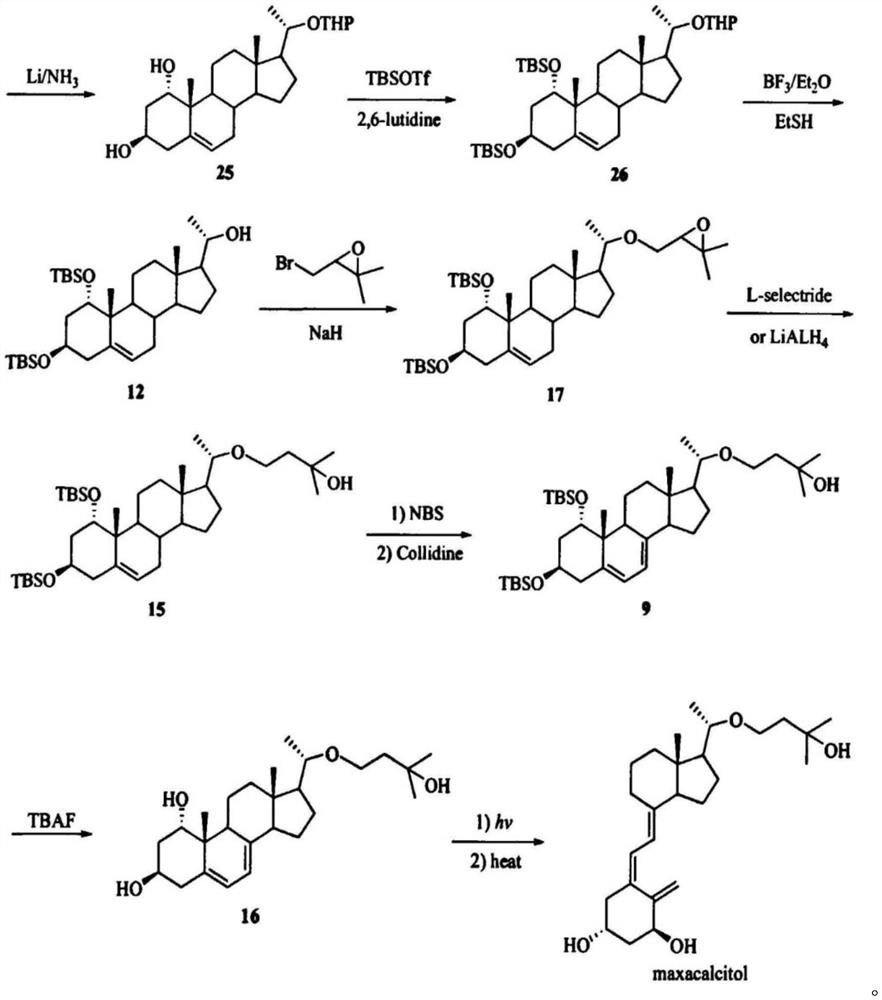
[0107] Preparation o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com