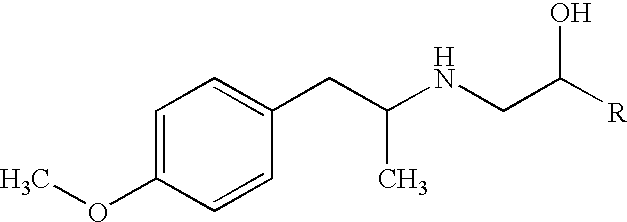
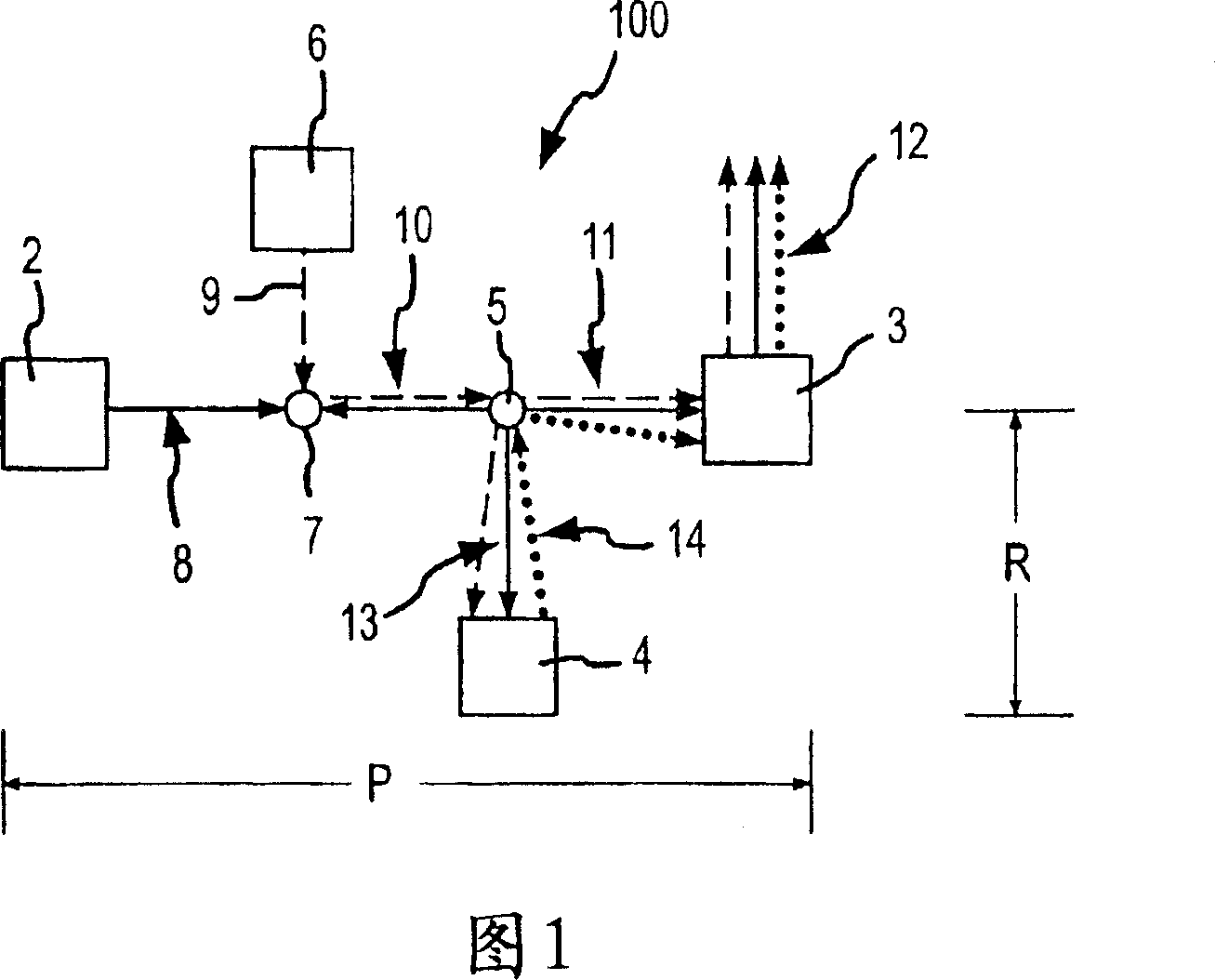
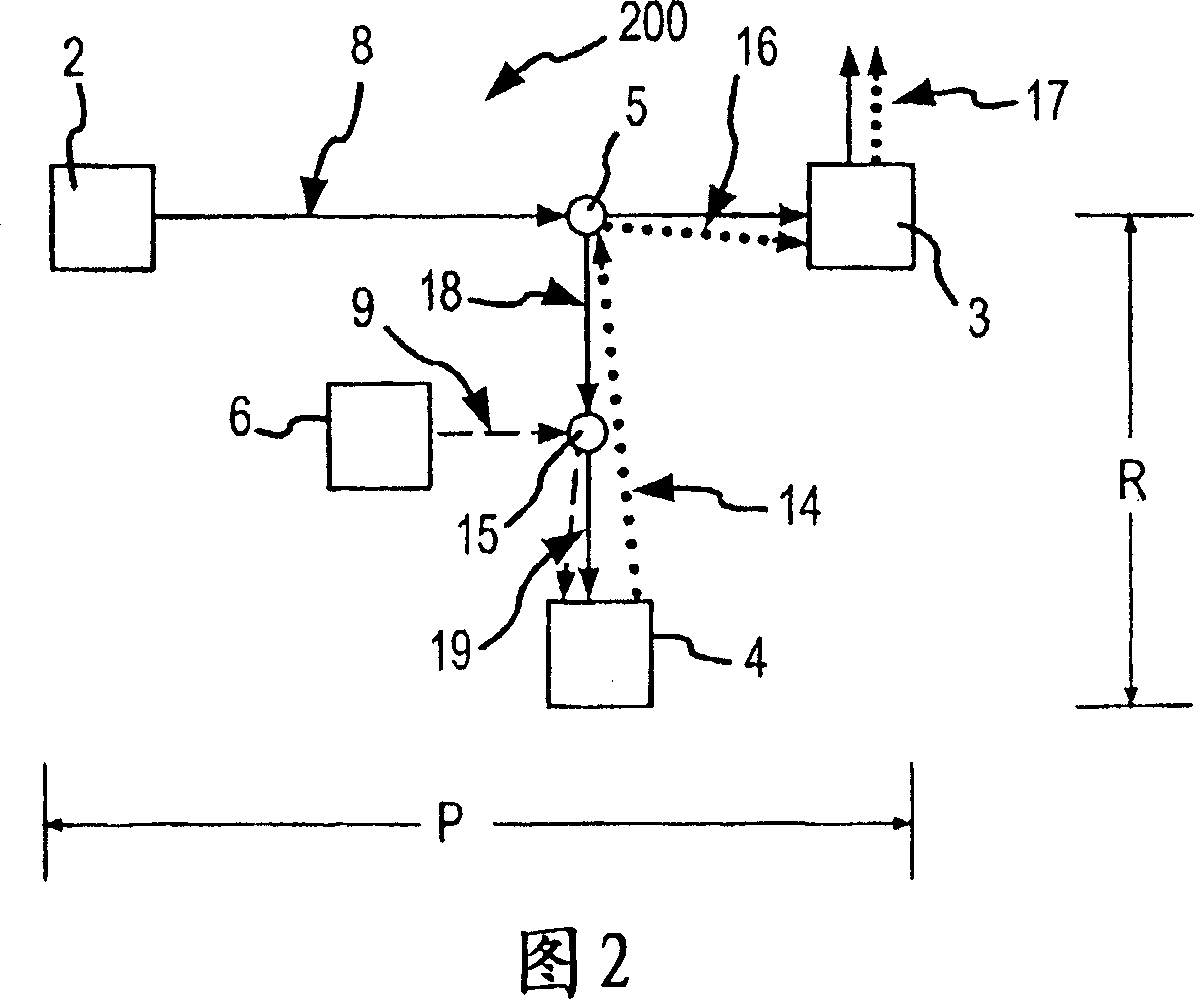
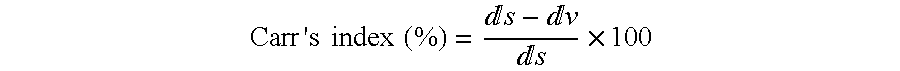Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1612 results about "Respiratory airway" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
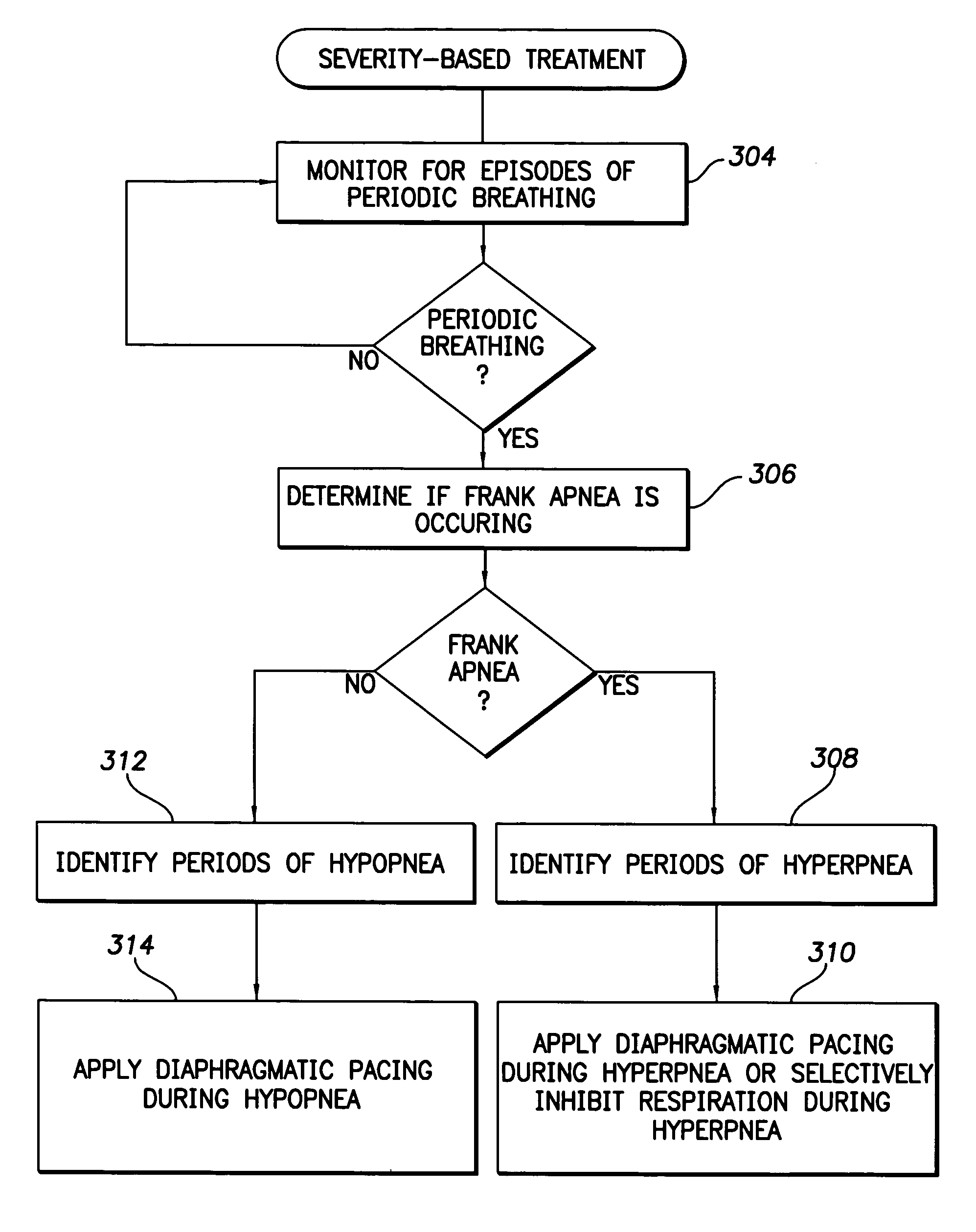
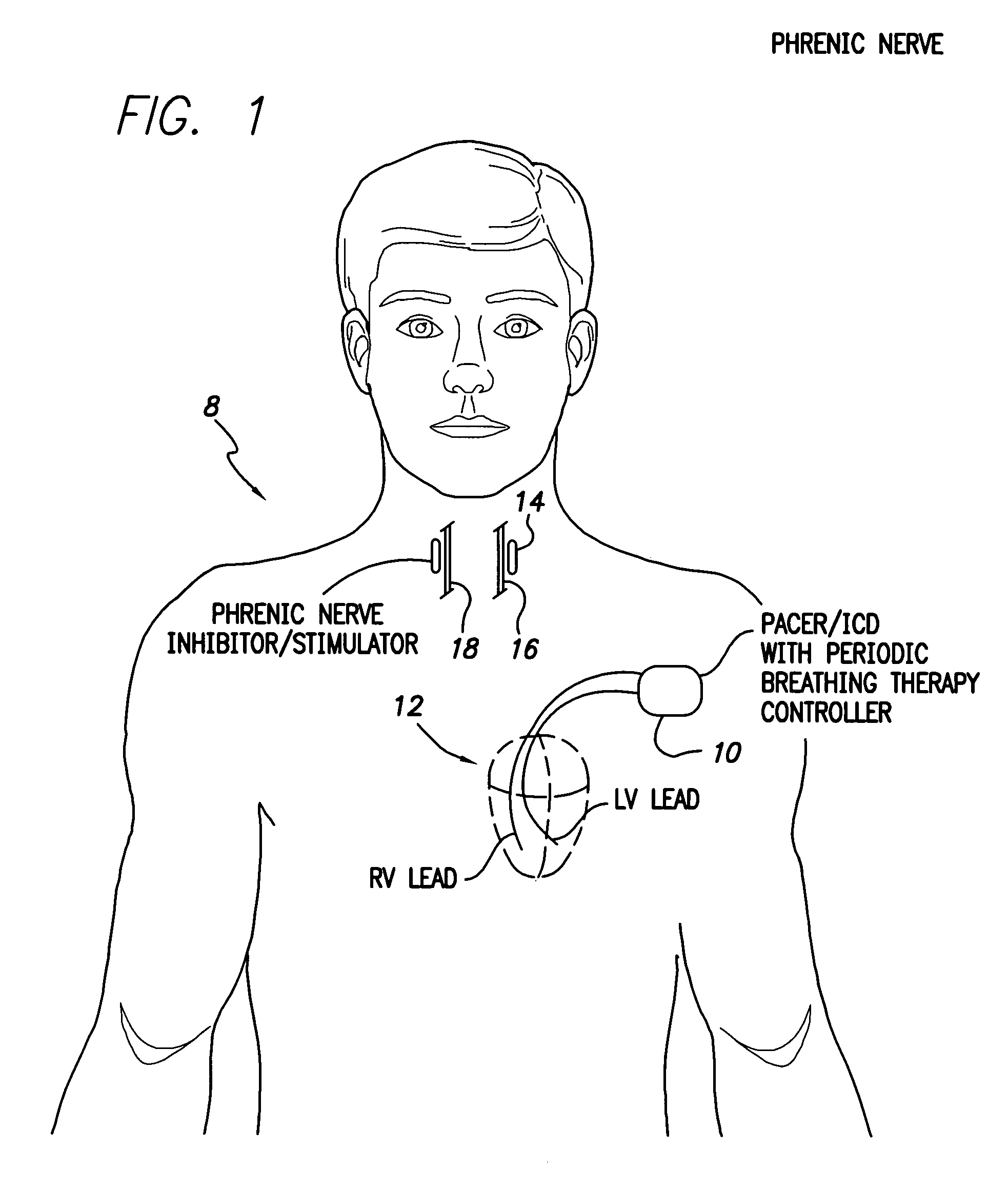
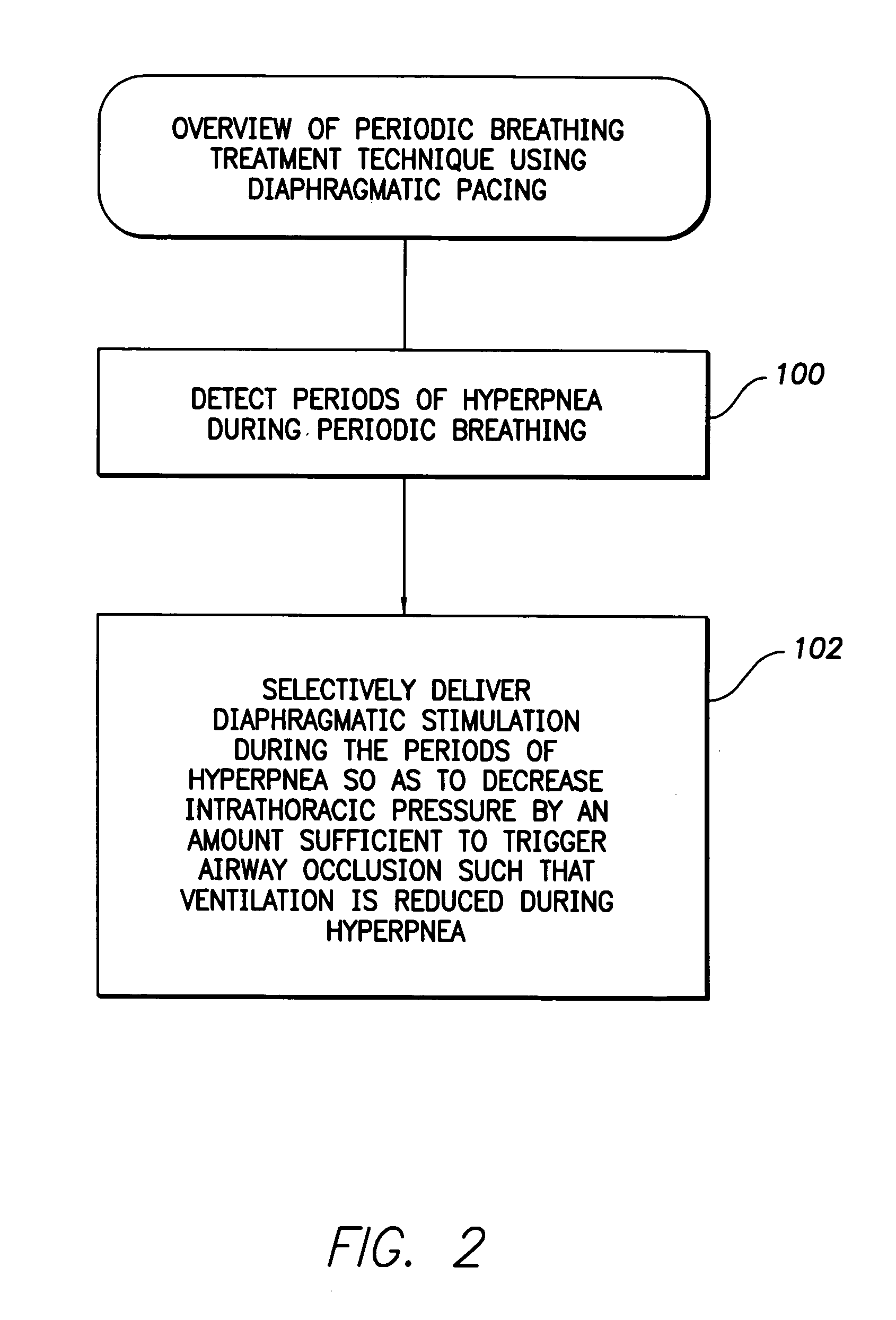
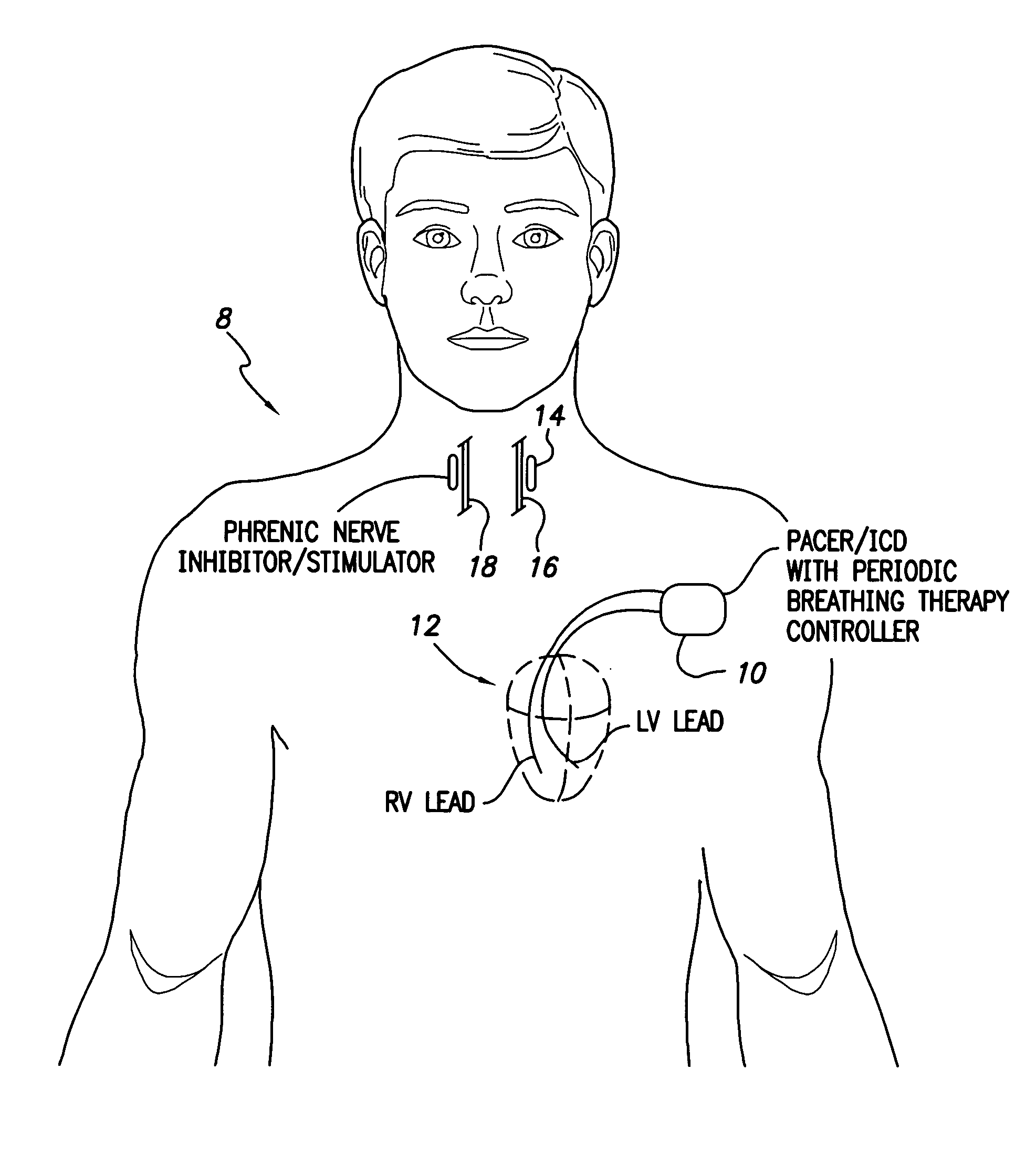
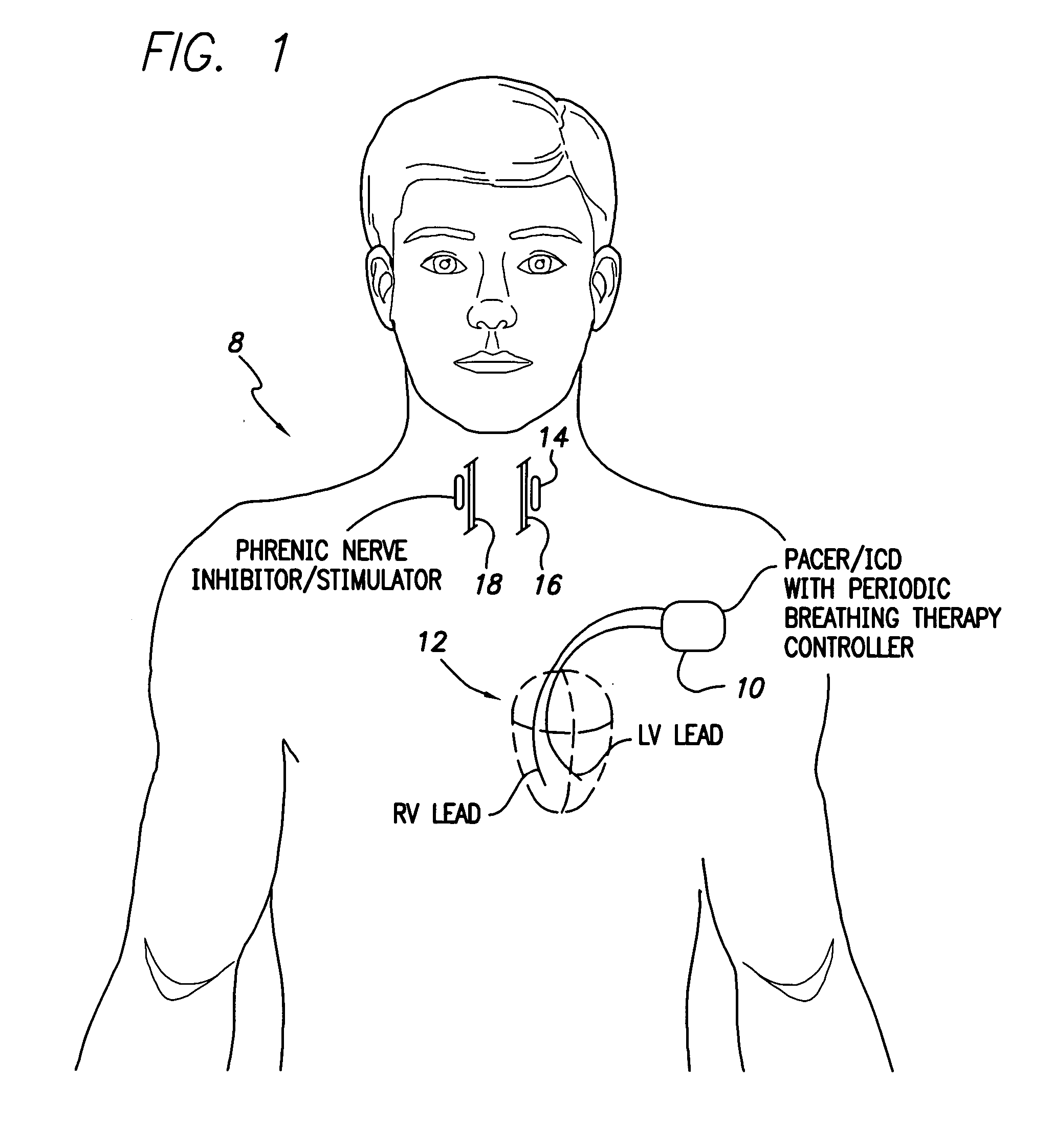
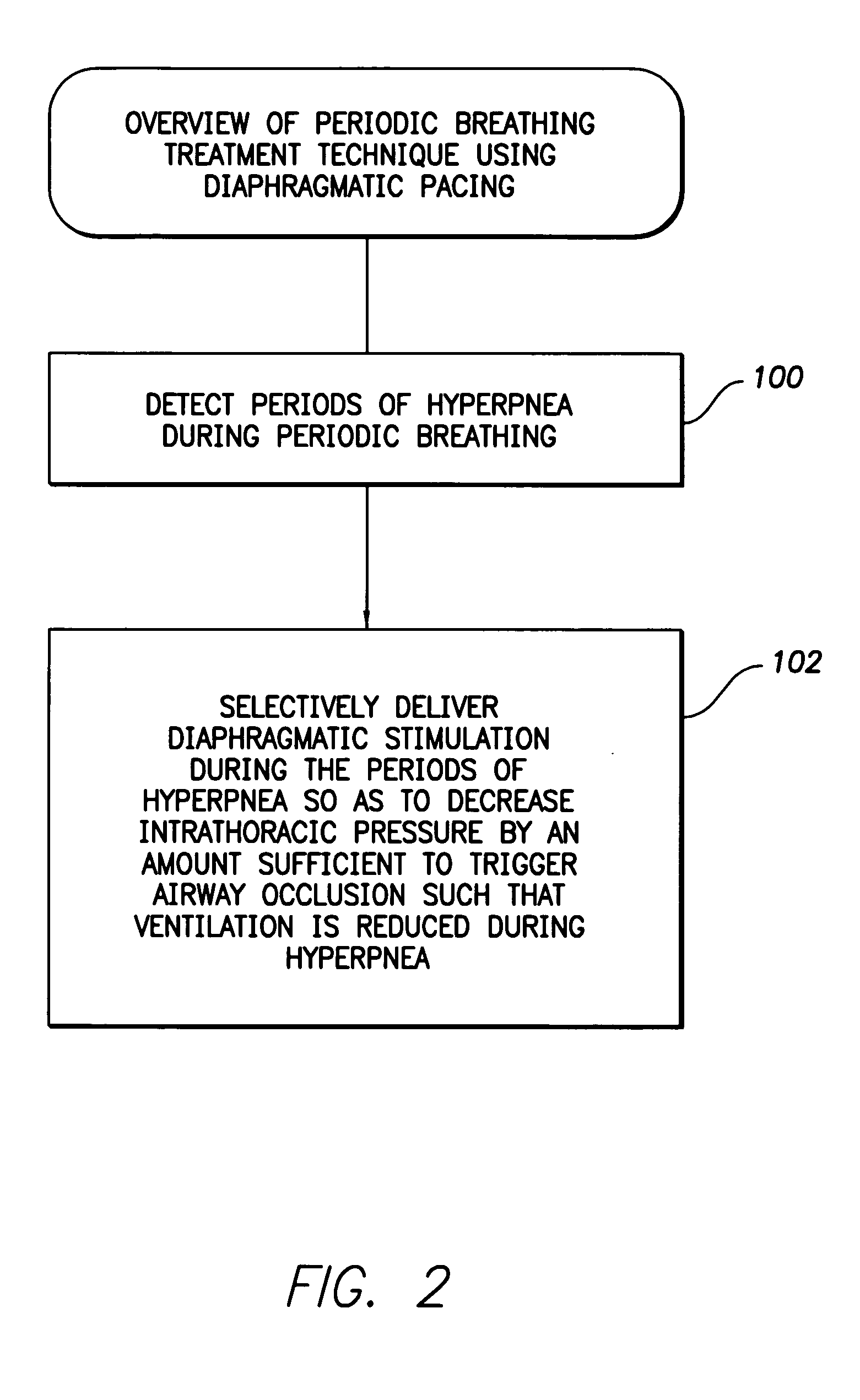
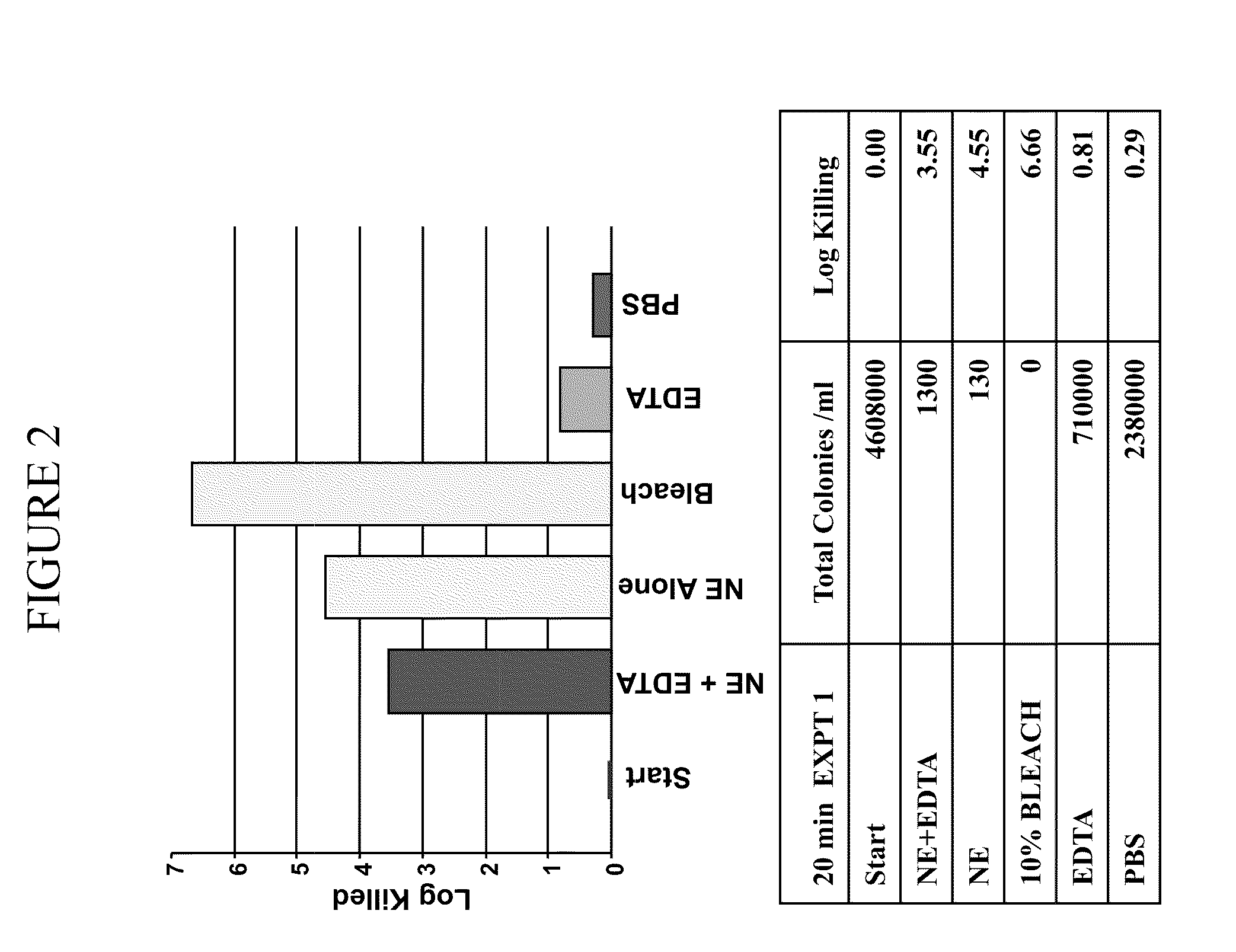
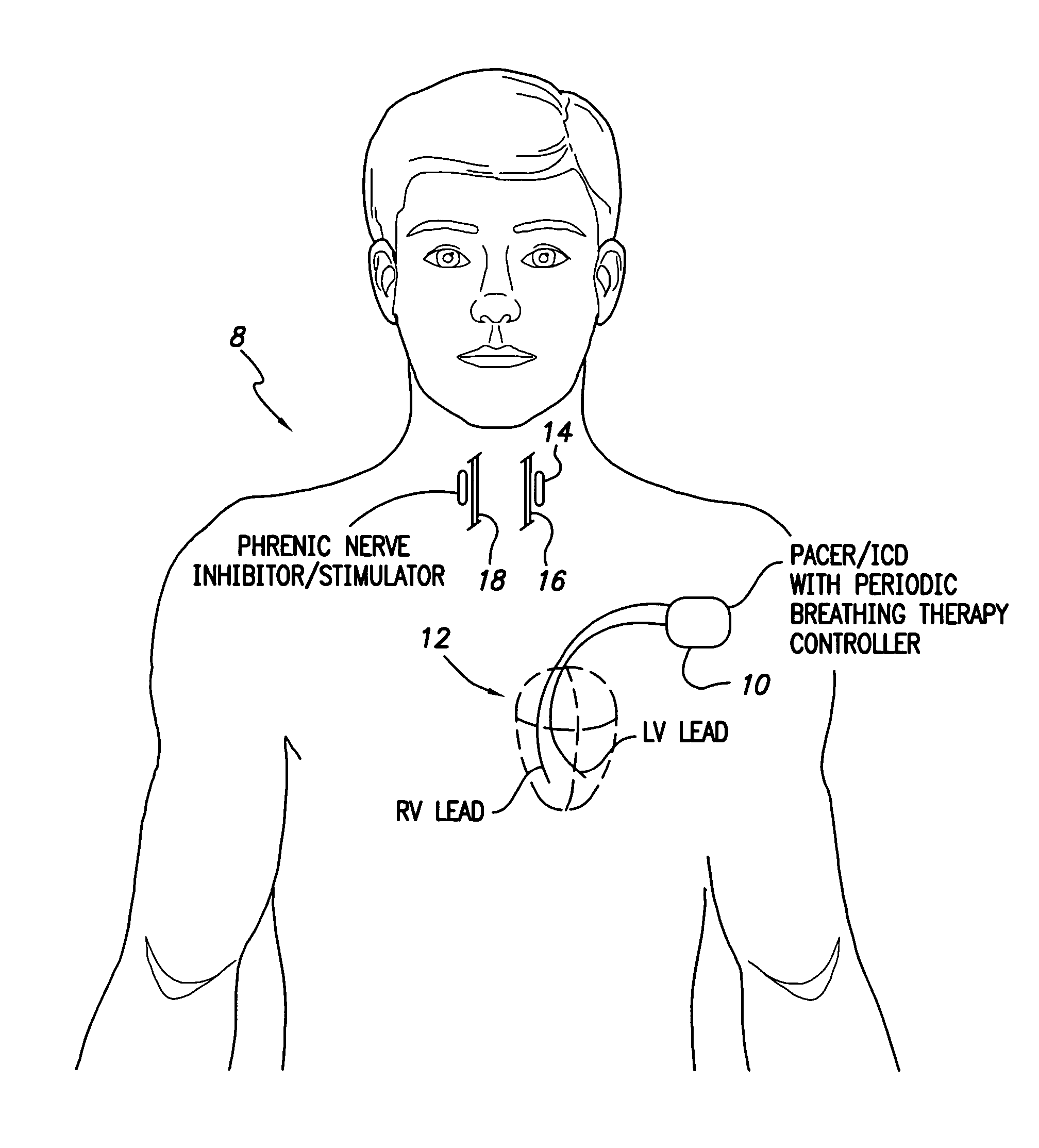
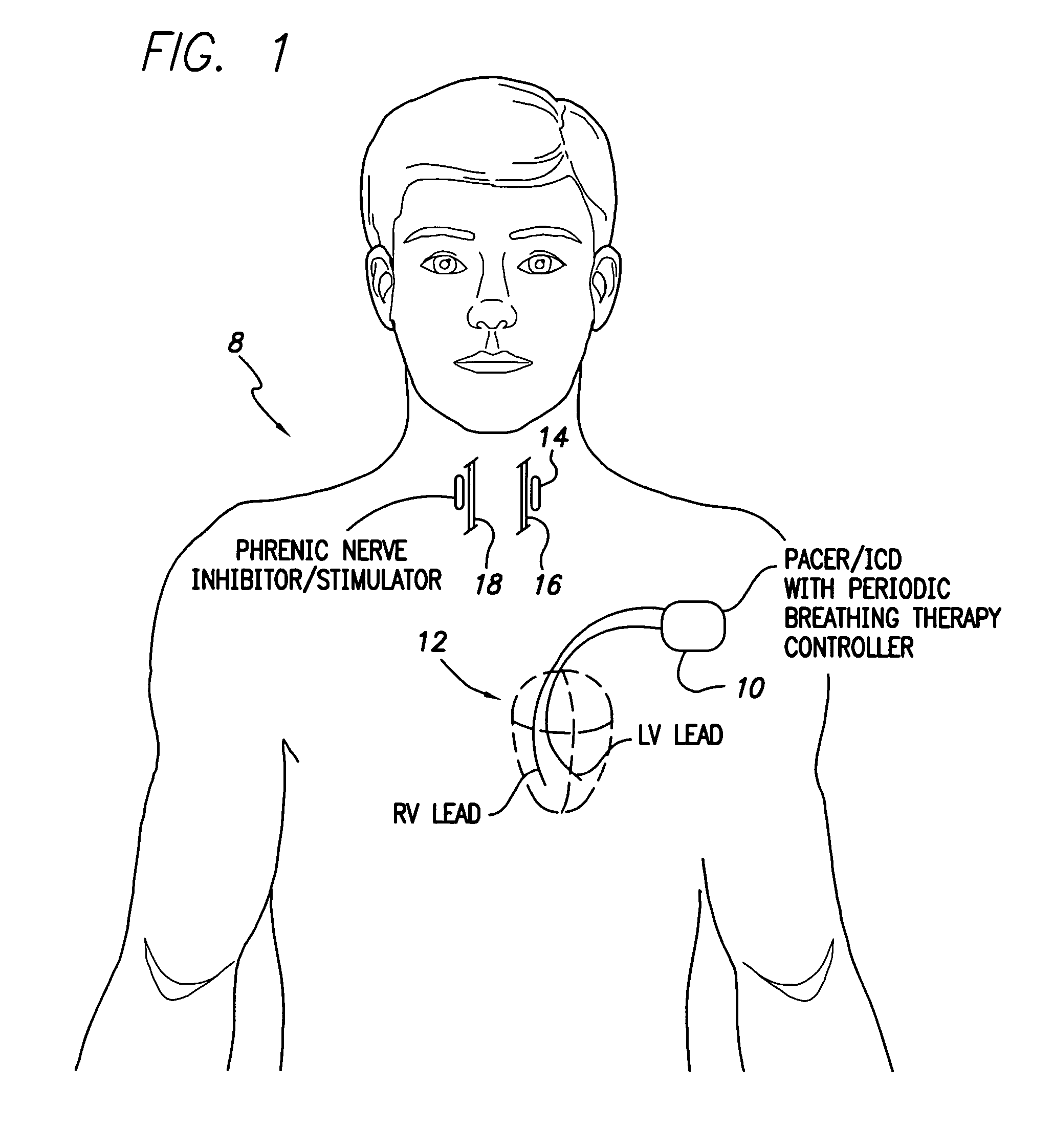
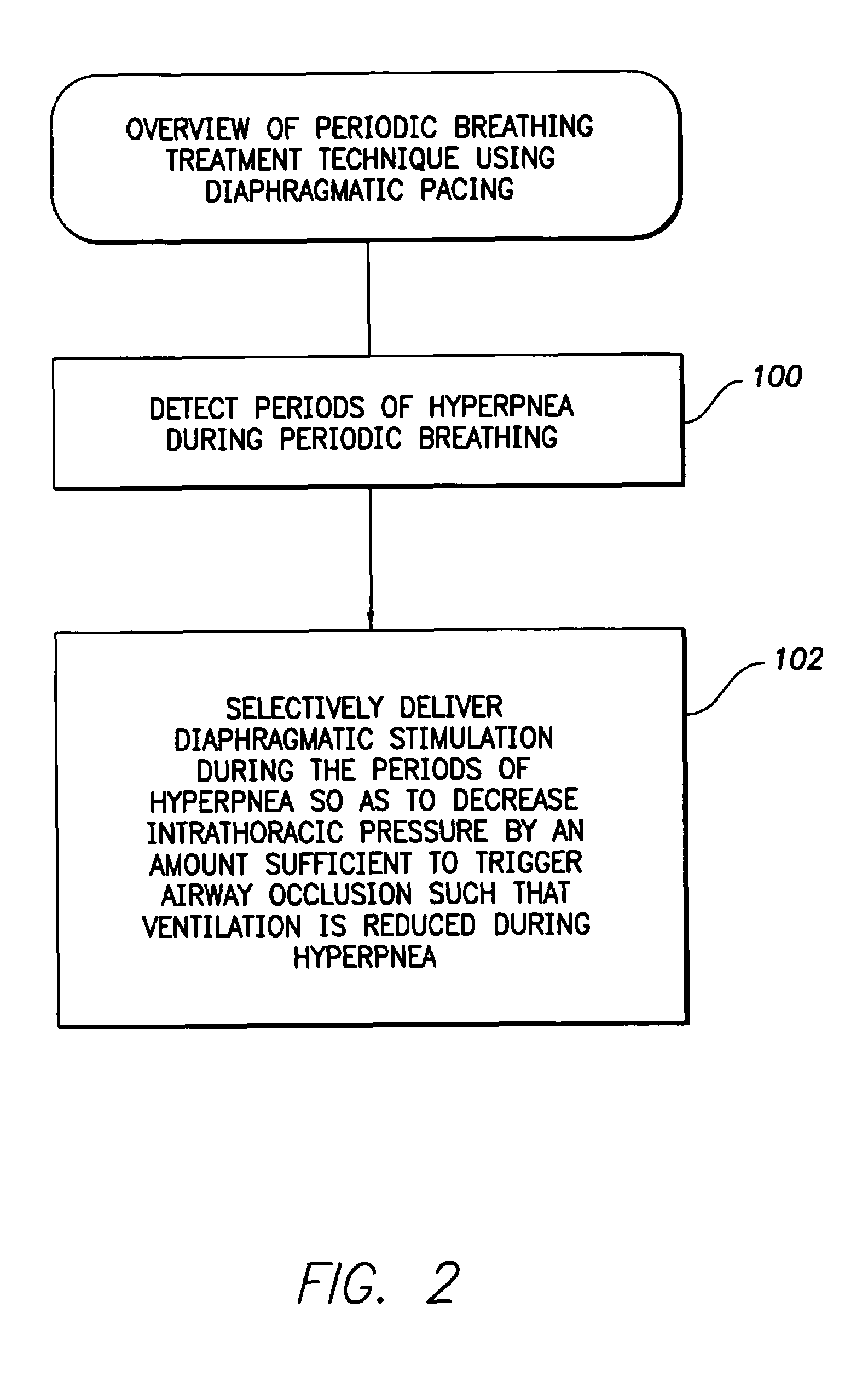
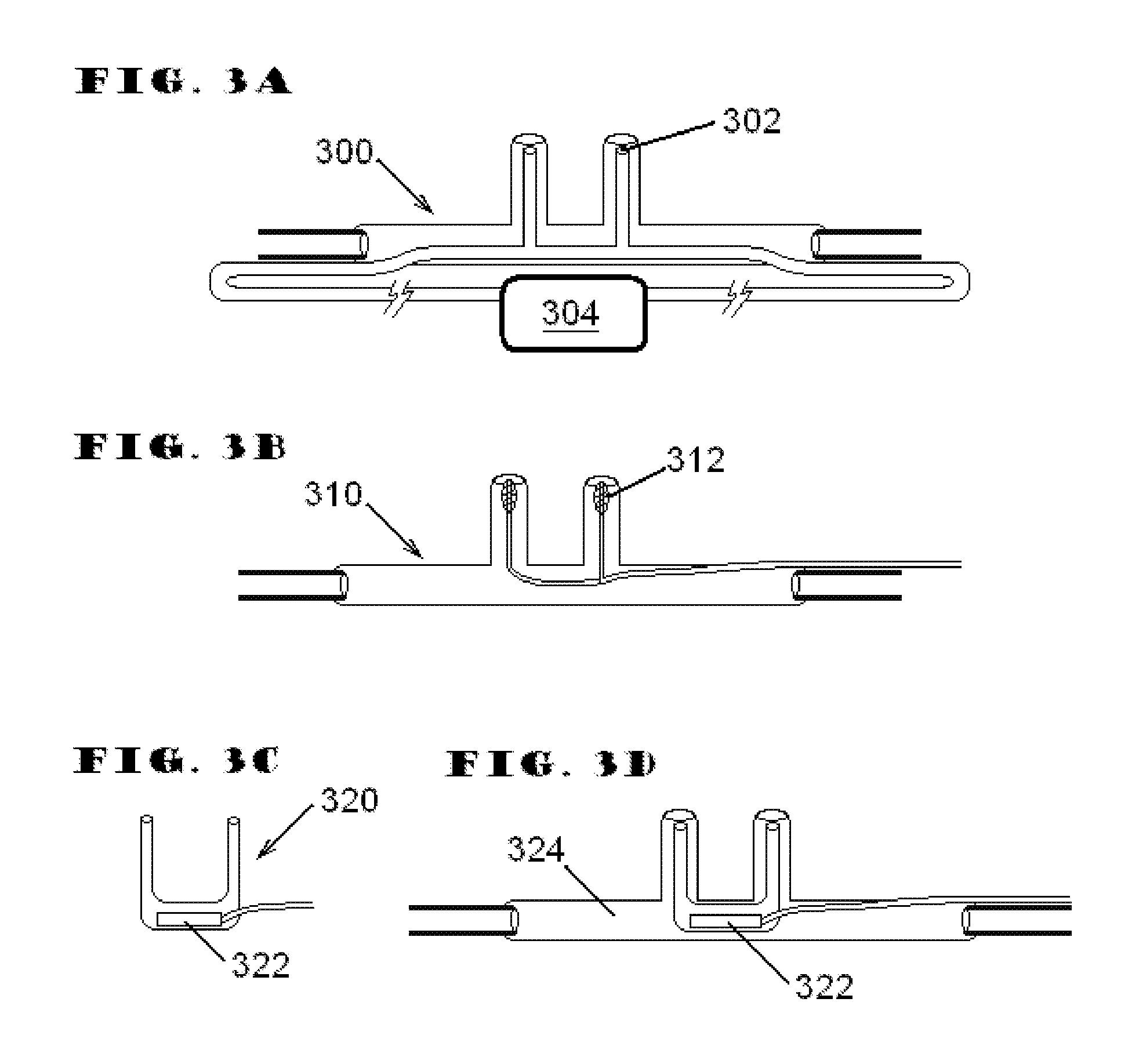
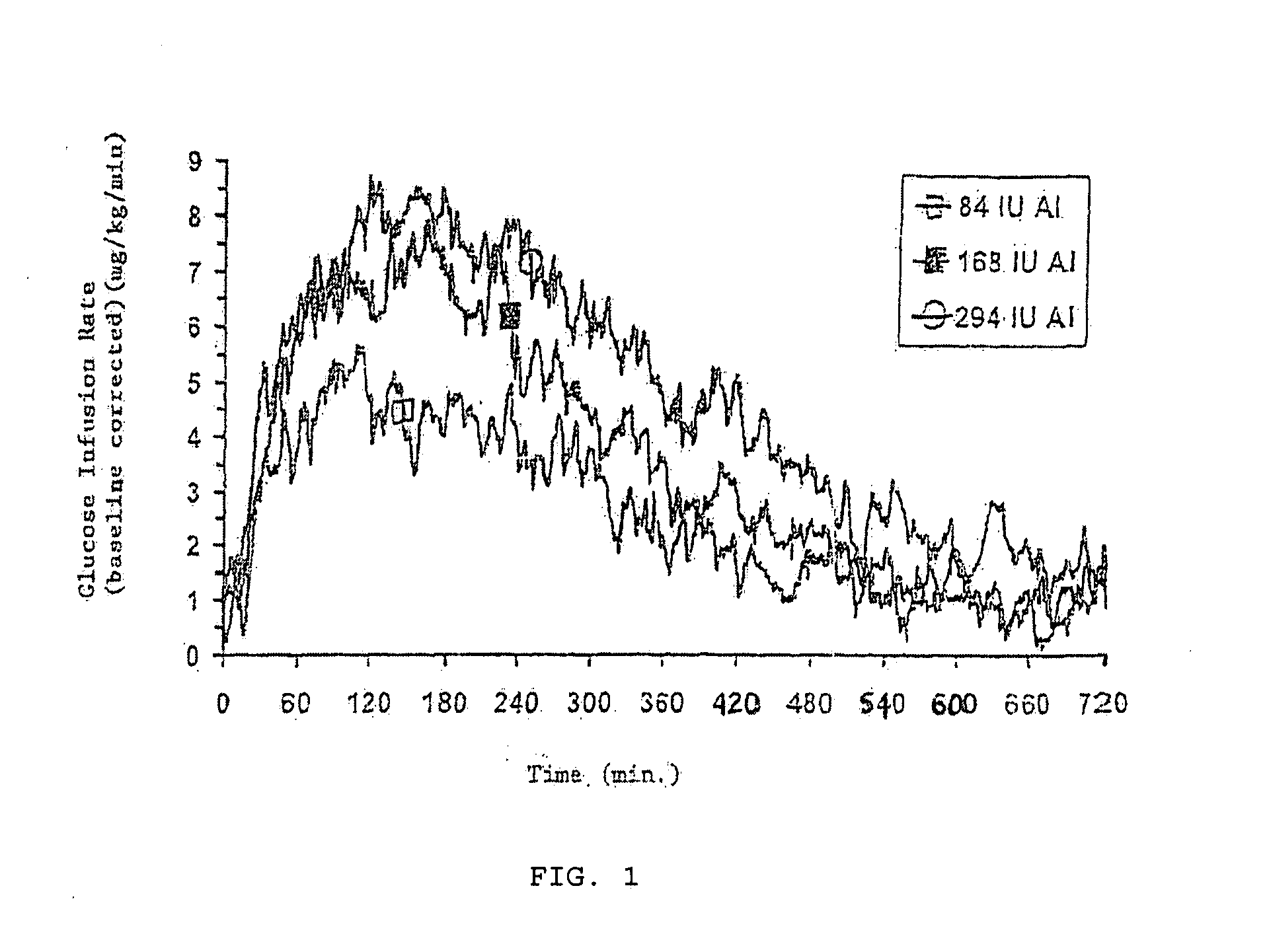
System and method for applying therapy during hyperpnea phase of periodic breathing using an implantable medical device
InactiveUS7082331B1Reducing cyclic blood chemistry imbalanceReduce ventilationElectrotherapyCheyne–Stokes respirationTreatment typology
Techniques are provided for treating periodic breathing, such as Cheyne-Stokes Respiration, using an implantable medical system. In one technique, diaphragmatic stimulation is delivered during a hyperpnea phase of periodic breathing via electrical stimulation of the phrenic nerves. Diaphragmatic stimulation is synchronized with intrinsic inspiration so as to increase the amplitude of diaphragmatic contraction during inspiration. This tends to decrease intrathoracic pressure leading to occlusion of the respiratory airway. Occlusion reduces actual ventilation during hyperpnea, thus reducing the cyclic blood chemistry imbalance that sustains periodic breathing so as to either mitigate periodic breathing or eliminate it completely. In another technique, respiration is instead inhibited during the hyperpnea phase of periodic breathing by blocking phrenic nerve signals to the extent necessary to reduce ventilation to terminate periodic breathing or at least mitigate its severity. Techniques are also described for controlling the type of therapy applied in response to periodic breathing.
Owner:PACESETTER INC
Aerosol-forming porous membrane with certain pore structure
InactiveUS6543442B2Improved size distributionMaximizing conversionRespiratorsLiquid spraying apparatusPorous membraneBiomedical engineering
A nozzle comprised of a thin, flexible membrane material having a plurality of pores is disclosed. In one embodiment, the pores have an unflexed exit aperture diameter in the range of about 0.5 to about 2 microns (preferably about 1 micron) and are positioned substantially uniformly in the material, preferably about 50 microns apart. The nozzle preferably has a conical or trumpet-shaped cross-section. In another aspect of the invention, the exit aperture of the nozzle is surrounded by an elevated area protruding above the substantially planar exit side of the membrane in order to prevent intrusion of liquid back into the nozzle. The nozzle can be used to form an aerosol containing a pharmaceutical composition from the exit side of the nozzle upon forcible application of the composition to the entrance side of the nozzle. This aerosol can be used to administer the pharmaceutical composition, for example, to the eye or to a selected portion of the respiratory tract. The nozzle is preferably a component of a container which holds a formulation of drug.
Owner:ARADIGM
Pulmonary delivery of aminoglycosides
InactiveUS7368102B2Eliminate side effectsReduce in quantityBiocidePowder deliveryRespiratory infectionTopical treatment
Owner:BGP PROD OPERATIONS GMBH +1
Compositions & formulations with an epiandrosterone or a ubiquinone & kits & their use for treatment of asthma symptoms & for reducing adenosine/adenosine receptor levels
A composition and various formulations comprise preventative or therapeutic amounts of an epiandrosterone, analogue thereof or salt thereof, and / or a ubiquinone or salt thereof, and a pharmaceutically or veterinarily acceptable carrier or diluent. The composition and formulations are useful for treating bronchoconstriction, respiratory tract inflammation and allergies, asthma, and cancer. A method of treating diseases associated with low adenosine levels or adenosine depletion comprises administering folinic acid or a pharmaceutically acceptable salt hereof in a preventative or therapeutic amount, or an amount effective to treat adenosine depletion.
Owner:EAST CAROLINA UNIVERISTY
Method for stabilizing biomolecules in liquid formulations
InactiveUS20020110524A1Improve stabilityOrganic active ingredientsFactor VIIActive proteinPharmaceutical medicine
The invention is directed to a stable formulation of a biologically active protein useful for aerosol delivery to the respiratory tract of a patient in need of treatment comprising: (a) a carrier liquid comprising from about 10% to from about 100% V / V water and from about 0% to from about 90% V / V of an organic liquid; (b) a biologically effective amount of a protein suspended or dissolved in a carrier liquid; and (c) a stabilizing effective amount of a derivatized carbohydrate stabilizing agent suspended or dissolved in said carrier liquid. The stable formulations of the invention may optionally contain about 0.1% to about 5.0% W / V of a pharmaceutically acceptable excipient.
Owner:BATTELLE MEMORIAL INST
Pharmaceutical formulations for dry powder inhalers in the form of hard-pellets
InactiveUS20030180227A1Reduce intensityEfficient deliveryPowder deliveryDispersion deliveryPrillInhalation
The invention provides a formulation to be administered as dry powder for inhalation suitable for efficacious delivery of active ingredients into the low respiratory tract of patients suffering of pulmonary diseases such as asthma. In particular, the invention provides a formulation to be administered as dry powder for inhalation freely flowable, which can be produced in a simple way, physically and chemically stable and able of delivering either accurate doses and high fine particle fraction of low strength active ingredients by using a high- or medium resistance device.
Owner:CHIESI FARM SPA
Compositions and methods for treating asthma and other lung disorders
Provided are compositions and methods for treating or preventing lung or respiratory disorders or conditions characterized by airflow obstruction or limitation, or a symptom thereof (e.g., asthma, rhinitis, allergic rhinitis (e.g. nose respiratory tract), and chronic obstructive pulmonary disease (COPD) and COPD-associated conditions (e.g., bronchitis, emphysema, asthma), emphysema, pneumonia, bronchitis, influenza, SARS, tuberculosis, and whooping cough (pertussis), and the like) in a subject in need thereof by administering a therapeutic composition comprising at least one electrokinetically generated fluid (including gas-enriched electrokinetically generated fluids) as disclosed herein, the electrokinetically altered aqueous fluid suitable to alter cellular membrane structure or function sufficient to provide for modulation of intracellular signal transduction, wherein treating a lung disorder or a symptom thereof is thereby afforded. Additional aspects relate to therapeutic compositions, and combination treatment methods comprising administration of at least one electrokinetically generated fluid in combination with at least one additional therapeutic agent.
Owner:REVALESIO CORP
Methods of testing for bronchial asthma or chronic obstructive pulmonary disease
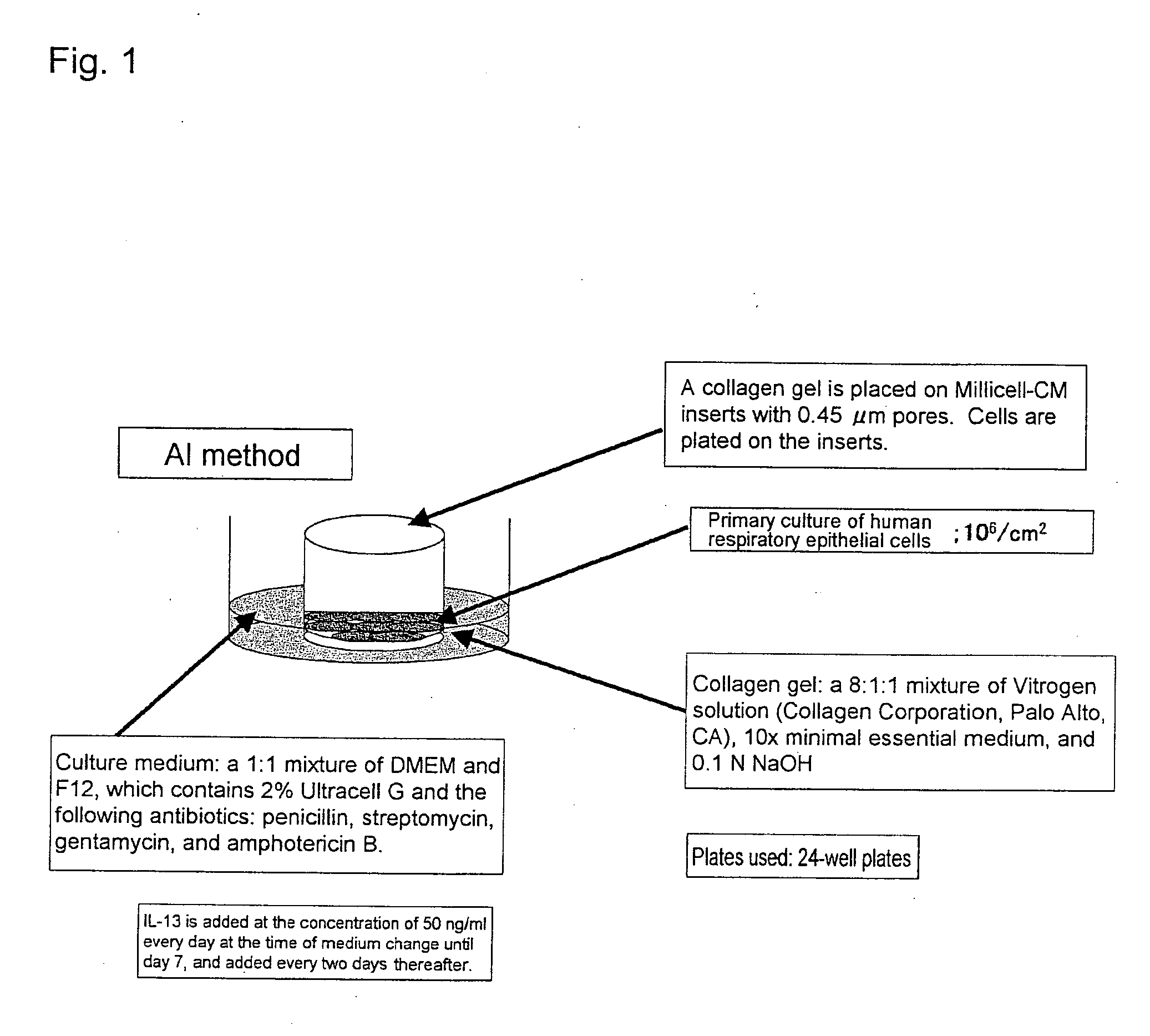
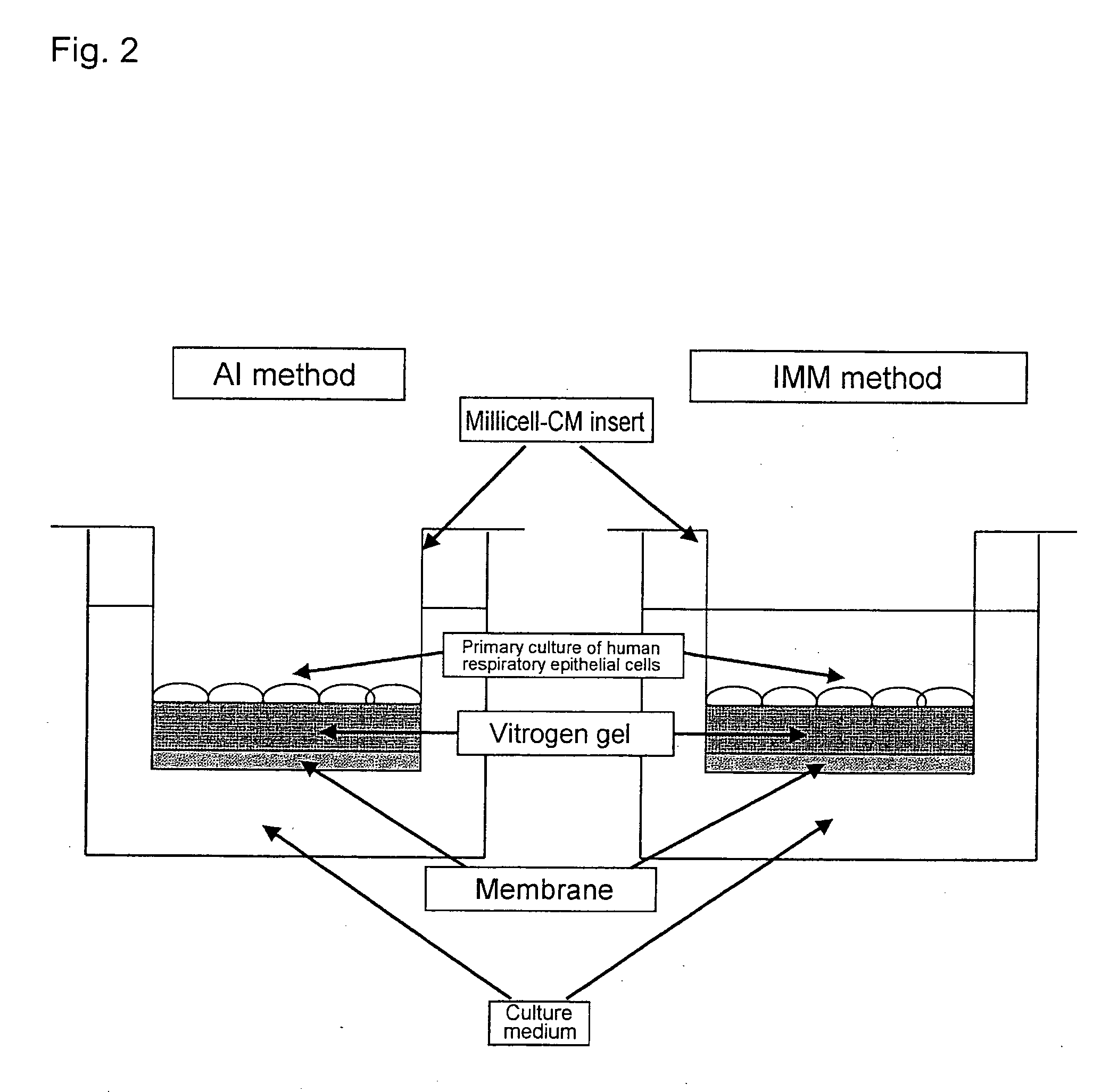
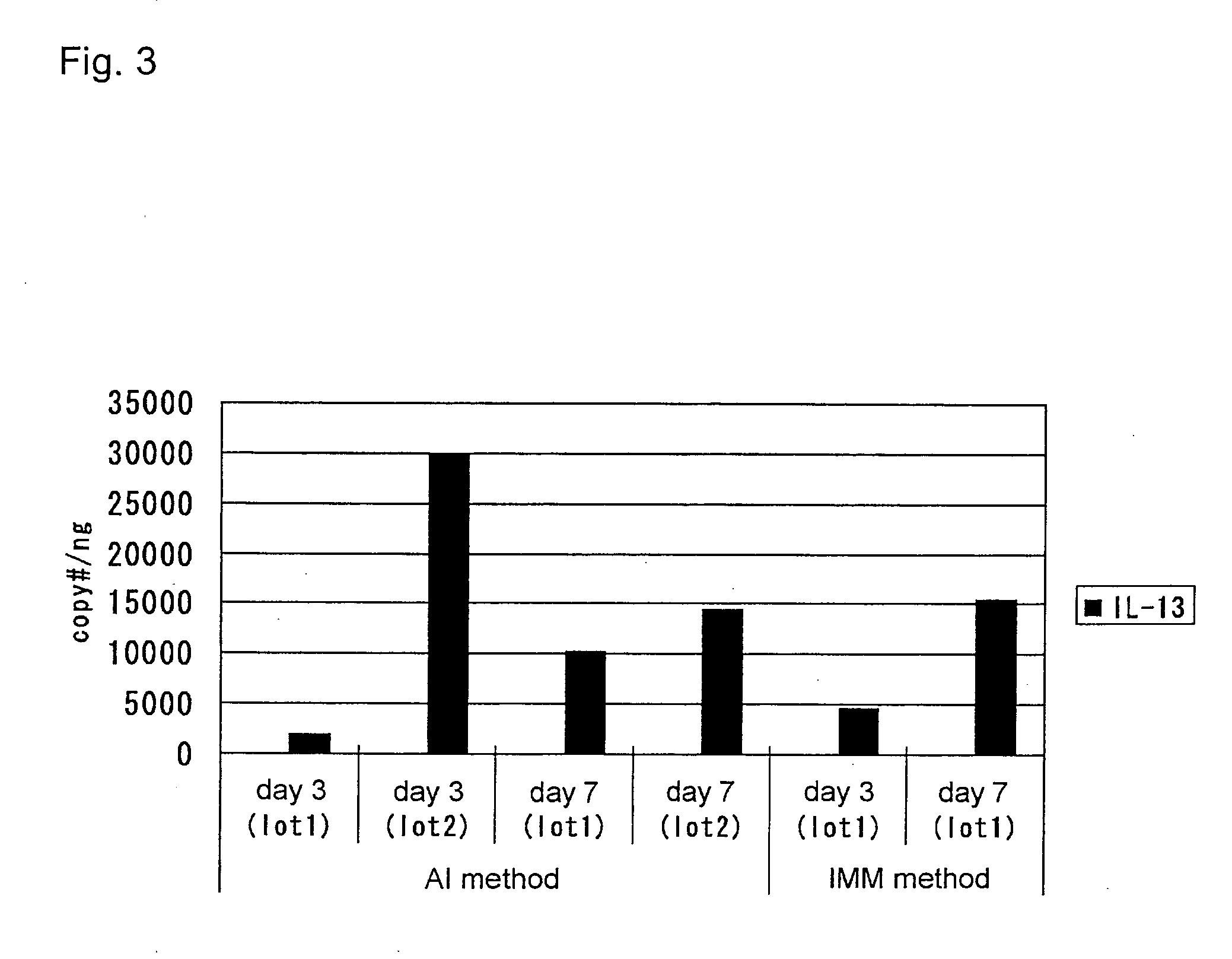
An objective of the present invention is to provide a method of testing for bronchial asthma or chronic obstructive pulmonary disease, a method of screening for candidate compounds for treating bronchial asthma or chronic obstructive pulmonary disease, and a pharmaceutical agent for treating bronchial asthma or chronic obstructive pulmonary disease. The present invention identified genes whose expression levels varied between respiratory epithelial cells that had been stimulated by IL-13 to induce the goblet cell differentiation, and unstimulated respiratory epithelial cells. The respiratory epithelial cells were cultured according to the air interface method. The genes were revealed to be useful as markers for testing for bronchial asthma or chronic obstructive pulmonary disease and screening for therapeutic agents for such diseases. Specifically, the present invention provides methods of testing for bronchial asthma or chronic obstructive pulmonary disease and methods of screening for compounds to treat the diseases based on the comparison of the expression levels of marker genes identified as described above.
Owner:GENOX RES
System and method for applying therapy during hyperpnea phase of periodic breathing using an implantable medical device
ActiveUS20050240240A1Reducing cyclic blood chemistry imbalanceLowering intrathoracic pressureElectrotherapyRespiratory organ evaluationCheyne–Stokes respirationTreatment typology
Techniques are provided for treating periodic breathing, such as Cheyne-Stokes Respiration, using an implantable medical system. In one technique, diaphragmatic stimulation is delivered during a hyperpnea phase of periodic breathing via electrical stimulation of the phrenic nerves. Diaphragmatic stimulation is synchronized with intrinsic inspiration so as to increase the amplitude of diaphragmatic contraction during inspiration. This tends to decrease intrathoracic pressure leading to occlusion of the respiratory airway. Occlusion reduces actual ventilation during hyperpnea, thus reducing the cyclic blood chemistry imbalance that sustains periodic breathing so as to either mitigate periodic breathing or eliminate it completely. In another technique, respiration is instead inhibited during the hyperpnea phase of periodic breathing by blocking phrenic nerve signals to the extent necessary to reduce ventilation to terminate periodic breathing or at least mitigate its severity. Techniques are also described for controlling the type of therapy applied in response to periodic breathing.
Owner:PACESETTER INC
Nanoemulsion therapeutic compositions and methods of using the same
The present invention relates to therapeutic nanoemulsion compositions and to methods of utilizing the same. In particular, nanoemulsion compositions are described herein that find use in the treatment and / or prevention of infection (e.g., respiratory infection (e.g., associated with cystic fibrosis)), in burn wound management, and in immunogenic compositions (e.g., comprising a Burkholderia antigen) that generate an effective immune response (e.g., against a bacterial species of the genus Burkholderia) in a subject administered the immunogenic composition. Compositions and methods of the present invention find use in, among other things, clinical (e.g. therapeutic and preventative medicine), industrial, and research applications.
Owner:NANOBIO CORP
Method for treating a respiratory disease
The invention provides a novel method of treating respiratory diseases, e.g., pediatric asthma, in a continuing regimen with not more than one daily dose of the drug budesonide using a nebulizer.
Owner:ASTRAZENECA AB
System and method for applying therapy during hyperpnea phase of periodic breathing using an implantable medical device
ActiveUS7245971B2Reduce ventilationReducing the cyclic blood chemistry imbalanceElectrotherapyRespiratory organ evaluationCheyne–Stokes respirationElectrical stimulations
Techniques are provided for treating periodic breathing, such as Cheyne-Stokes Respiration, using an implantable medical system. In one technique, diaphragmatic stimulation is delivered during a hyperpnea phase of periodic breathing via electrical stimulation of the phrenic nerves. Diaphragmatic stimulation is synchronized with intrinsic inspiration so as to increase the amplitude of diaphragmatic contraction during inspiration. This tends to decrease intrathoracic pressure leading to occlusion of the respiratory airway. Occlusion reduces actual ventilation during hyperpnea, thus reducing the cyclic blood chemistry imbalance that sustains periodic breathing so as to either mitigate periodic breathing or eliminate it completely. In another technique, respiration is instead inhibited during the hyperpnea phase of periodic breathing by blocking phrenic nerve signals to the extent necessary to reduce ventilation to terminate periodic breathing or at least mitigate its severity. Techniques are also described for controlling the type of therapy applied in response to periodic breathing.
Owner:PACESETTER INC
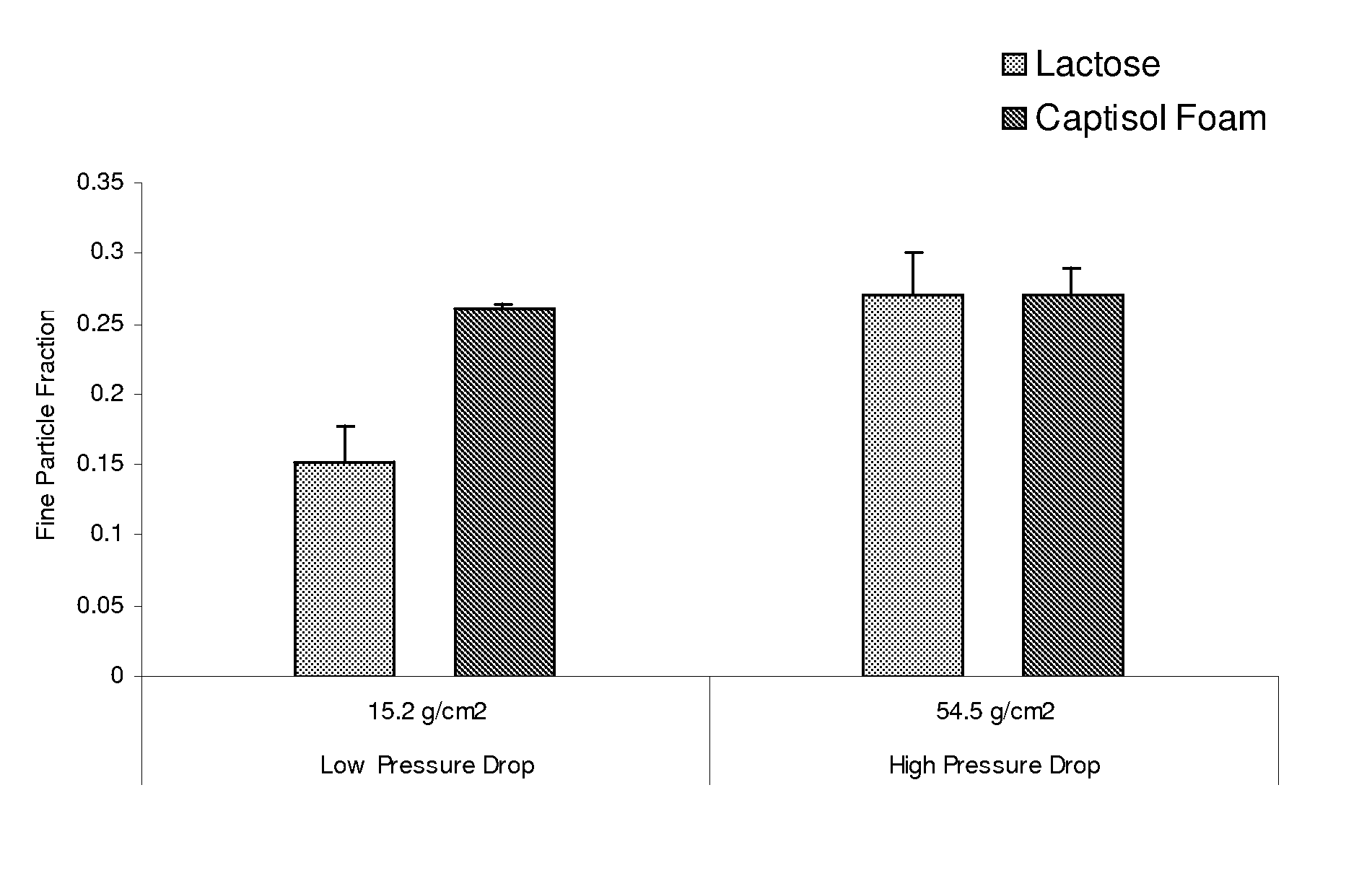
Dpi formulation containing sulfoalkyl ether cyclodextrin
ActiveUS20070175472A1Increase pressure dropSuitable for useOrganic active ingredientsPowder deliveryThroatActive agent
An inhalable dry powder formulation containing SAE-CD and an active agent is provided. The formulation is adapted for administration by DPI. The SAE-CD serves as a carrier rather than as an absorption enhancer. The average particle size of the SAE-CD is large enough to preclude (for the most part) pulmonary deposition thereof. Following release from the DPI device, the SAE-CD-containing particles dissociate from the active agent-containing particles in the buccal cavity or throat, after which the active agent-containing particles continue deeper into the respiratory tract. The physicochemical and morphological properties of the SAE-CD are easily modified to permit optimization of active agent and carrier interactions. Drugs having a positive, neutral or negative electrostatic charge can be delivered by DPI when SAE-CD is used as a carrier.
Owner:CYDEX PHARMACEUTICALS INC
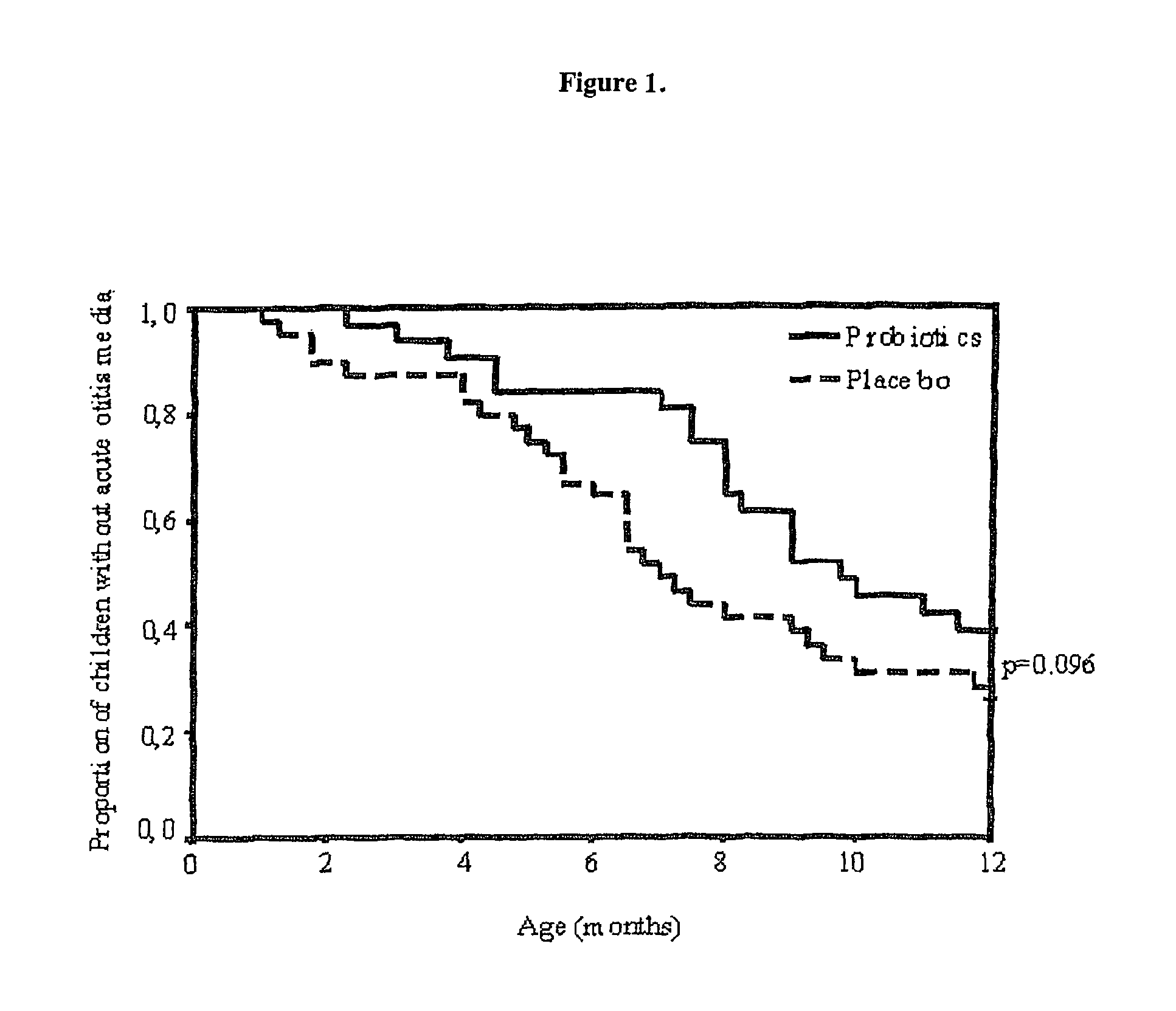
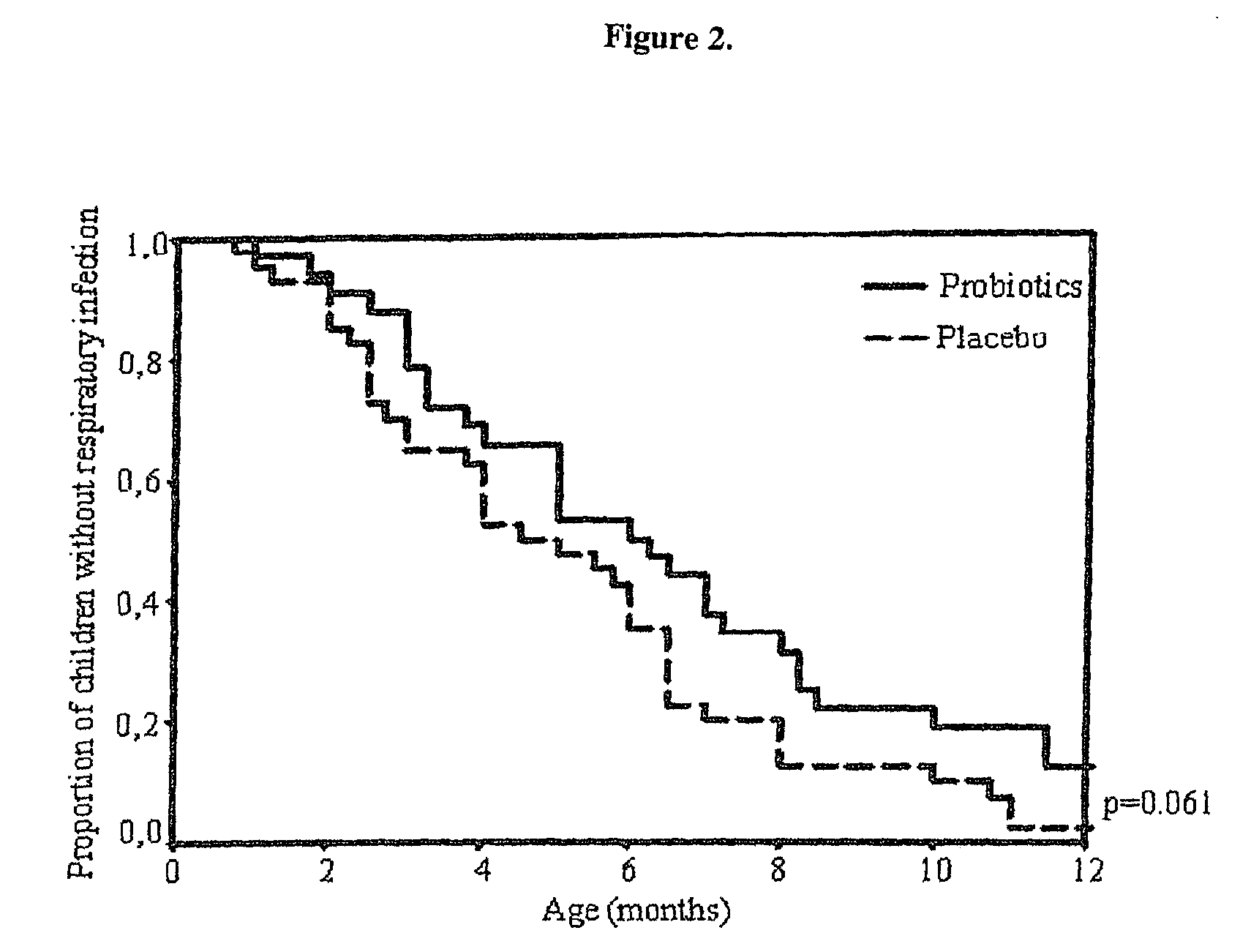
Method for preventing or treating respiratory infections and acute otitis media in infants using Lactobacillus rhamnosus LGG and Bifidobacterium lactis Bb-12
ActiveUS7862808B2Easy adhesionPromote growthBiocideSenses disorderLactobacillus rhamnosusProbiotic bacterium
The present invention is directed to a novel method for preventing or treating respiratory infections and acute otitis media in infants. The method comprises the administration of a therapeutically effective amount of a Bifidobacteria strain and an adherence-promoting probiotic, such as LGG, to the infant.
Owner:MEAD JOHNSON NUTRITION
Device and method for opening an airway
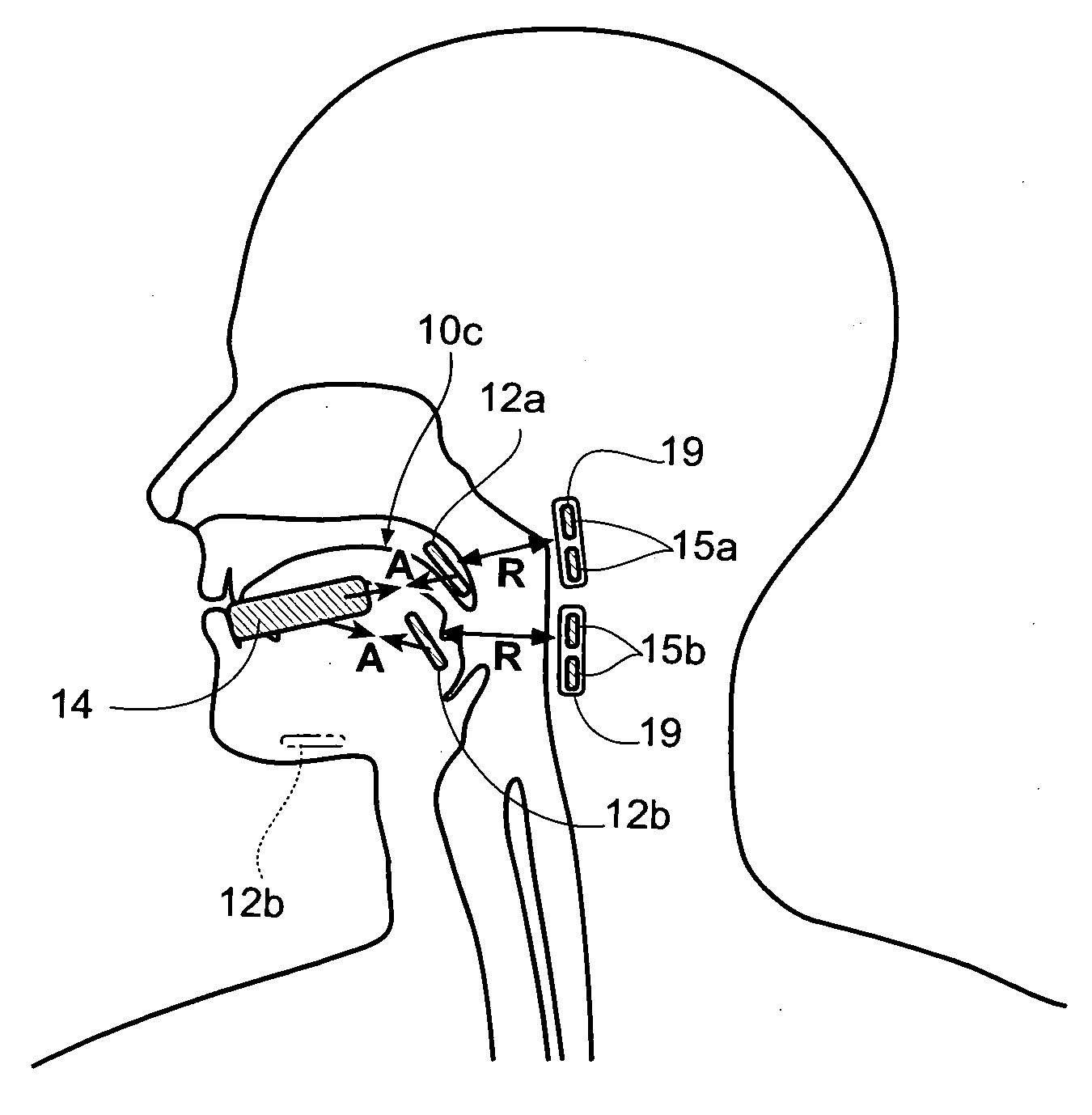
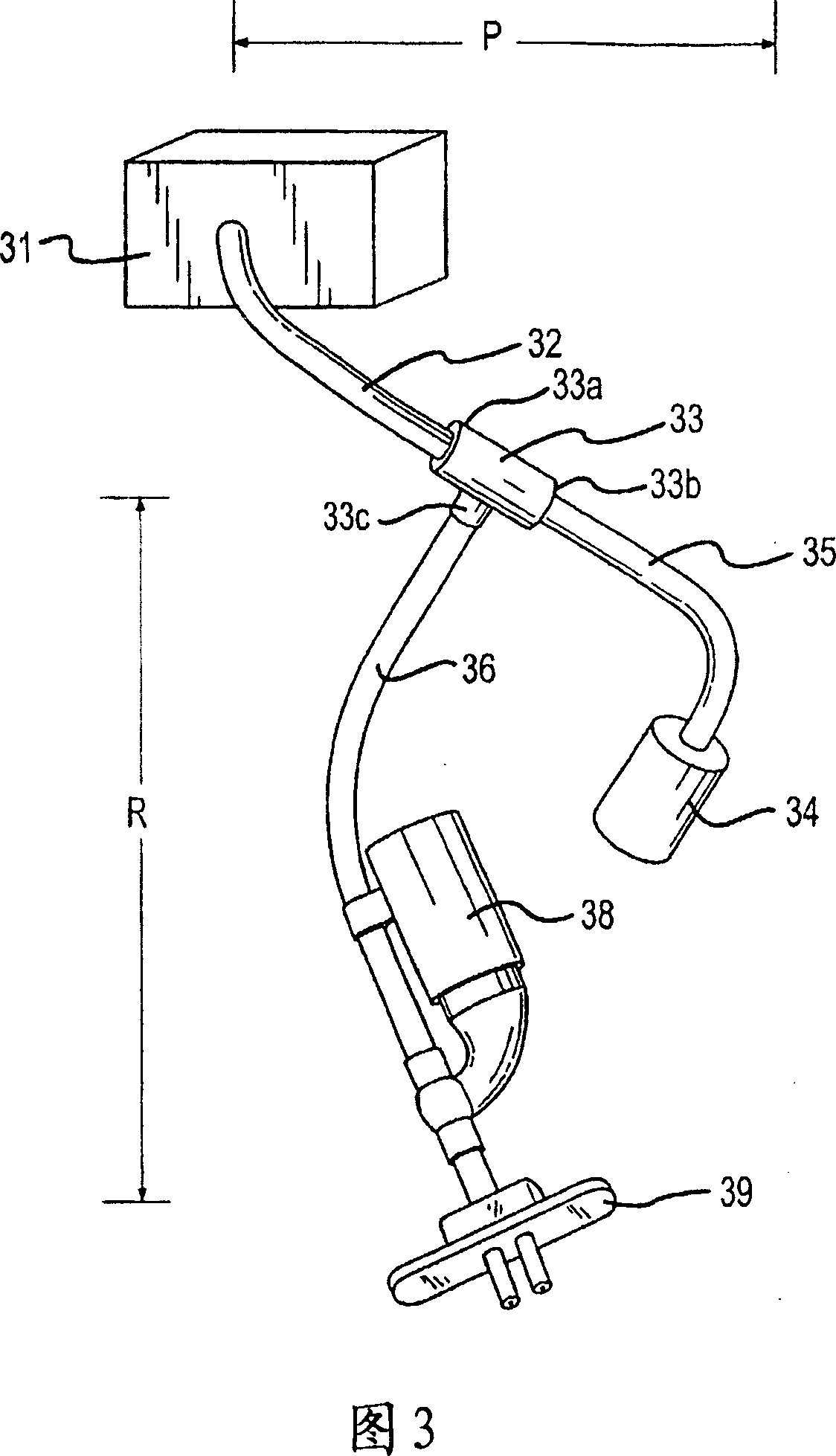
ActiveUS20110066086A1Convenient and smoothEasy to replaceSnoring preventionSuction-kneading massageChinAnterior triangle
A device and a method for creating and / or maintaining an obstruction free upper respiratory passages. The device is configured to fit under the chin of a subject adjacent to the subject's neck at an external location corresponding approximately with the subject's internal soft tissue associated with the neck's anterior triangle. The device is capable of exerting negative pressure on the surface of a subject's neck, displacing the soft tissue forward and enlarging the airway.
Owner:SOMMETRICS INC
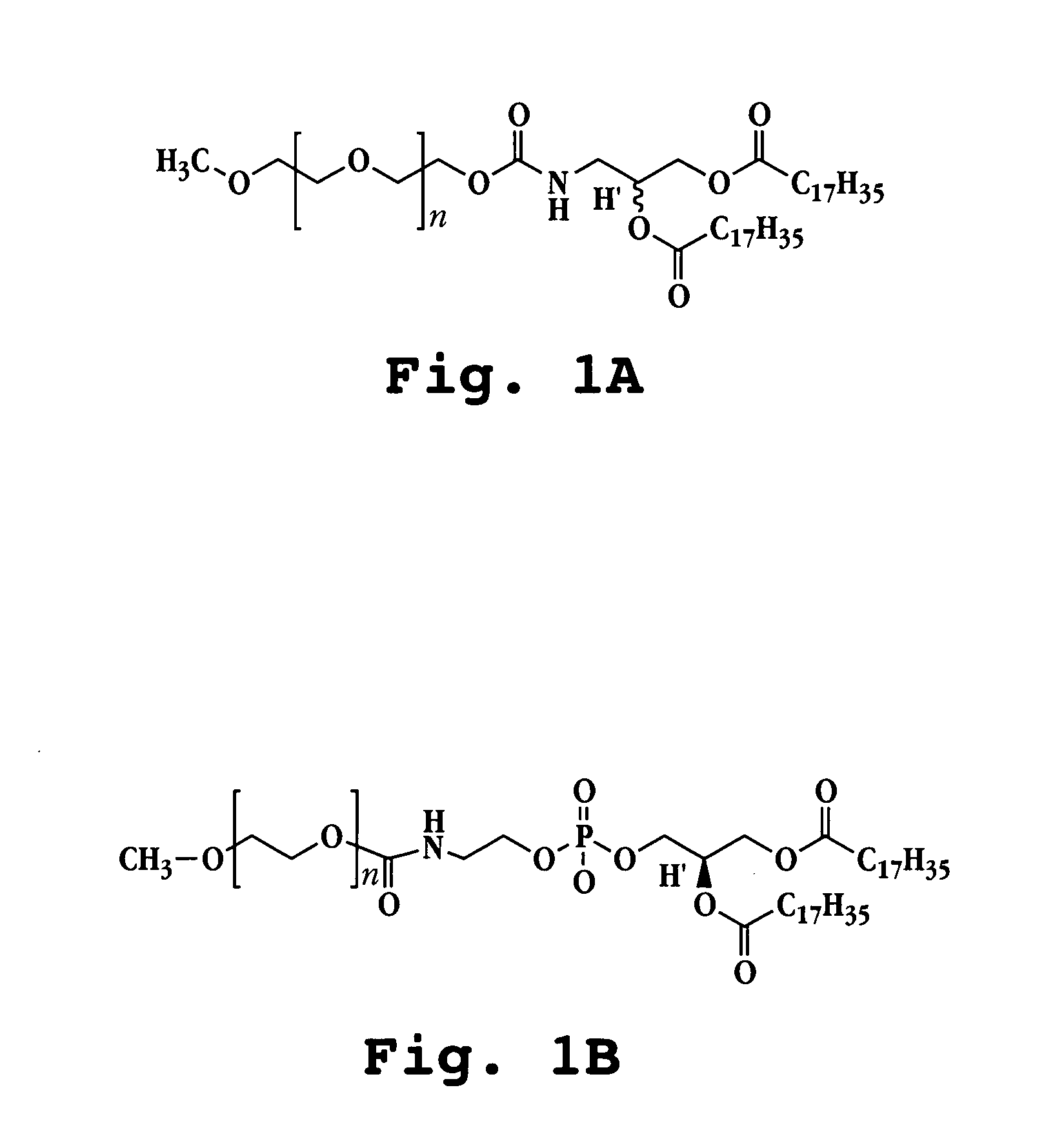
Method of pulmonary administration of an agent
A method for administering a therapeutic or diagnostic agent to a subject is described. The method includes providing a suspension of liposomes comprised of one or more of vesicle-forming lipids selected from (i) a vesicle-forming lipid derivatized with a hydrophilic polymer and (ii) a neutral lipopolymer, said liposomes being associated with said therapeutic or diagnostic agent, forming an aerosol of said liposome suspension; and administering the aerosol to the subject by inhalation. The liposome formulation delivers intact liposomal particles to the respiratory tract of said subject to form a depot of therapeutic agent therein with no observable provocation of an immune response, as measured by neutrophil or macrophage cell count in the lung after administration.
Owner:ALZA CORP
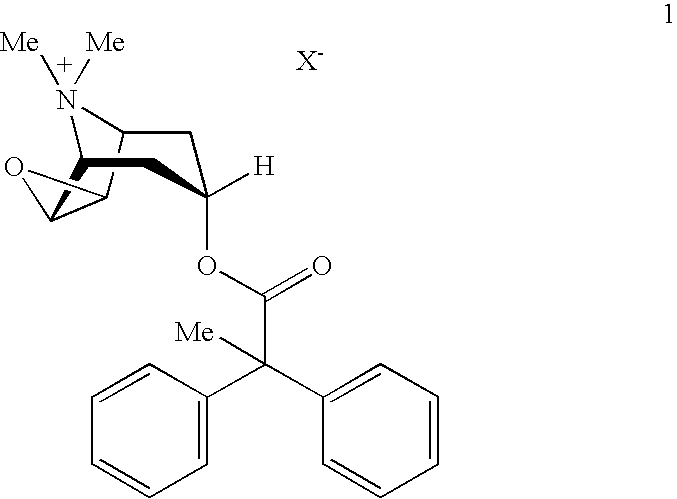
Anticholinergic powder formulations for inhalation
An inhalable powder comprising:(a) an active substance consisting essentially of a compound of formula 1 wherein X− is a pharmaceutically acceptable anion; and(b) a physiologically acceptable excipient having an average particle size of 10 μm to 50 μm,processes for preparing the inhalable powder, and methods of administration for the treatment of respiratory complaints, particularly for the treatment of chronic obstructive pulmonary disease (COPD) and asthma.
Owner:BOEHRINGER INGELHEIM INT GMBH
Mosquito-repellent incense made of Chinese-medicinal herbs
A mosquito-repellent incense is prepared from 12 Chinese-medicinal materials including Herba polygoni hydropiperis, argyi leaf, dehurian angelica root, atractylodes rhizome, etc. through drying, pulverizing, mixing, beating, shaping and baking. Its advantages include high effect and more functions of refreshing air, preventing diseases in respiratory tract and sobering brain.
Owner:宋建祥 +3
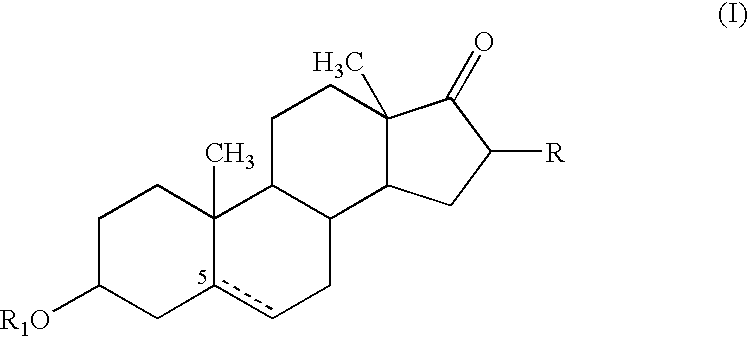
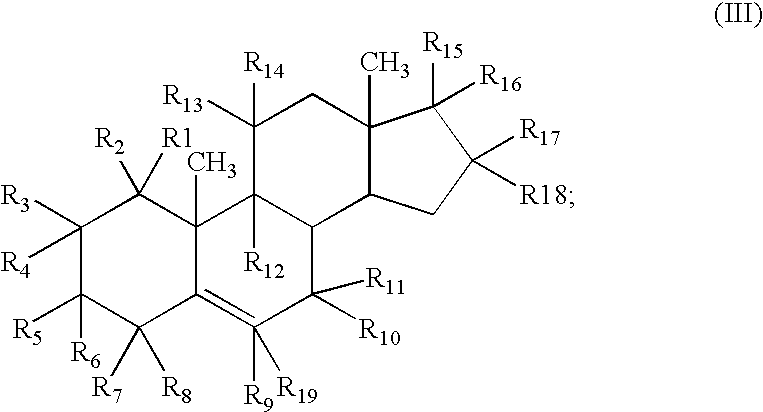
Agent for treating respiratory diseases containing 4-hydroxypiperidine derivative as active ingredient
InactiveUS7494987B2Potent antitussive actionImprove securityAntibacterial agentsBiocideDiseaseCarboxyl radical
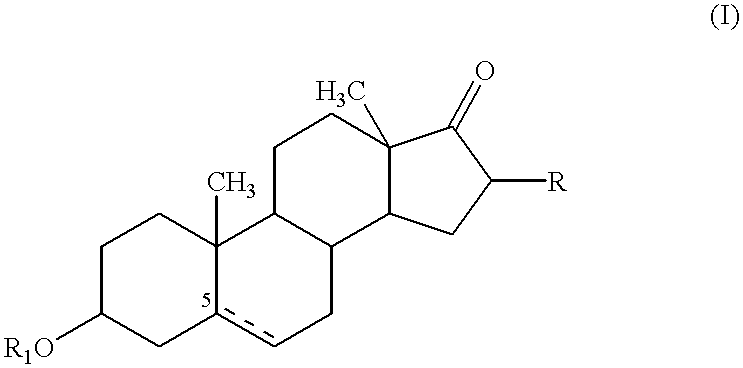
An agent for preventing / treating respiratory diseases contains, as an active ingredient, a compound represented by following Formula (I):wherein A is a group represented by L-W [wherein L is a bond or CH2; and W is O, SOn (wherein n is 0 to 2), or —NR7— (wherein R7 is hydrogen or lower alkyl)]; each of G1 and G2 is (CH2)r (wherein r is 0 to 2), provided that when n is 1, G1 and G2 may be bridged by lower alkylene; Y is a lower alkylene or (substituted) benzylidene; Z is a bond or O, provided that when Z is a bond, Y may form a 5- or 6-membered ring with carbon on the benzene ring; R1 is, for example, NO2, a lower alkoxycarbonyl, (substituted) carbamoyl, (protected) hydroxyl group, (protected) carboxyl, or (protected) N-hydroxycarbamoyl; each of R2 and R3 is hydrogen, halogen, (halogenated) lower alkyl, (halogenated) lower alkoxy or NO2; each of R4 and R5 is, for example, hydrogen, halogen, (halogenated) lower alkyl, (halogenated) lower alkoxy, CN, or lower alkylsulfonyl; and R6 is hydrogen or lower alkyl, a salt thereof or a solvate of them. It has excellent antitussive activity when used as an agent for preventing / treating respiratory diseases such as lung cancer, common cold syndrome, pulmonary tuberculosis, pneumonia, acute bronchitis or chronic bronchitis.
Owner:MOCHIDA PHARM CO LTD
Device, systems and methods having mobile ferromagnetic structures
InactiveUS20070144531A1Prevent tissue collapseSnoring preventionNon-surgical orthopedic devicesCombined useEngineering
A carrier is sized and configured for placement in or on a tissue region. The carrier includes at least one interior compartment. At least one ferromagnetic material is carried within the compartment. The compartment and the ferromagnetic material are mutually sized and configured to allow movement of the ferromagnetic material within the compartment in response to magnetic interaction with a magnetic material located outside the carrier. The magnetic interaction can include either a magnetic attracting force or a magnetic repelling force. The carrier can be used in association with a source of magnetism sized and configured for placement in or on a tissue region outside the carrier for magnetic interaction with the ferromagnetic material carried within the compartment, e.g., to stabilize the orientation of a tissue region within an airway.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Pharmaceutical formulations for dry powder inhalers in the form of hard-pellets
InactiveUS6884794B2High fine particle fractionReduce intensityBiocidePowder deliveryDiseasePowder Inhaler
The invention provides a formulation to be administered as dry powder for inhalation suitable for efficacious delivery of active ingredients into the low respiratory tract of patients suffering of pulmonary diseases such as asthma. In particular, the invention provides a formulation to be administered as dry powder for inhalation freely flowable, which can be produced in a simple way, physically and chemically stable and able of delivering either accurate doses and high fine particle fraction of low strength active ingredients by using a high- or medium resistance device.
Owner:CHIESI FARM SPA
Aerosol delivery apparatus, method and preparation for pressure-assisted breathing systems
An improved pressure-assisted breathing system for delivering aerosolized medication. In addition, the present invention also provides methods and formulations for the treatment of respiratory diseases.
Owner:AEROGEN
System and method for collecting human health data in real time
PendingCN111210886AConvenient prevention and controlNo traffic jamsEpidemiological alert systemsCo-operative working arrangementsEmergency medicineHuman health
The invention discloses a system and method for collecting human health data in real time. The system comprises a health data measurer carried by a user, a data reader, an execution mechanism, a datamonitoring center, and an epidemic situation control center. Health code data can be transmitted to the health data measurer. The health data measurer is worn on a hand of a user. A wrist temperaturesensor is arranged in the health data measurer; the wrist temperature sensor can detect the temperature of the wrist of a human body in real time; health code data can be transmitted into the health data measurer through mobile phone Bluetooth communication. With the system and method, respiratory tract infectious diseases with large transmissibility can be prevented; the people with abnormal health data and people in close contact with the people with abnormal health data within 0-30 days in the past can be locked rapidly, and isolation measures can be taken rapidly, so that the infection source is isolated and a propagation path is cut off.
Owner:宁波一川交通科技有限公司
Lift shaft capable of ventilating by wind power and lift ventilating method
InactiveCN102514998AUniform air supplyLighting and heating apparatusElevatorsWind powerIndustrial engineering
The invention relates to a lift shaft capable of ventilating by wind power and a lift ventilating method. The lift shaft is provided with a new air pipeline and an air exhausting pipeline. A new air port is arranged corresponding to a position of a floor and the upper part of a lift car. When the lift runs, a lower exhaust blower stops running. A new air shutter in the lift car is started facing to wind based on the running direction. Air flow with ascending air supplying flow and descending air exhausting flow is formed in the internal part of the lift car, so that energy consumption is saved and respiratory tract infection of passengers and bad influence of odor are effectively prevented. The shortcomings that the lift shaft is damp and the lift car is stuffy are overcome. Therefore, the method disclosed by the invention can be widely applied to ventilation of lift shaft and lift car and the lift shaft can be manufactured in an industrial, standard, universal and serial manner. The invention belongs to the fields of buildings, building ventilation and lift manufacturing.
Owner:TIANJIN ARCHITECTURE DESIGN INST +2
Combination of dehydroepiandrosterone or dehydroepiandrosterone-sulfate with an anti-IgE antibody for treatment of asthma or chronic obstructive pulmonary disease
A pharmaceutical or veterinary composition, comprises a first active agent selected from a dehydroepiandrosterone and / or dehydroepiandrosterone-sulfate, or a salt thereof, and a second active agent comprising an anti-IgE antibody for the treatment of asthma, chronic obstructive pulmonary disease, or any other respiratory disease. The composition is provided in various formulations and in the form of a kit. The products of this patent are applied to the prophylaxis and treatment of asthma, chronic obstructive pulmonary disease, or any other respiratory disease.
Owner:EPIGENESIS PHARMA LLC
Dissociated Discharge EHD Sprayer With Electric Field Shield
The invention is directed to devices and methods for electrohydrodynamic (EHD) aerosolization of liquids utilizing a dissociated discharge electrode and an electric field shield disposed between the nozzle and the discharge electrode. Preferred embodiments are designed as inhalers suitable for administration of therapeutic compounds to the respiratory tract of a patient, preferably the lungs.
Owner:EFIELD INNOVATIONS
Micro-fluidic chip reagent kit for detecting ten respiratory tract infection pathogens and use method of reagent kit
ActiveCN107603866AStrong specificityBioreactor/fermenter combinationsBiological substance pretreatmentsFluorescent pcrBiology
The invention provides a micro-fluidic chip reagent kit for detecting ten respiratory tract infection pathogens and a use method of the reagent kit. The reagent kit adopts a combination of a Taqman probe fluorescent PCR (polymerase chain reaction) technology and a micro-fluidic chip, detects the ten common respiratory tract infection pathogens, can obtain a detection result within 2h, and is highin specificity, and the sensitivity can reach 100 copies / microliter. The kit comprises a sample introduction chamber, at least ten reaction chambers and a micro-fluidic flow channel, wherein the reaction chambers are mutually independent; each reaction chamber is provided with a reagent dry powder for amplifying one respiratory tract pathogen in advance; each reagent dry powder comprises a primerfor amplifying the corresponding respiratory tract pathogen and a TaqMan probe; the reagent dry powder arranged in each reaction chamber in advance can amplify any one of the ten respiratory tract pathogens; the respiratory tract pathogens amplified by the reagent dry powder in all the reaction chambers can include the ten respiratory tract pathogens.
Owner:NANJING LANSION BIOTECH CO LTD
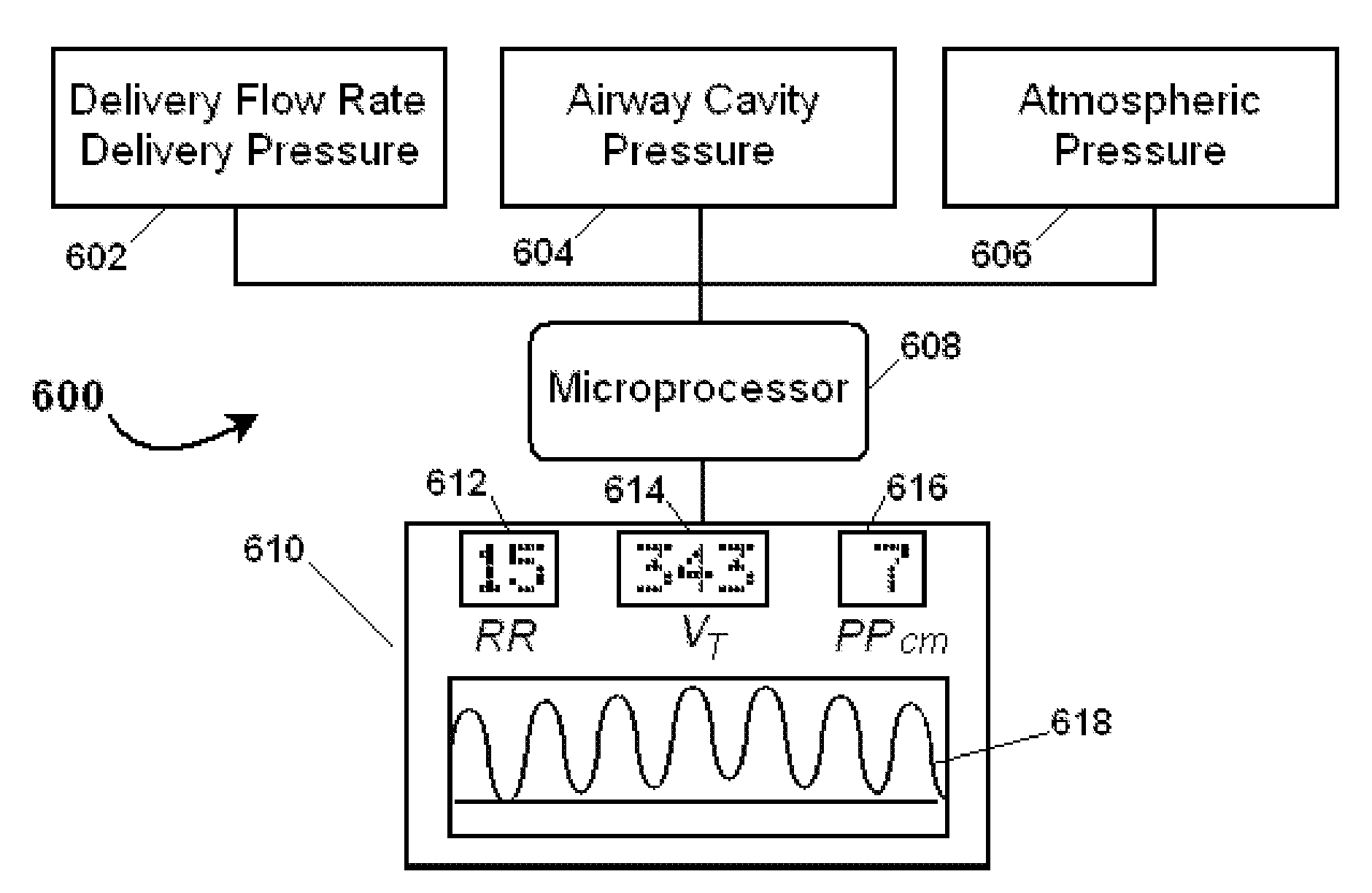
Clinical monitoring in open respiratory airways
A novel and non-obvious method, system and apparatus for determining respiratory volume flow rate of a subject and associated parameters such as tidal volume, minute volume, and respiratory rate. The method for determining respiratory volume flow rate of a subject can include selecting an airway cavity of the subject, measuring delivery volume flow rate of respiratory gas delivered to the airway cavity, measuring pressure within the airway cavity and calculating a respiratory volume flow rate of the subject using the measured delivery volume flow rate of respiratory gas delivered to the airway cavity and the measured pressure within the airway cavity. The method further can include generating a warning signal selected from the group consisting of an indicator that a respiratory volume flow value is outside of an expected value for the subject and an indicator that an airway cavity measurement value does not conform to an expected value.
Owner:MERGENET MEDICAL
Particles for inhalation having rapid release properties
InactiveUS20080226730A1Facilitated releaseShorten the timePowder deliveryOrganic active ingredientsActive agentPhospholipid
The invention generally relates to formulations having particles comprising phospholipids, bioactive agent and excipients and the pulmonary delivery thereof. Dry powder inhaled insulin formulations are disclosed. Improved formulations comprising DPPC, insulin and sodium citrate which are useful in the treatment of diabetes are disclosed. Also, the invention relates to a method of for the pulmonary delivery of a bioactive agent comprising administering to the respiratory tract of a patient in need of treatment, or diagnosis an effective amount of particles comprising a bioactive agent or any combination thereof in association, wherein release of the agent from the administered particles occurs in a rapid fashion.
Owner:CIVITAS THERAPEUTICS
Method of producing a nicotine medicament and a medicament made by the method
InactiveUS20120042886A1Efficiently conveyedImprove efficiencyTobacco preparationPowder deliveryNicotineSpray drying
A method of producing a nicotine medicament for use in an inhaler comprises combining nicotine, a non-spheronized sugar and a liquid carrier including water to produce a flowable mixture and spray drying the flowable mixture at conditions to produce particles of the nicotine medicament suitable for delivery to the alveoli and lower airways of the person. Also disclosed is a nicotine medicament made by the method. The nicotine composition produced by this method is a composite particle suitable for tobacco replacement or withdrawal therapy.
Owner:SANSA BARBADOS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com