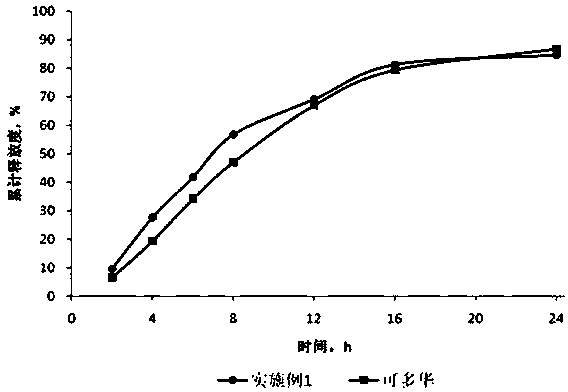
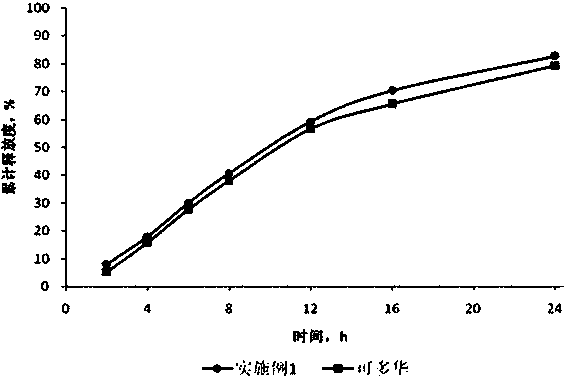
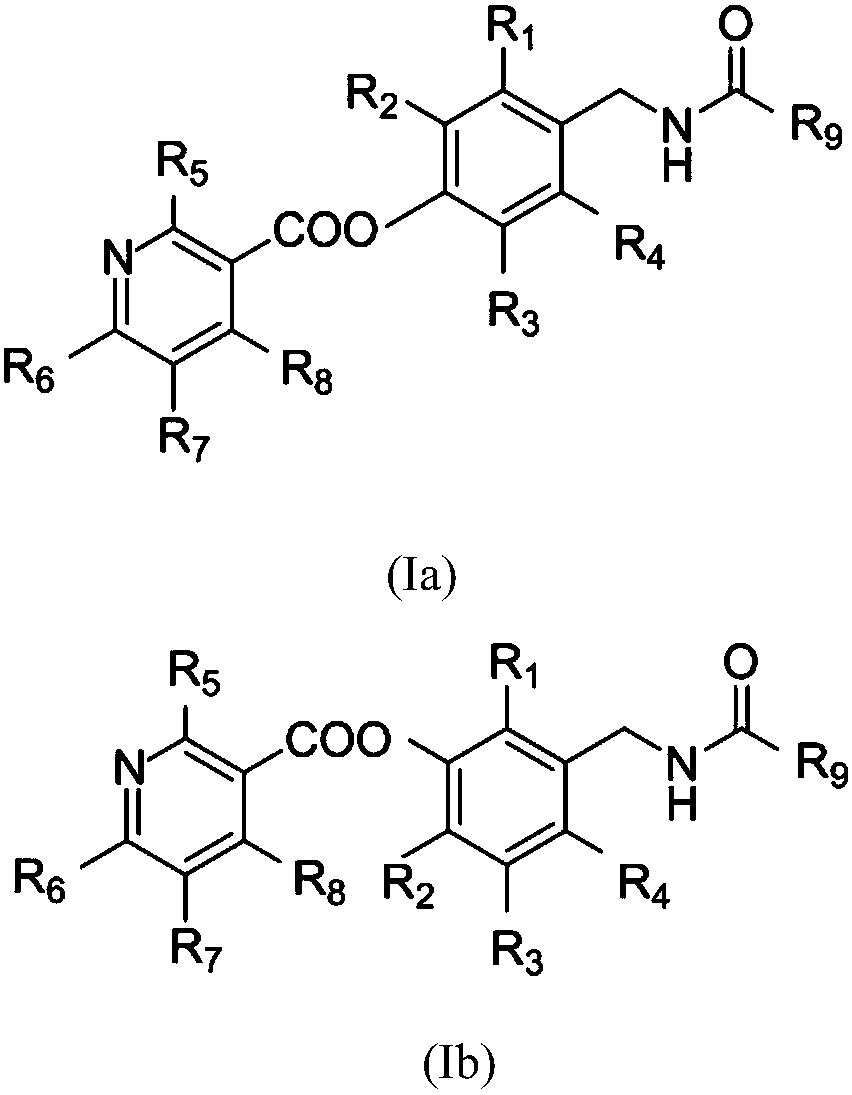
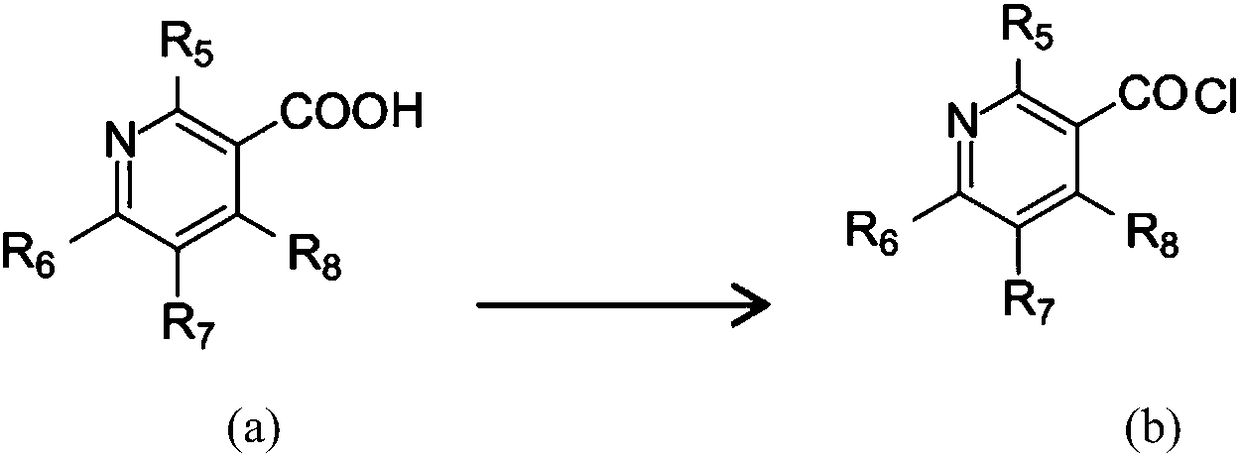
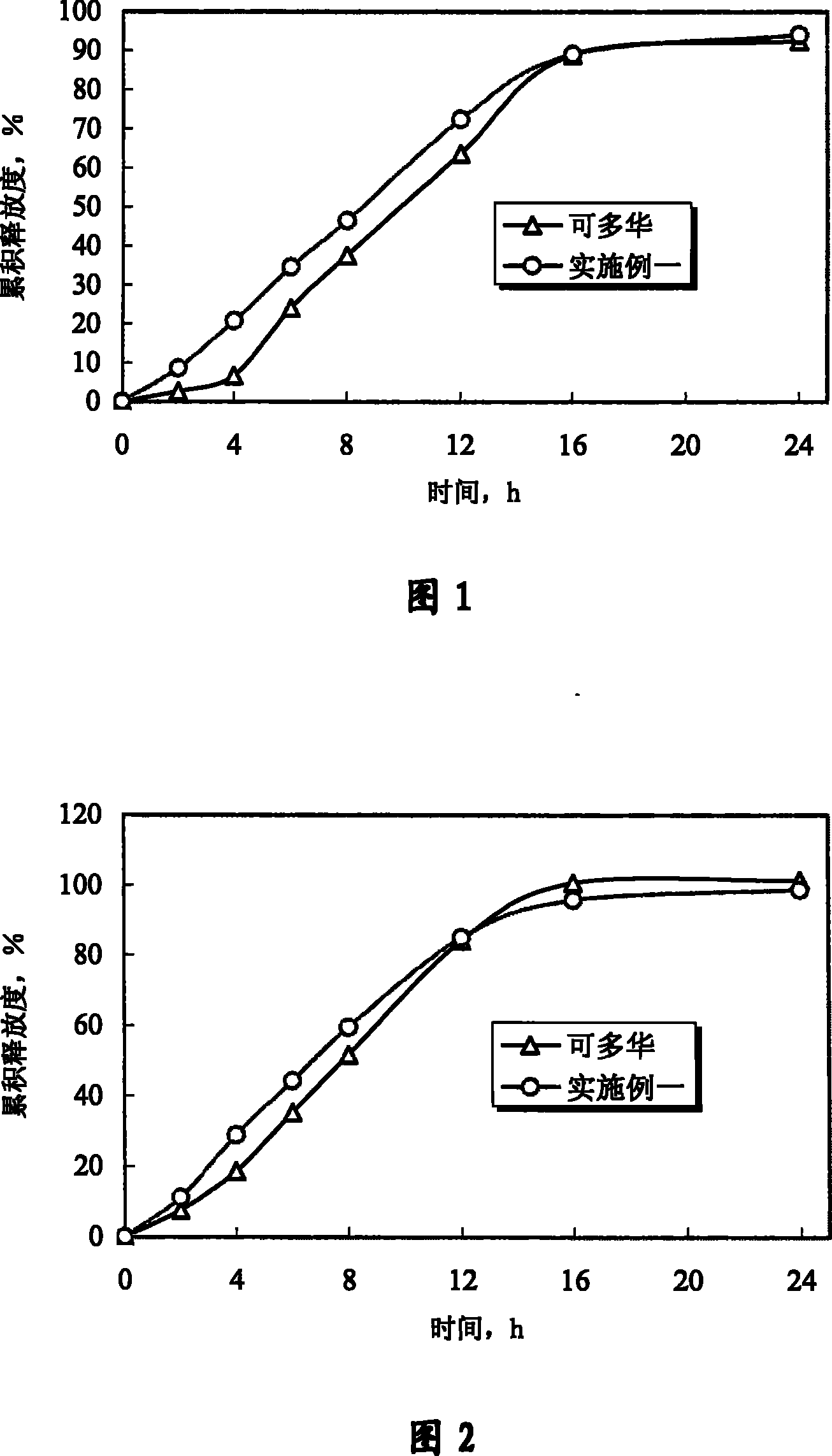
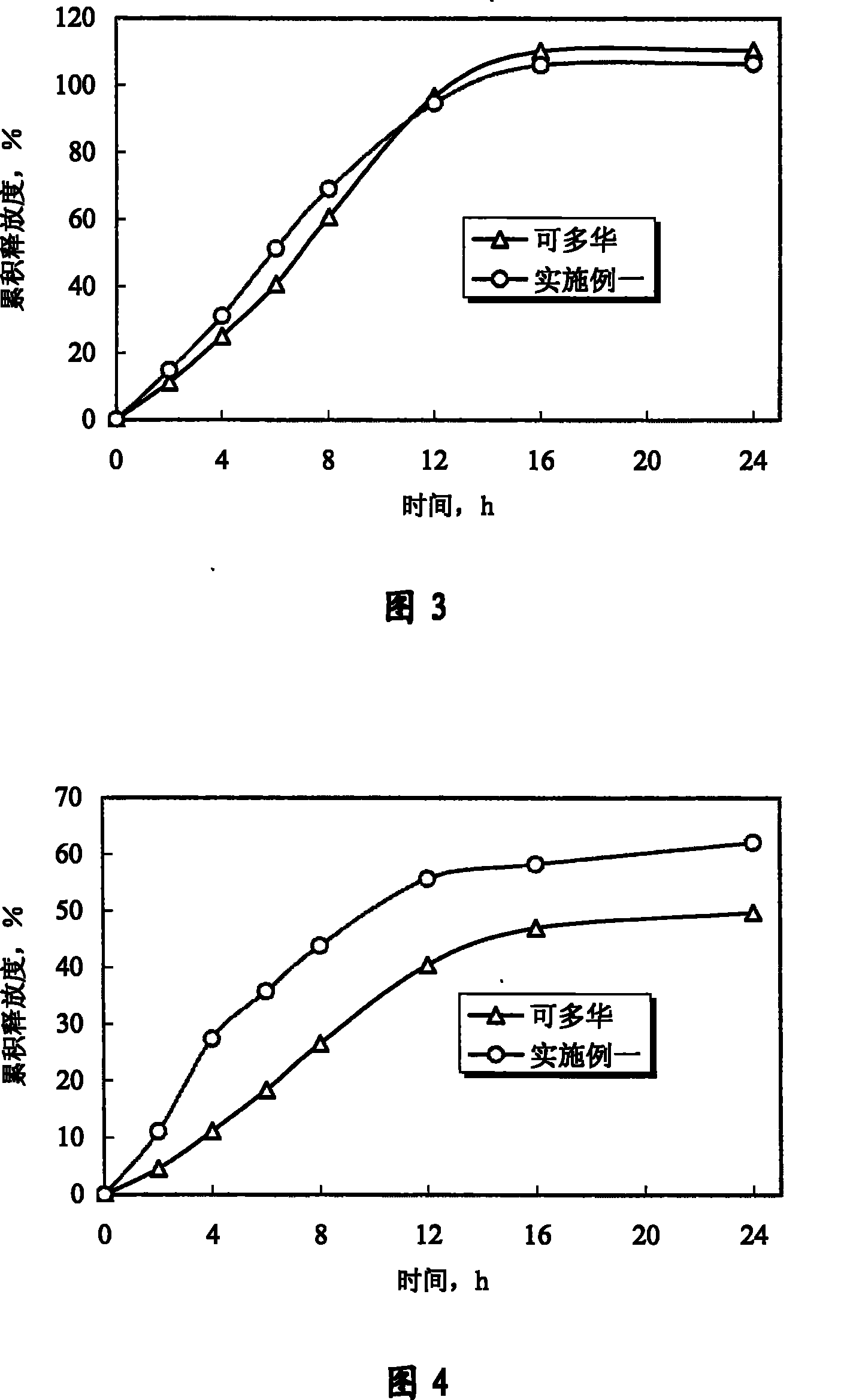
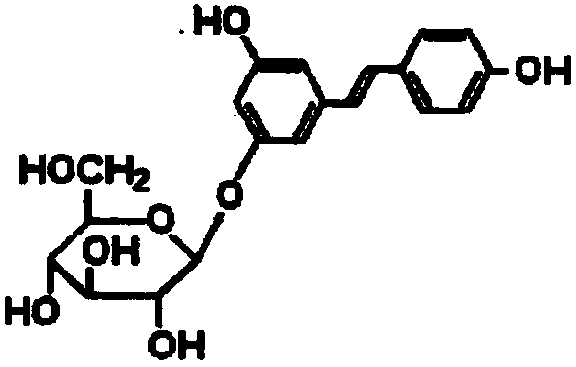
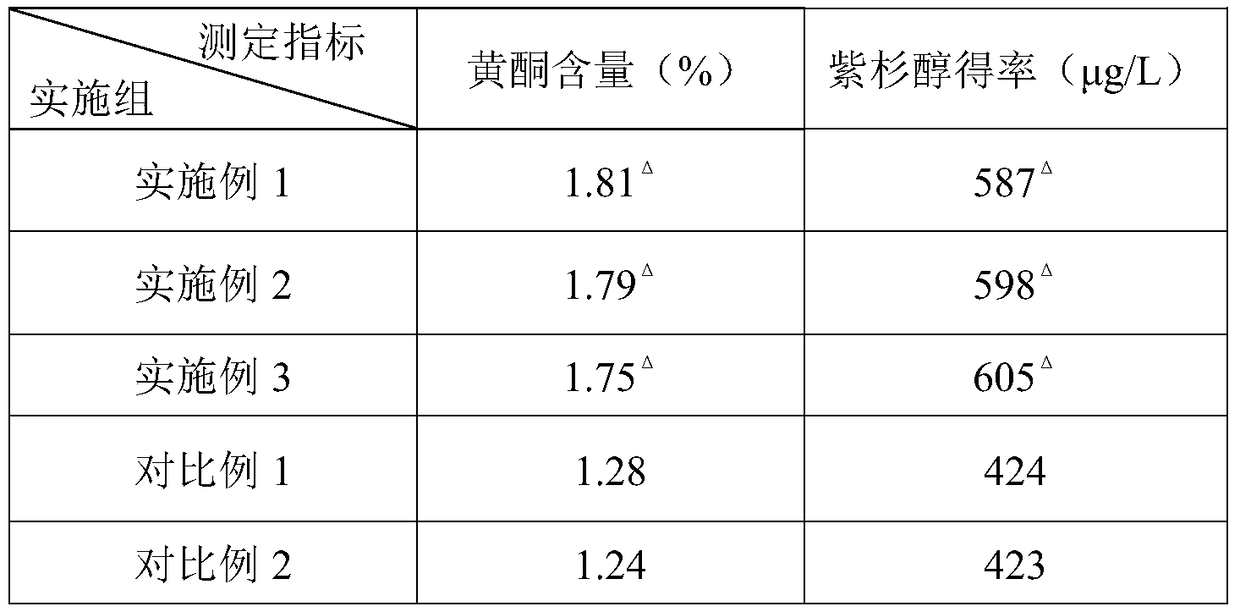
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33results about How to "Meet the needs of clinical medicine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
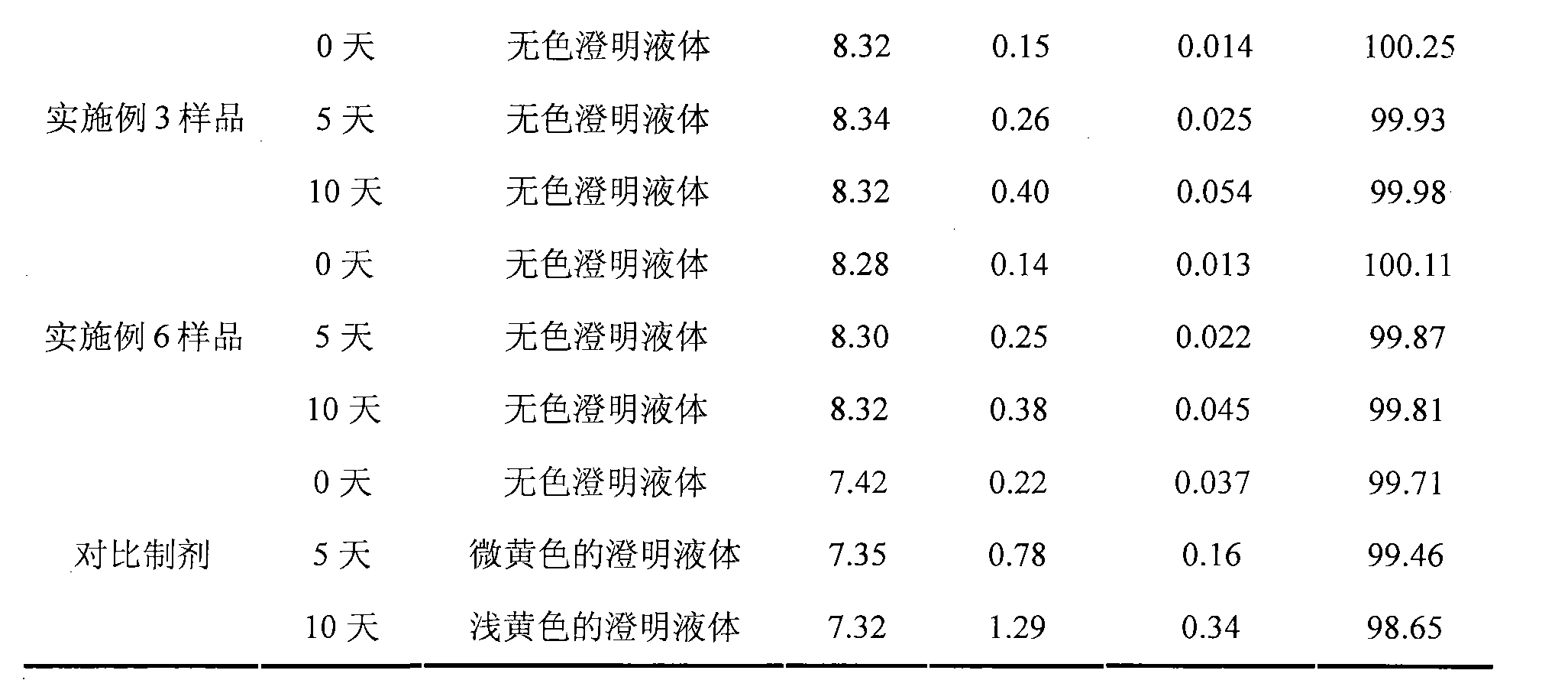
Ibuprofen injection composite and preparation method thereof
ActiveCN101966147AImprove stabilityMeet the needs of clinical medicineOrganic active ingredientsAntipyreticIbuprofen InjectionMedicine
The invention discloses an ibuprofen injection composite which can obviously improve the quality stability of products and meet the clinical compatibility demand and a preparation method thereof to overcome the defect of the traditional ibuprofen injection. The injection is prepared by mixing ibuprofen and cosolvent according to a molar ratio of 1: 1.001-2, and the pH value of the injection is 7.5-9.0. The ibuprofen injection prepared through the method has obviously improved tolerance capability to high temperature and strong light and better stability and no irritant to vessels, and can effectively meet the requirement of the clinical intravenous drip. Moreover, the preparation method of the invention has the advantages of simple process and good stability of the prepared product.
Owner:四川阳光润禾药业有限公司
Etimicin sulfate preparation and its preparing method
InactiveCN1569010AAdd new varietiesExpand the scope of clinical applicationOrganic active ingredientsAntipyreticChemistrySulfuric acid
The invention discloses etimicin sulfate preparation and its preparing method which comprises, charging acids or alkali as pH conditioning agent so as to increase the dissolving degree of the etimicin, charging right amount of isoosmotic adjustment agent, thus resulting the action of quick sterilization.
Owner:ZHEJIANG UNIV
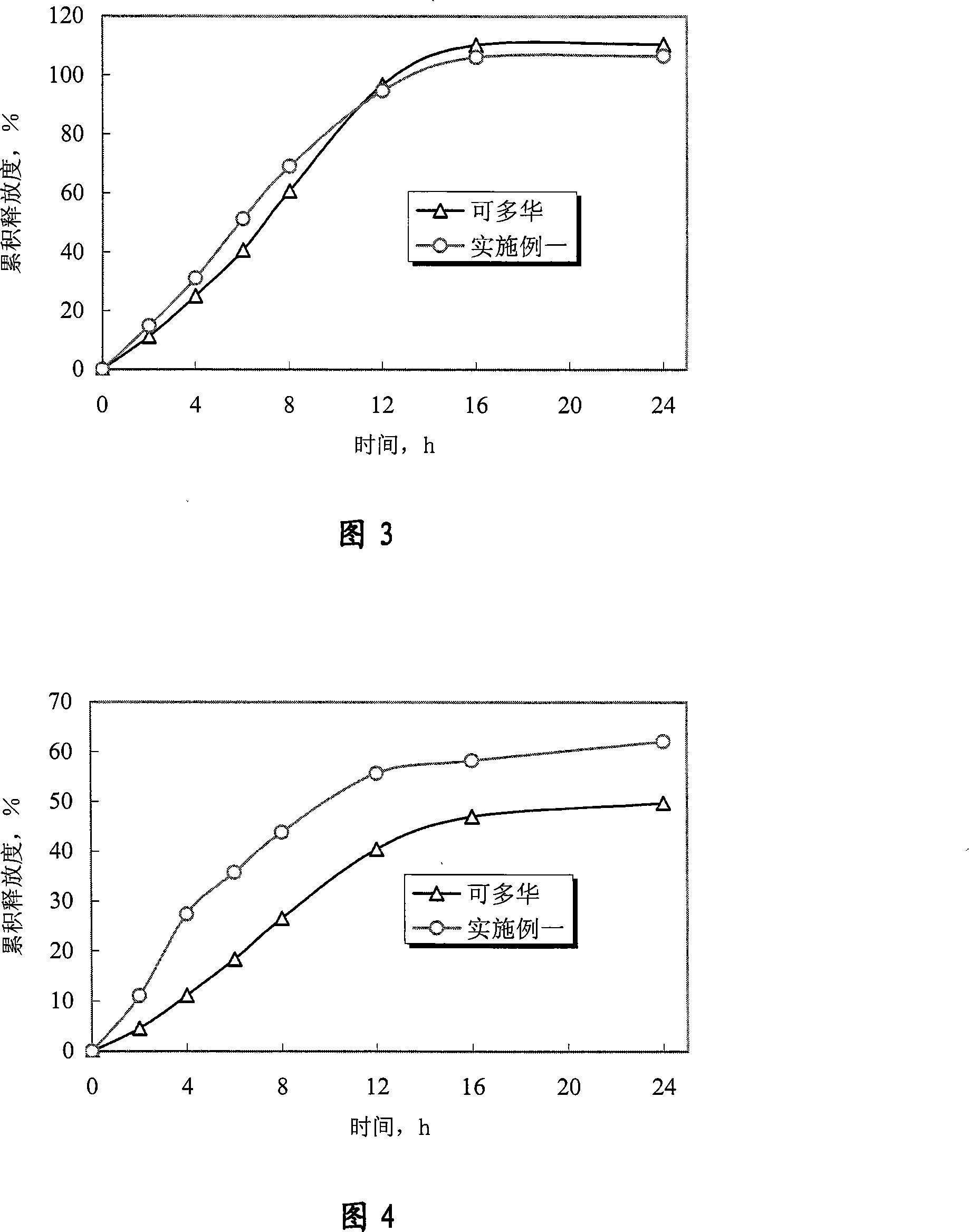
Doxazosin-mesylate controlled-releasing tablet and preparation method thereof
ActiveCN101167728AImprove complianceStable blood concentrationOrganic active ingredientsPharmaceutical delivery mechanismSide effectGeneration rate
The invention discloses methanesulfonic acid doxazosin controlling releasing tablets and a process for preparation, which contains a single-layer core, insoluble semi-permeable membrane, single drug-releasing eyelet on each side of the tablet, and depends on the osmotic pressure difference between inside and outside of the semi-permeable membrane medium. The invention realizes the control of linear releasing of methanesulfonic acid doxazosin by single-layer core structure, and can remain effective and steady blood and drug concentration. The curative effect is improved, the generation rate of side effect is reduced, and the productive technology is greatly simplified.
Owner:HEFEI LIFEON PHARMA
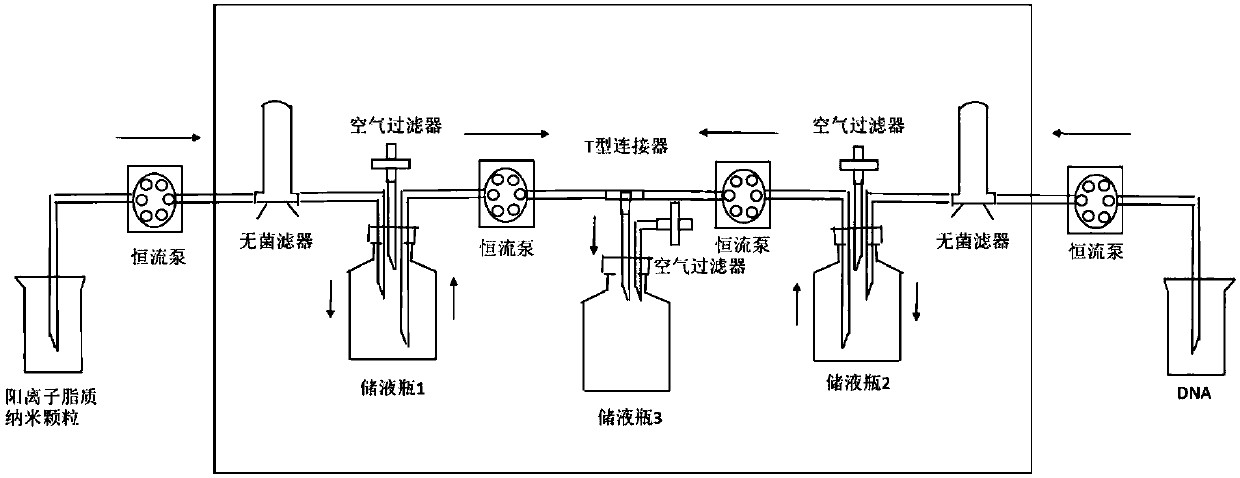
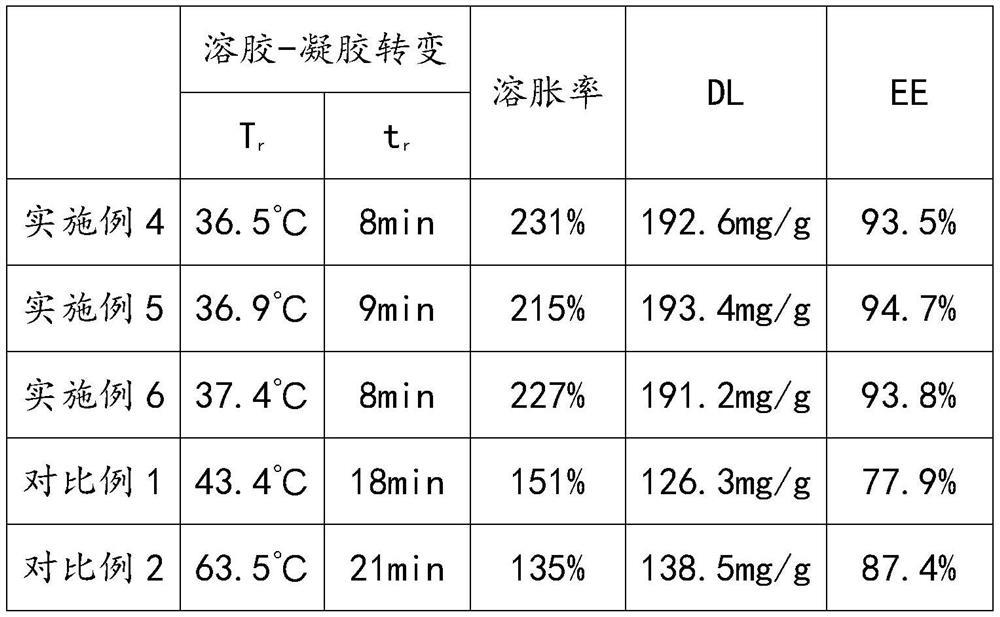
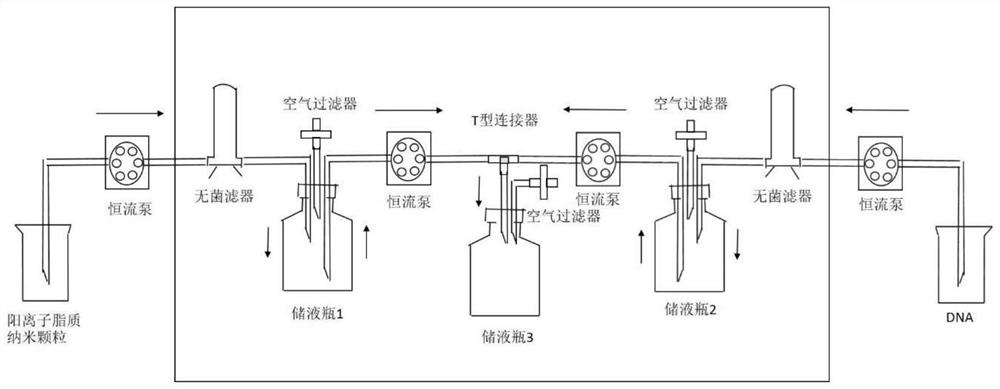
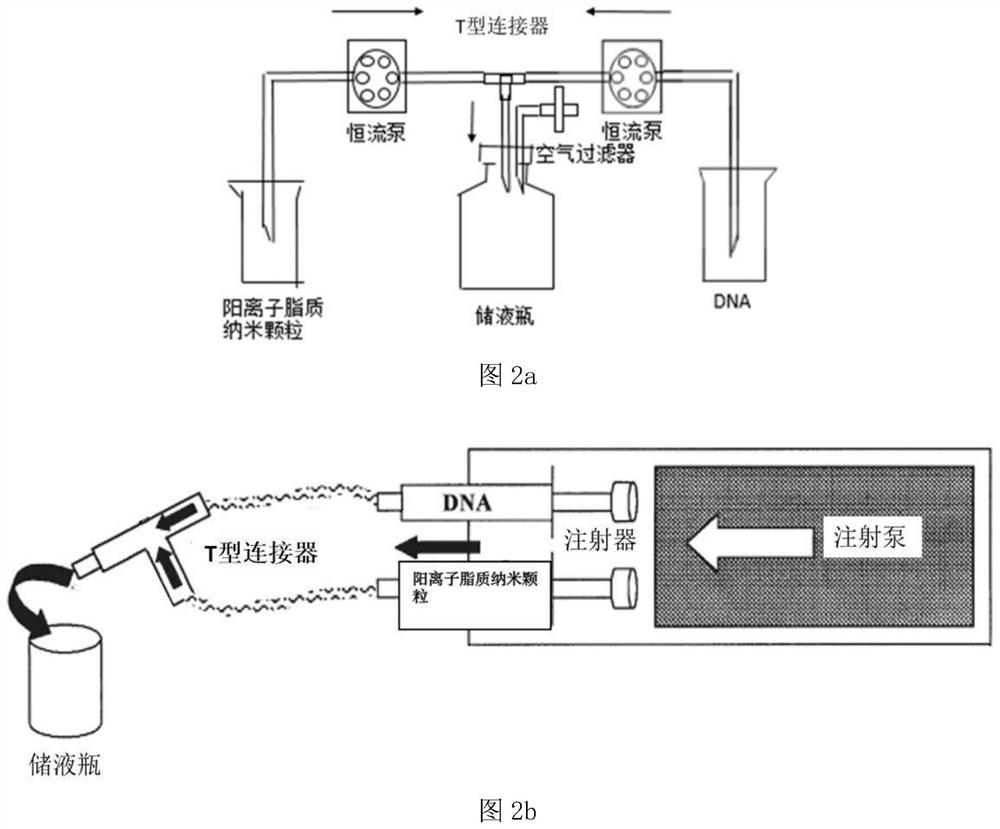
Positive ion lipid nanometer particle/DNA compound and preparation method thereof
ActiveCN109893664AEvenly distributedMonodisperseGenetic material ingredientsPharmaceutical non-active ingredientsDNA SolutionsAlcohol
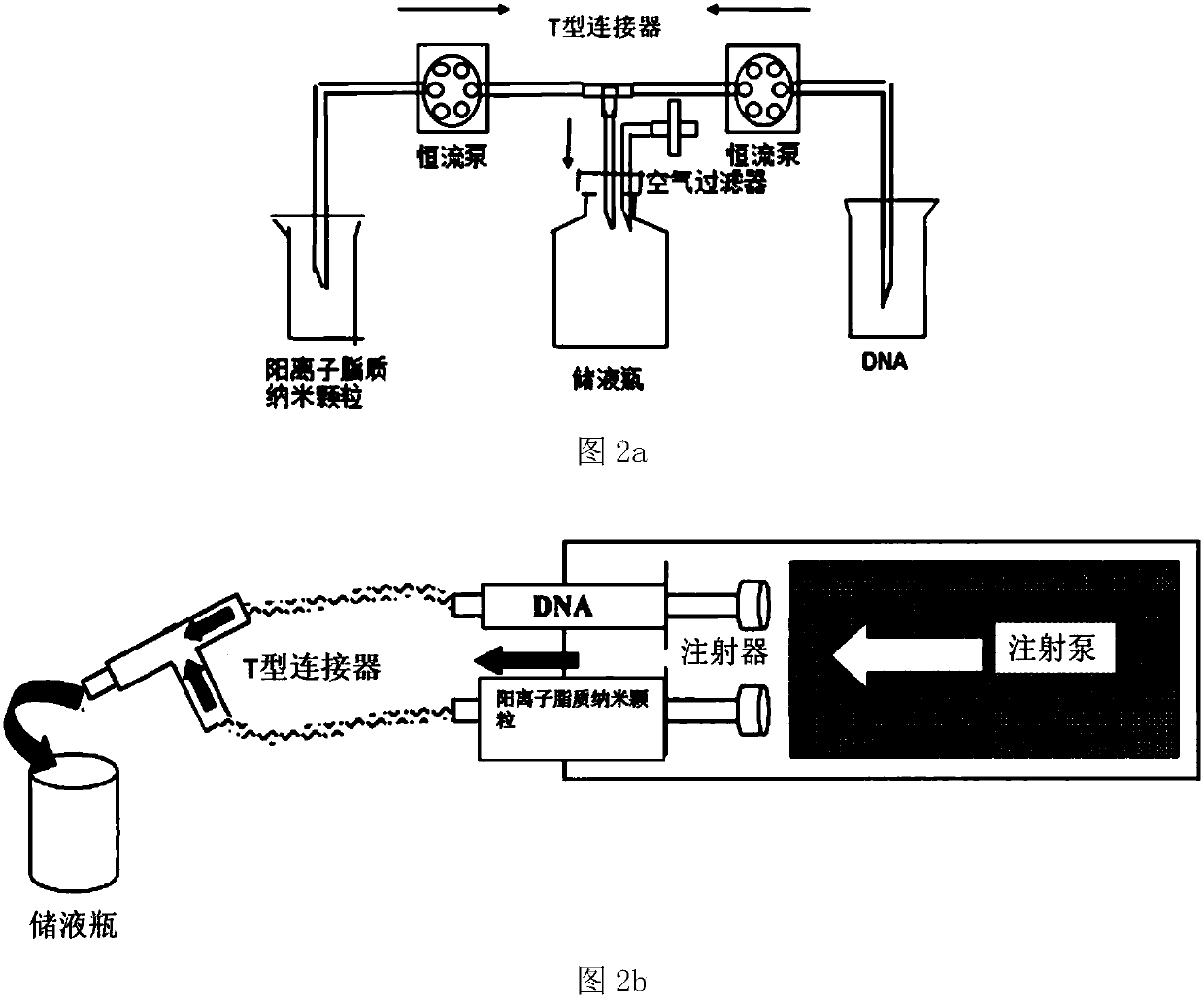
The invention provides a positive ion lipid nanometer particle / DNA compound and a preparation technology thereof. The preparation technology comprises the following steps that (1) a positive ion lipidmaterial is dissolved in absolute ethyl alcohol through heating; (2) an ethyl alcohol solution prepared in the step (1) is added to an aqueous phase solution dropwise, and positive ion lipid nanometer particles are formed through self-assembling; (3) residue ethyl alcohol in the positive ion lipid nanometer particles of the step (2) is removed; (4) filtering is carried out; (5) a DNA solution isprepared; (6) the prepared positive ion lipid nanometer particles in the step (4) and the DNA solution prepared in the step (5) are mixed according to a certain weight ratio to form the positive ion lipid nanometer particle / DNA compound; and (7) filtering is carried out. According to the preparation technology of the positive ion lipid nanometer particle / DNA compound, the operation of the preparation method is easy and rapid, the particle diameter of the prepared positive ion lipid nanometer particle / DNA compound is 50-150 nm, PDI<0.3, the positive ion lipid nanometer particle / DNA compound isdistributed in a mono-dispersion mode, the structure is stable, the compound is subjected to filtering sterilization at a terminal, and the security of the compound in clinic application of preparation of tumor curing medicine is effectively ensured.
Owner:SICHUAN UNIV
New strain and method of producing pacilitaxel using said strain
The invention relates to a new fungus that could produce taxales mellow-Alternaria alternate var.monosporus ST-026-R CGMCC No.0899. The invention also discloses the method to produce taxales mellow that includes the following steps: cultivating CGMCC 0899 in culture medium to produce and gather taxales mellow in the strain cell and the culture medium. Thus, the taxales mellow could be recycle and purify in the cell and culture medium. It could solve the technology problem of taxales mellow and protect natural resource.
Owner:SUNGEN BIOSCIENCE CO LTD
Method for increasing yield of paclitaxel in endophytic fungus fermentation product
ActiveCN105400842AIncrease contentEfficient degradationMicroorganism based processesFermentationBiotechnologyValeriana jatamansi
The invention discloses a method for increasing the yield of paclitaxel in an endophytic fungus fermentation product. The method comprises the steps that an endophytic fungus seed solution for producing paclitaxel is inoculated into an endophytic fungus solid state fermentation medium containing taxus media and valeriana jatamansi for culture, the mixture is then put into an alternating magnetic field environment for further culture, and a endophytic fungus solid state fermentation product is obtained; then the endophytic fungus seed solution for producing paclitaxel is inoculated into a liquid fermentation medium containing the endophytic fungus solid state fermentation product for culture, and the final endophytic fungus fermentation product is obtained. According to the method, raw materials are natural, no pollution is caused to the environment, a cycle is short, the yield is high, the method is suitable for industrial production, and compared with a common endophytic fungus fermentation product, the yield of paclitaxel in the endophytic fungus fermentation product is obviously increased.
Owner:ZHEJIANG FORESTRY ACAD
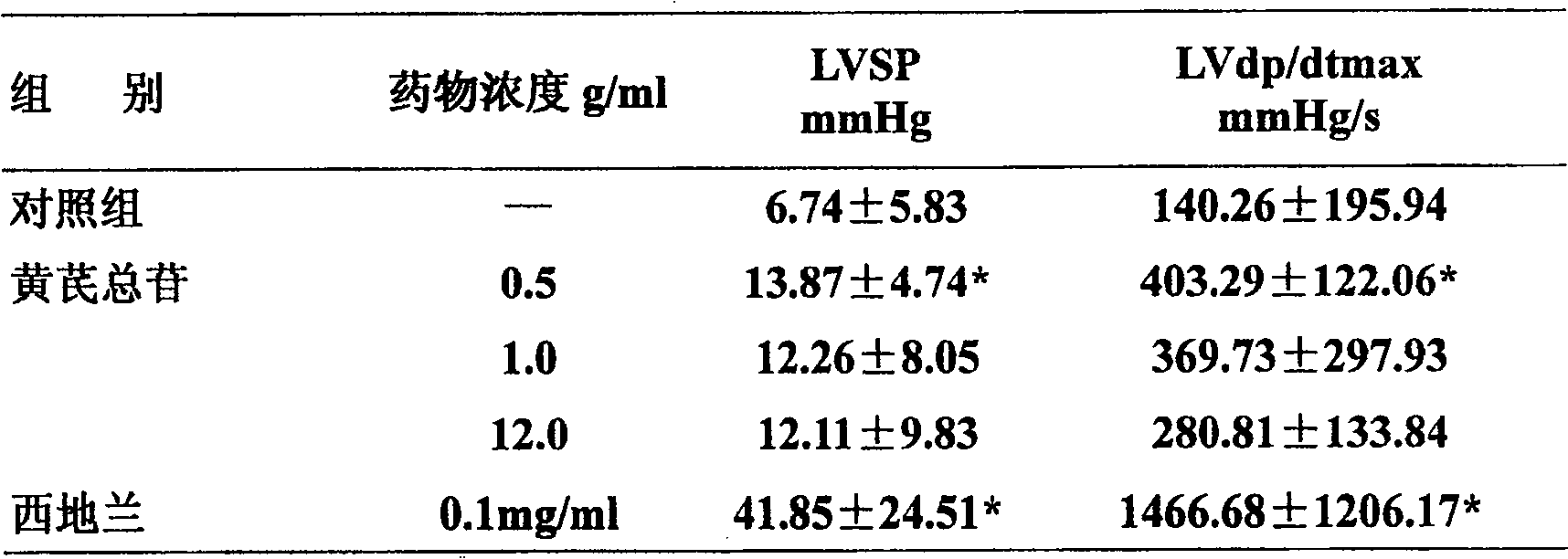
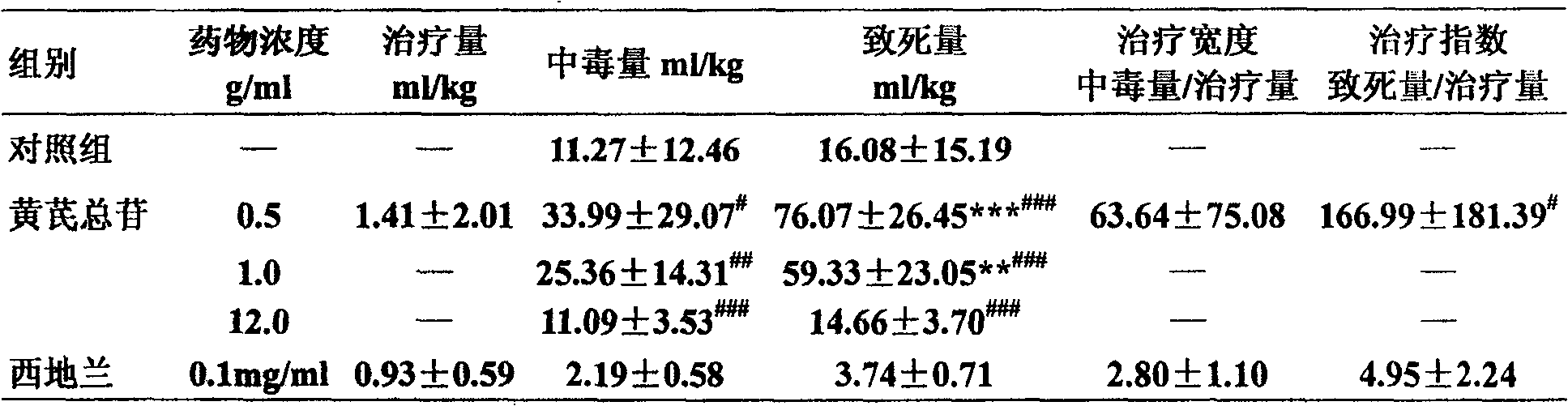
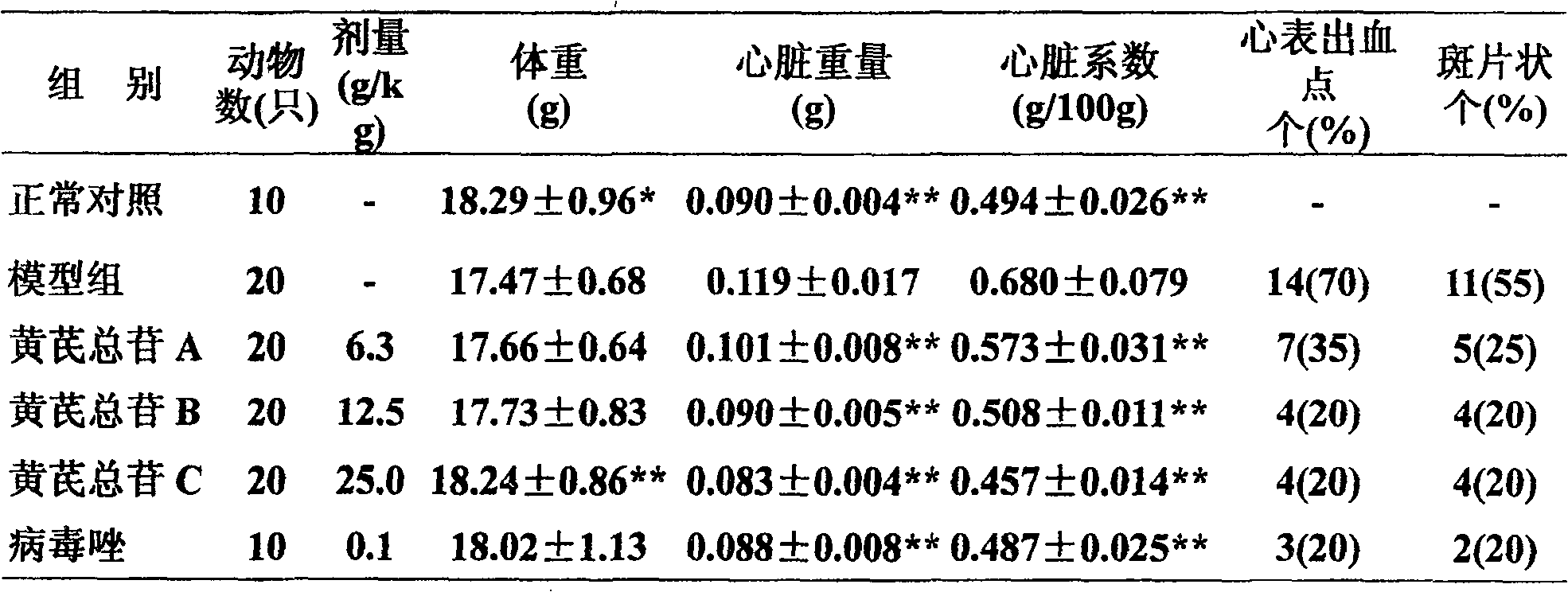
Astragaloside injection and preparation method thereof
ActiveCN1839920AImprove stabilityQuality is easy to controlOrganic active ingredientsAntiviralsAstragalosideGlycoside formation
The invention relates to an injection using astragalus root glycosides extract as the active ingredient, which also comprises pharmaceutically acceptable adjunct for injection, the content of the astragalus root glycosides in the total astragalus root extract is 80-100%, each unit of the preparation contains astragalus root glycosides 90-300mg.
Owner:CHENGDU DIAO JIUHONG PHARMA FAB
Pharmaceutical preparation containing temozolomide, pharmaceutically acceptable salts or other derivatives thereof
InactiveCN104274412ASolve solubilitySolve the shortcomings of poor reconstitution effectOrganic active ingredientsPowder deliveryWater basedFreeze-drying
The invention discloses a pharmaceutical preparation containing temozolomide or pharmaceutically acceptable salts or other derivatives thereof, at least one water-based diluent and at least one cosolvent which is sufficient to substantially dissolve the temozolomide or pharmaceutically acceptable salts or other derivatives thereof and a preparing process thereof, and in particular, discloses the freeze-dried preparation containing the temozolomide.
Owner:北京恒瑞康达医药科技发展有限公司
Medicinal compound composition of irbesartan and hydrochlorothiazide and preparation method thereof
InactiveCN103655579ADissolution unchangedImprove stabilityOrganic active ingredientsCardiovascular disorderUse medicationCoated tablets
The invention discloses a compound composition of irbesartan and hydrochlorothiazide. The composition can be made into granules, capsules, tablets and coated tablets. By screening and adopting a specific adhesive, the dissolution rate of the active ingredient irbesartan can exceed 94%, and the dissolution rate of hydrochlorothiazide can exceed 96%; after the composition is placed for 10 days at a high temperature of 60 DEG C, the dissolution rates of the two active ingredients are basically kept unchanged; and therefore, the composition has good stability and can meet the needs of clinical medication.
Owner:石药集团中诺药业(石家庄)有限公司
Salicylic acid-containing capsaicin ester derivative, preparation method and application
ActiveCN108069870AImprove biological activityIncrease irritationOrganic active ingredientsOrganic compound preparationSalicylic acidCapsaicin
The invention provides a salicylic acid-containing capsaicin ester derivative with a general structural formula I and a preparation method thereof. The derivative has anti-inflammatory, analgesic, anti-cancer, antibacterial and hypolipidemic aspect applications.
Owner:NANHUA UNIV
Doxazosin mesylate sustained release tablet and preparation method thereof
InactiveCN105213347AControl releaseImprove complianceOrganic active ingredientsMetabolism disorderSustained Release TabletSide effect
The invention discloses a doxazosin mesylate sustained release tablet and a preparation method thereof. The doxazosin mesylate sustained release tablet contains doxazosin mesylate and pharmaceutically acceptable auxiliary materials, and comprises a tablet core and a sustained release coating, wherein the tablet core comprises a hydrophilic gel framework material and an acidifying agent, the hydrophilic gel framework material accounts for 5-25 percent, and the acidifying agent accounts for 10-30 percent; and the weight of the sustained coating layer is 1-5 percent of that of the tablet core. The prepared doxazosin mesylate sustained release tablet can well control release of doxazosin mesylate, has similar release curves to 'cardura XL' in different dissolution media, is not interfered and influenced by the pH of stomach and intestine, enables the plasma concentration to be stable, eliminates a peak-valley phenomenon, effectively reduces side effects and the dosage frequency, improves the compliance of patients and meets the clinical medication requirements.
Owner:LIAONING YAOLIAN PHARMA
Nicotinic acid-containing capsaicin ester derivative, preparation method and application thereof
ActiveCN108069898ALow toxicityIncrease appetiteOrganic active ingredientsOrganic chemistryAnti atherosclerosisCapsaicin
The invention provides a nicotinic acid-containing capsaicin ester derivative with a general structural formula I and a preparation method thereof. The derivative has analgesic, anti-tumor, anti-inflammatory, anti-atherosclerosis, hypolipidemic, appetite promoting, digestion improving, antibacterial and insecticide, anti-oxidant, anti-viral applications.
Owner:NANHUA UNIV
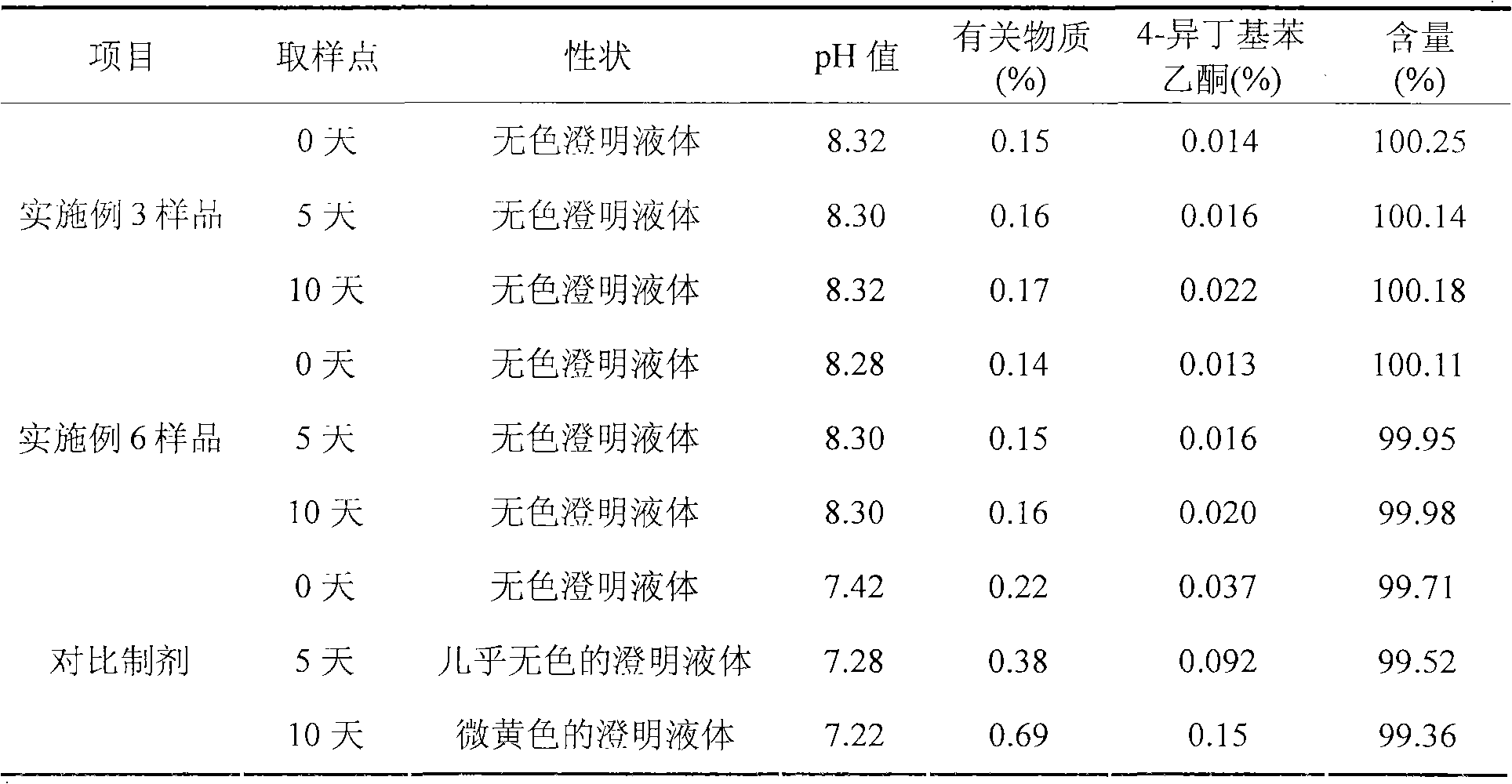
Ibuprofen injection composite and preparation method thereof
ActiveCN101966147BImprove stabilityLow in 4-isobutylacetophenoneOrganic active ingredientsAntipyreticIbuprofen InjectionMedicine
The invention discloses an ibuprofen injection composite which can obviously improve the quality stability of products and meet the clinical compatibility demand and a preparation method thereof to overcome the defect of the traditional ibuprofen injection. The injection is prepared by mixing ibuprofen and cosolvent according to a molar ratio of 1: 1.001-2, and the pH value of the injection is 7.5-9.0. The ibuprofen injection prepared through the method has obviously improved tolerance capability to high temperature and strong light and better stability and no irritant to vessels, and can effectively meet the requirement of the clinical intravenous drip. Moreover, the preparation method of the invention has the advantages of simple process and good stability of the prepared product.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD +1
Carbinoxamine maleate oral slow-release suspension and preparation method thereof
InactiveCN108420791AImprove complianceImprove Medication AdherenceOrganic active ingredientsDispersion deliveryMedicinePlasticizer
The invention provides a carbinoxamine maleate oral slow-release suspension which is prepared from impregnated carbinoxamine maleate-resin composite particles and a suspension matrix. The impregnatedcarbinoxamine maleate-resin composite particles are prepared by using an impregnating agent to impregnate a carbinoxamine-maleate-resin compound and performing wet-process granulation, and the impregnating agent comprises a slow-release material and a plasticizer. The invention further provides a preparation method of the suspension. The preparation method is simple; a novel administration systemtaking sodium polystyrene sulfonate as a carrier is adopted, medicine is combined with sodium polystyrene sulfonate, and the objectives of slow release and taste masking are achieved after impregnating through the impregnating agent and wet-process granulation.
Owner:SHANDONG DYNE MARINE BIOTECHCAL PHARM HLDG CO LTD
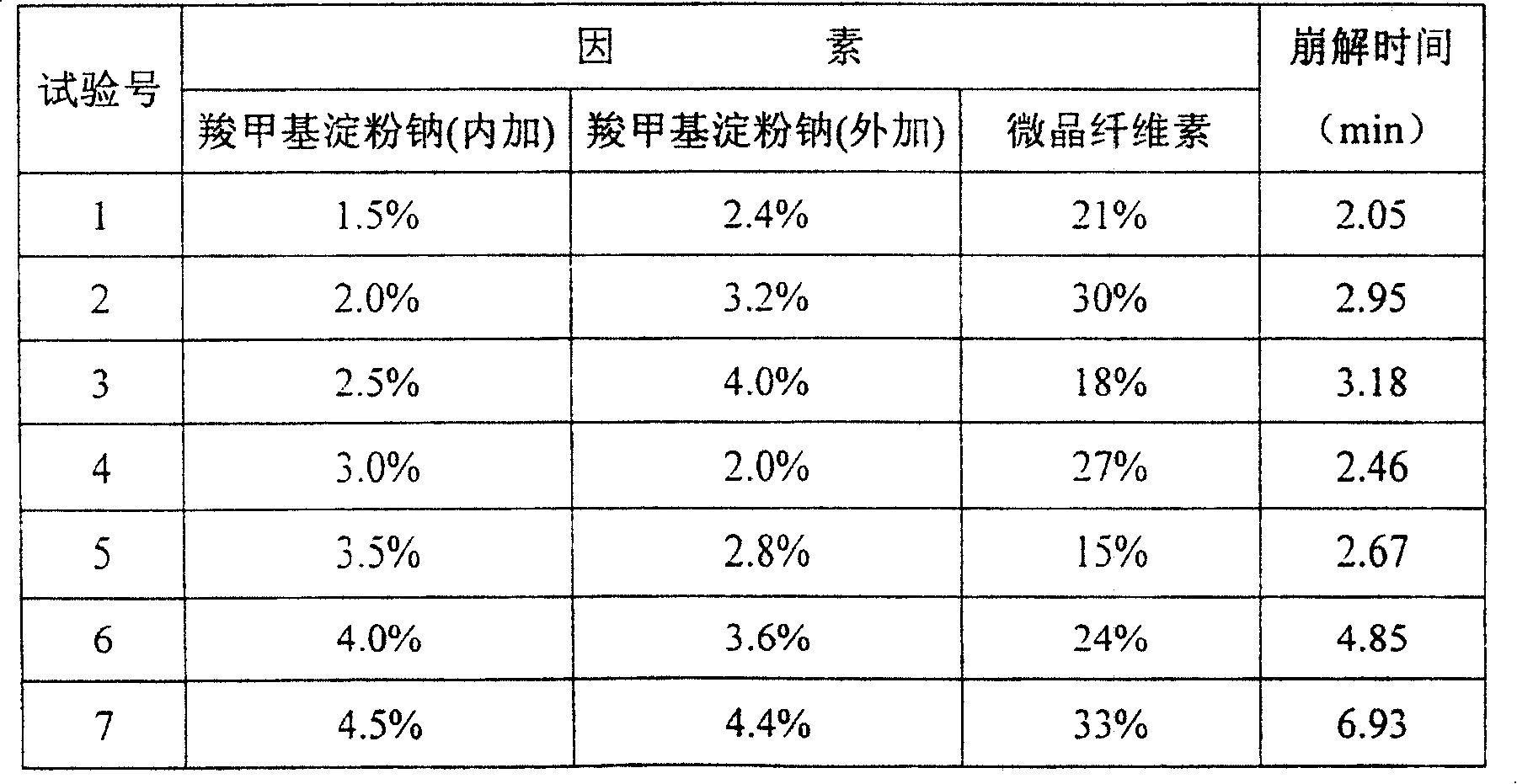
Heart beneficial keton dispersion tablet and its preparation method
InactiveCN1823944AFast disintegrationImprove bioavailabilityPill deliveryCardiovascular disorderSodium carboxymethyl starchLactose
A dispersing tablet of Yixintong is prepared from the micropowdered Yixintong, lactose, microcrystalline cellulose, polyvinyl pyrrolidone, sodium laurylsulfate and carboxymethyl starch sodium through mixing, pulverizing, adding magnesium stearate and residual carboxymethyl starch sodium, stirring and die pressing.
Owner:INST OF PHARMACY SHANDONG PROV ACAD OF MEDICAL SCI
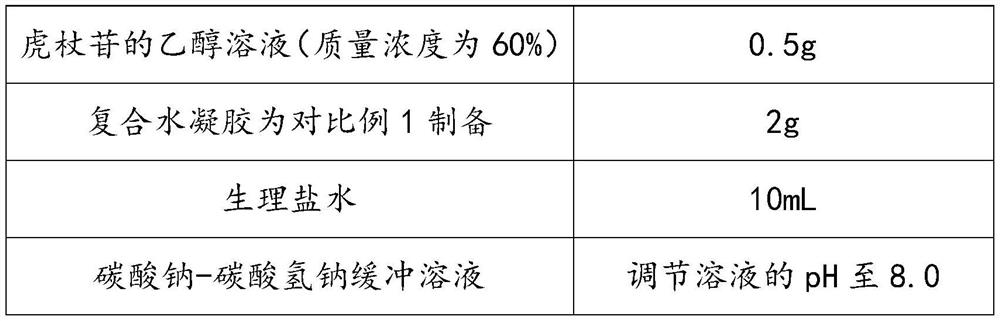
Polydatin-containing composition and application thereof
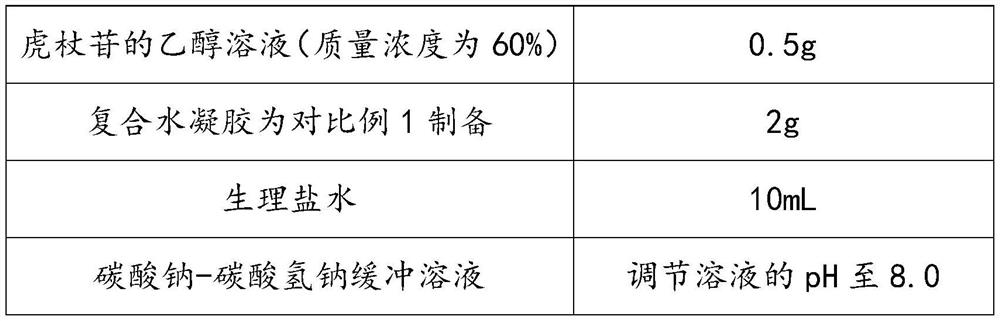
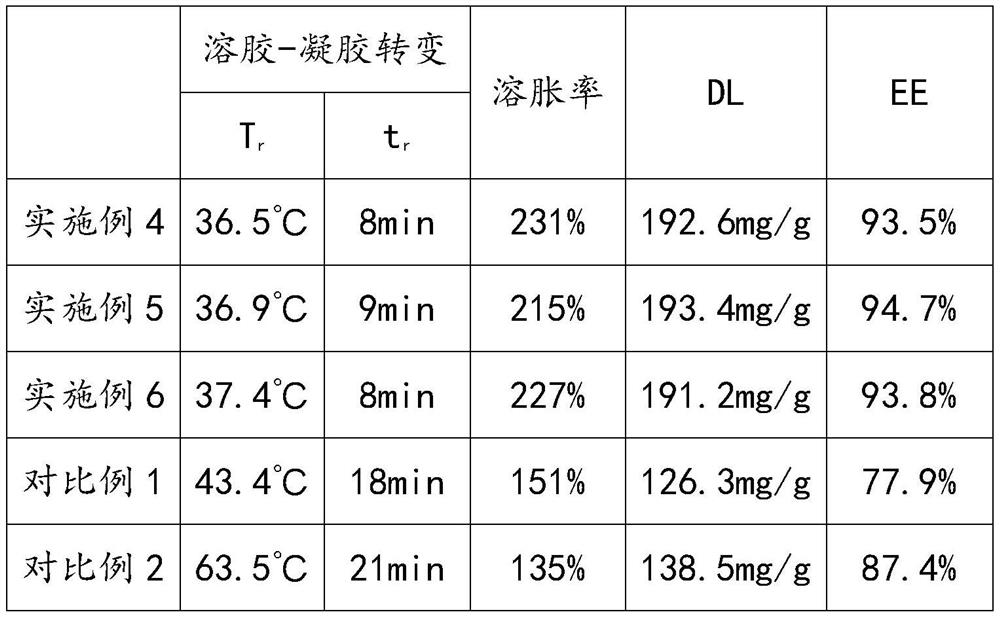
ActiveCN113332232AImprove solubilityMeet the needs of clinical medicineOrganic active ingredientsAerosol deliveryPolymer scienceCarboxyl radical
The invention discloses a polydatin-containing composition and application thereof, belongs to the technical field of polydatin application, and aims to solve the technical problem that the solubility of polydatin in pure water or a normal saline solution is limited. The polydatin-containing composition adopts composite hydrogel as a drug carrying agent, so that the solubility of polydatin in an aqueous solution or the normal saline solution is improved, and the polydatin-containing composition meets the clinical medication requirements of polydatin; and according to the composite hydrogel, the reverse reversible thermal gelling property of poloxamer is utilized, silk fibroin and carboxymethyl chitosan are introduced, carboxyl on the carboxymethyl chitosan reacts with amino on the silk fibroin and hydroxyl on the poloxamer at the same time, an interpenetrating network is formed in a composite gel system, the mechanical strength of the composite hydrogel is improved, and hydrophilic chain segments of the silk fibroin and the poloxamer exist in the whole gel system, so that the gel-sol transition temperature tends to body temperature, and the composite hydrogel is suitable for human body administration.
Owner:HUNAN NUSTREETCARAX
A cationic lipid nanoparticle/DNA complex and its preparation method
ActiveCN109893664BEvenly distributedMonodisperseGenetic material ingredientsPharmaceutical non-active ingredientsDNA SolutionsPolyester
The invention provides a cationic lipid nanoparticle / DNA complex and a preparation process thereof, the preparation process comprising the following steps: (1) dissolving the cationic lipid material in absolute ethanol by heating; (2) dissolving the step ( 1) The prepared ethanol solution is added dropwise to the aqueous phase solution to self-assemble to form cationic lipid nanoparticles; (3) remove the residual ethanol in step (2) cationic lipid nanoparticles; (4) filter. (5) prepare DNA solution; (6) mix the cationic lipid nanoparticle that step (4) prepares and the DNA solution that step (5) prepares according to certain mass ratio, to form cationic lipid nanoparticle / DNA complex ; (7) filter. The preparation method of the present invention is simple and quick to operate, and the prepared cationic lipid nanoparticle / DNA complex has a particle size of 50-150 nm, a PDI<0.3, a monodisperse distribution, and a stable structure, and the complex is sterilized by terminal filtration, The safety of the complex in the preparation of clinical application of tumor medicine is effectively guaranteed.
Owner:SICHUAN UNIV
A kind of hydration icariin nanoparticle and its preparation method and application
ActiveCN109276544BImprove biological activityEvenly dispersedOrganic active ingredientsPowder deliveryDrug utilisationIcaricia icarioides
The invention relates to the technical field of medicine, in particular to hydrated icariin nanoparticles and a preparation method and application thereof. The hydrated icariin nanoparticles comprisehydrated icariin and a stabilizer. According to the hydrated icariin nanoparticles and the preparation method and application thereof, the stabilizer is adopted to enable water-insoluble hydrated icariin drugs to be uniformly dispersed in a water phase in the form of nano-sized particles, so that the problems of insolubility and difficult administration are solved, and the bioavailability of the hydrated icariin is improved; based on the hydrated icariin nanoparticles, the water-insoluble hydrated icariin drugs can be further prepared into various clinically common dosage forms through a modern preparation process so as to meet various clinical medication requirements; and meanwhile, the stabilizer can improve the biological activity of hydrated icariin and further improve the bioavailability of the hydrated icariin.
Owner:INST OF MEDICINAL PLANT DEV CHINESE ACADEMY OF MEDICAL SCI
A kind of polydatin pharmaceutical composition without organic solvent and preparation method thereof
ActiveCN108670950BImprove solubilitySolve the problem of prone to bubble headPowder deliveryOrganic active ingredientsPolyethylene glycolHydroxystearic Acid
The invention relates to the field of pharmaceutical preparations, and in particular, relates to a polydatin pharmaceutical composition containing no organic solvent and a preparation method thereof.The polydatin pharmaceutical composition comprises polydatin, a solubilizing agent and a solvent. The solubilizing agent is polyethylene glycol dodecyl hydroxy stearate, and the solvent is water for injection or a pH buffer solution or a sodium chloride solution or a glucose solution. According to the polydatin pharmaceutical composition containing no organic solvent, the dosage form is a pharmaceutically acceptable conventional dosage form such as water for injection or pre-serviced water or an aqueous solution freeze-dried product prepared from normal saline. The preparation method of the polydatin pharmaceutical composition mainly comprises the steps of dissolution, filtration, sealed potting, sterilization, lamp examination and the like.
Owner:SHENZHEN NEPTUNUS PHARMA RES INST CO LTD
Derivatives containing capsaicin salicylate, preparation method and use
ActiveCN108069870BImprove biological activityIncrease irritationOrganic active ingredientsMetabolism disorderLabor painSalicylic acid
The invention provides a class of capsaicin-containing salicylate derivatives with general structural formula I and a preparation method thereof, and the derivatives have the uses of anti-inflammation, labor pain, anti-cancer, antibacterial and hypolipidemic.
Owner:NANHUA UNIV
Doxazosin-mesylate controlled-releasing tablet and preparation method thereof
ActiveCN101167728BImprove complianceStable blood concentrationOrganic active ingredientsPharmaceutical delivery mechanismDoxazosin mesilateCurative effect
The invention discloses methanesulfonic acid doxazosin controlling releasing tablets and a process for preparation, which contains a single-layer core, insoluble semi-permeable membrane, single drug-releasing eyelet on each side of the tablet, and depends on the osmotic pressure difference between inside and outside of the semi-permeable membrane medium. The invention realizes the control of linear releasing of methanesulfonic acid doxazosin by single-layer core structure, and can remain effective and steady blood and drug concentration. The curative effect is improved, the generation rate ofside effect is reduced, and the productive technology is greatly simplified.
Owner:HEFEI LIFEON PHARMA
Polydatin pharmaceutical composition containing no organic solvent and preparation method thereof
ActiveCN108670950AImprove solubilitySolve the problem of prone to bubble headPowder deliveryOrganic active ingredientsFreeze-dryingFiltration
The invention relates to the field of pharmaceutical preparations, and in particular, relates to a polydatin pharmaceutical composition containing no organic solvent and a preparation method thereof.The polydatin pharmaceutical composition comprises polydatin, a solubilizing agent and a solvent. The solubilizing agent is polyethylene glycol dodecyl hydroxy stearate, and the solvent is water for injection or a pH buffer solution or a sodium chloride solution or a glucose solution. According to the polydatin pharmaceutical composition containing no organic solvent, the dosage form is a pharmaceutically acceptable conventional dosage form such as water for injection or pre-serviced water or an aqueous solution freeze-dried product prepared from normal saline. The preparation method of the polydatin pharmaceutical composition mainly comprises the steps of dissolution, filtration, sealed potting, sterilization, lamp examination and the like.
Owner:SHENZHEN NEPTUNUS PHARMA RES INST CO LTD
A kind of method for improving the yield of paclitaxel in endophytic fungus fermentation product
ActiveCN105400842BIncrease contentEfficient degradationMicroorganism based processesFermentationBiotechnologyValeriana jatamansi
The invention discloses a method for increasing the yield of paclitaxel in an endophytic fungus fermentation product. The method comprises the steps that an endophytic fungus seed solution for producing paclitaxel is inoculated into an endophytic fungus solid state fermentation medium containing taxus media and valeriana jatamansi for culture, the mixture is then put into an alternating magnetic field environment for further culture, and a endophytic fungus solid state fermentation product is obtained; then the endophytic fungus seed solution for producing paclitaxel is inoculated into a liquid fermentation medium containing the endophytic fungus solid state fermentation product for culture, and the final endophytic fungus fermentation product is obtained. According to the method, raw materials are natural, no pollution is caused to the environment, a cycle is short, the yield is high, the method is suitable for industrial production, and compared with a common endophytic fungus fermentation product, the yield of paclitaxel in the endophytic fungus fermentation product is obviously increased.
Owner:ZHEJIANG FORESTRY ACAD
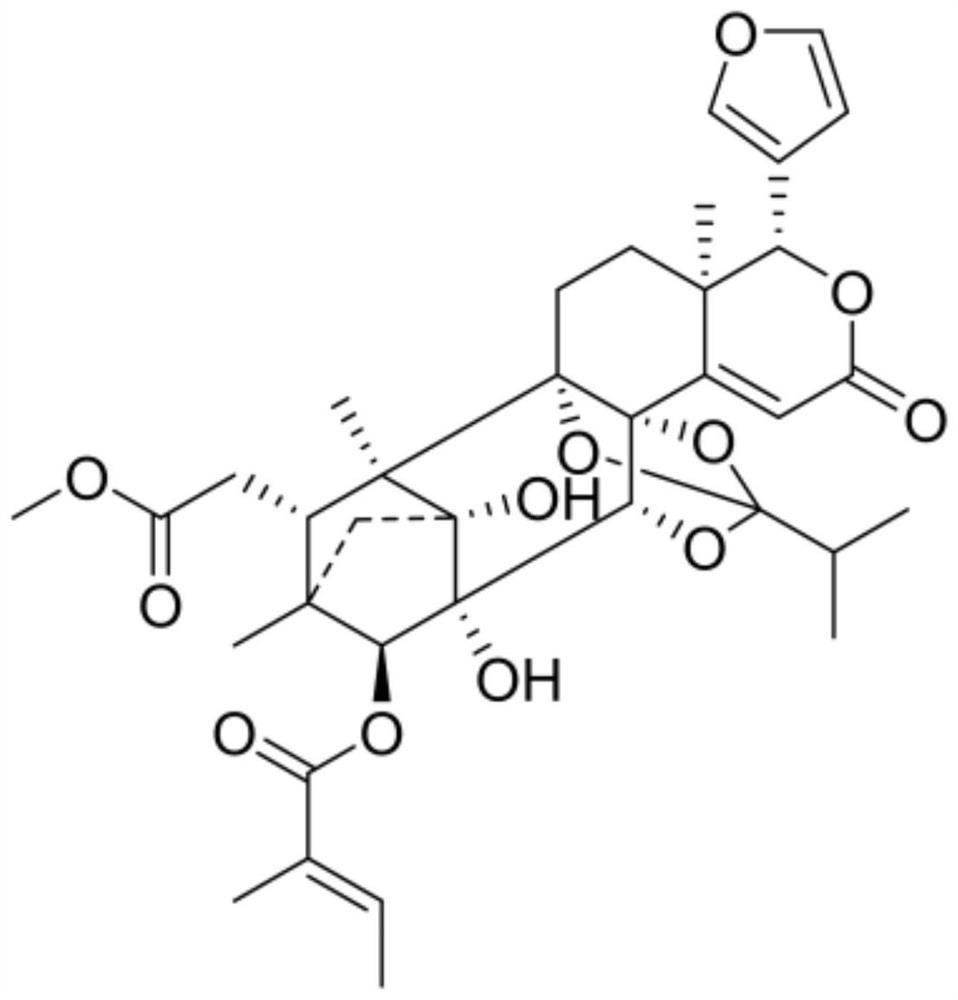
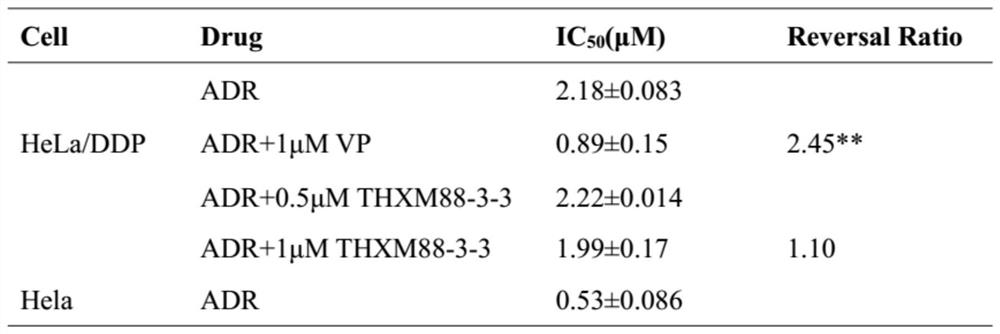
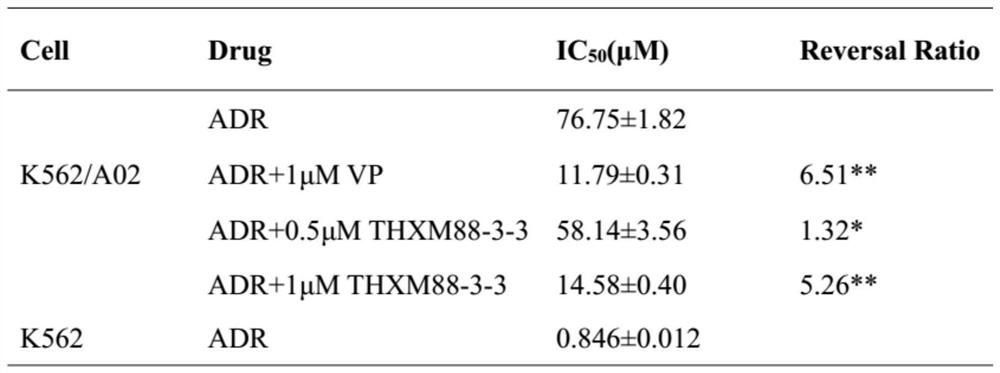
Application of terpenoid THXM 88-3-3 in peach blossom tree fruit as tumor multidrug resistance reversal agent
PendingCN113509463AMeet the needs of clinical medicineOrganic active ingredientsAntineoplastic agentsCancer cellOncology
The invention belongs to the technical field of medicines, relates to a novel application of THXM 88-3-3 as a multidrug resistance reversal agent, and concretely relates to a novel application of the THXM 88-3-3 in treating tumors with multidrug resistance and improving the sensitivity of tumor cells to anti-cancer drugs when the THXM 88-3-3 and the anti-cancer drugs are combined. The compound has the beneficial effects that the sensitivity of chemotherapeutic drugs to cancer cells is enhanced, the curative effect of the drugs is enhanced, and THXM 88-3-3 is expected to be developed into the tumor multidrug resistance reversal agent.
Owner:NANKAI UNIV
Heart beneficial keton dispersion tablet and its preparation method
InactiveCN100402060CFast disintegrationImprove bioavailabilityPill deliveryCardiovascular disorderCarboxymethyl starchLactose
A dispersing tablet of Yixintong is prepared from the micropowdered Yixintong, lactose, microcrystalline cellulose, polyvinyl pyrrolidone, sodium laurylsulfate and carboxymethyl starch sodium through mixing, pulverizing, adding magnesium stearate and residual carboxymethyl starch sodium, stirring and die pressing.
Owner:INST OF PHARMACY SHANDONG PROV ACAD OF MEDICAL SCI
New strain and method of producing pacilitaxel using said strain
The invention relates to a new fungus that could produce taxales mellow-Alternaria alternate var.monosporus ST-026-R CGMCC No.0899. The invention also discloses the method to produce taxales mellow that includes the following steps: cultivating CGMCC 0899 in culture medium to produce and gather taxales mellow in the strain cell and the culture medium. Thus, the taxales mellow could be recycle and purify in the cell and culture medium. It could solve the technology problem of taxales mellow and protect natural resource.
Owner:SUNGEN BIOSCIENCE CO LTD
A composition containing polydatin and its application
ActiveCN113332232BImprove solubilityMeet the needs of clinical medicineOrganic active ingredientsAerosol deliveryMedicineEngineering
The invention discloses a composition containing polydatin and its application, which belongs to the technical field of polydatin application and aims to solve the technical problem of the limited solubility of polydatin in pure water or physiological saline solution. And this kind of polydatin-containing composition adopts composite hydrogel as loading agent, improves the solubility of polydatin in aqueous solution or saline solution, and makes it meet the needs of clinical medicine of polydatin; and the composite hydrogel utilizes Poloxamer has the property of reverse reversible thermal gelation, and the introduction of silk fibroin and carboxymethyl chitosan utilizes the carboxyl group on carboxymethyl chitosan to combine with the amino group on silk fibroin and poloxamer The hydroxyl group on the hydrogel reacts to form an interpenetrating network in the composite gel system, which improves the mechanical strength of the composite hydrogel, and there are hydrophilic segments of silk fibroin and poloxamer in the entire gel system, making the gel ‑Sol transition temperature tends to body temperature, suitable for human administration.
Owner:HUNAN NUSTREETCARAX
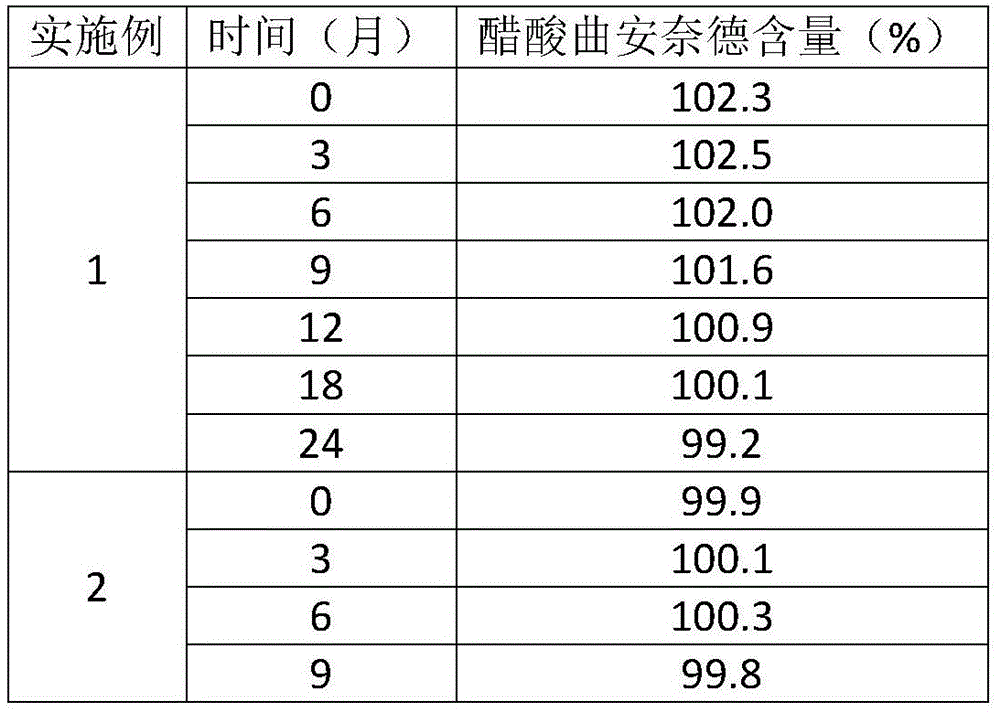
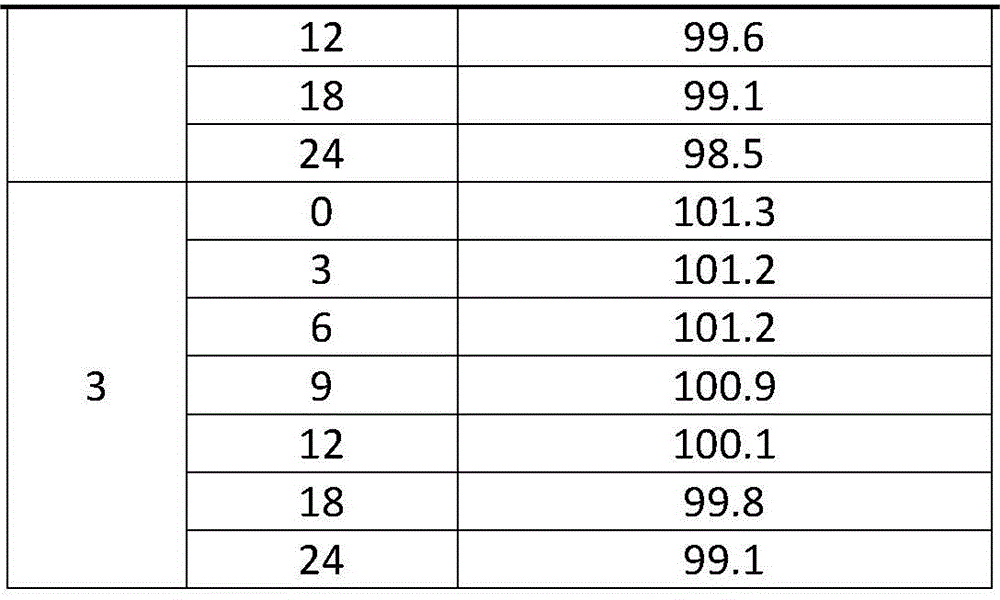
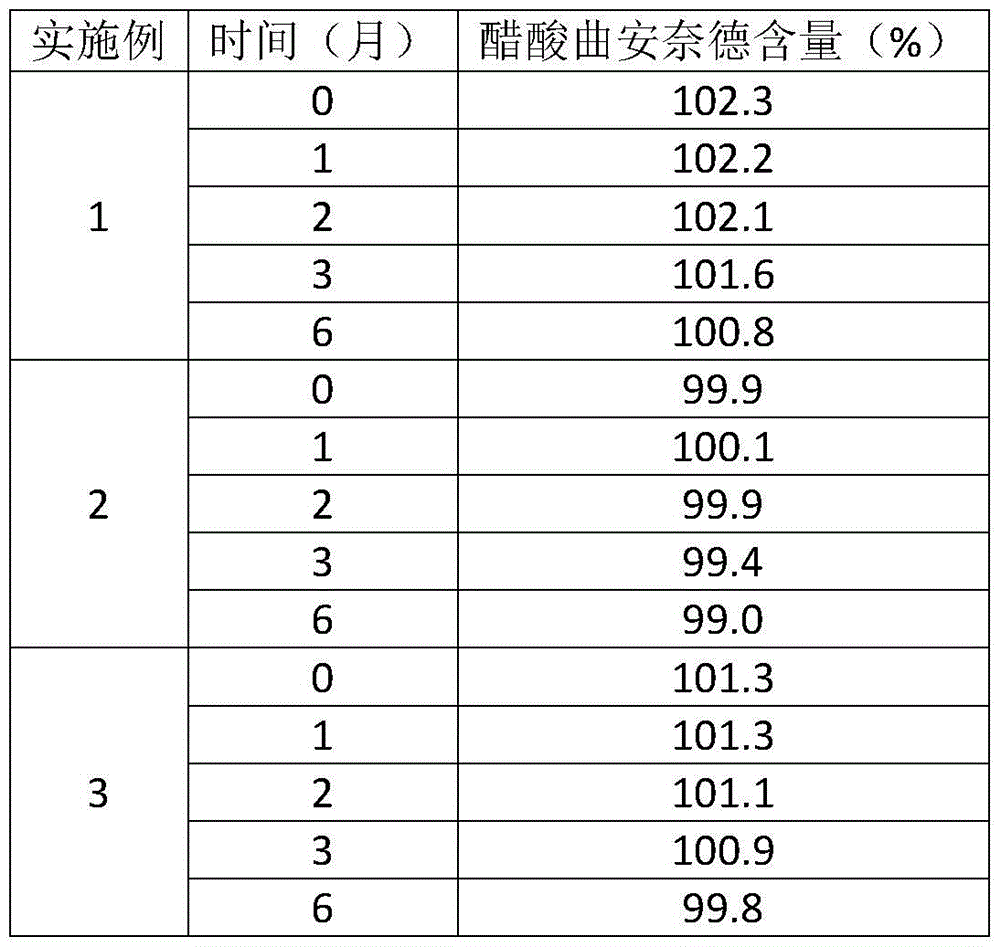
A kind of preparation method of compound triamcinolone acetonide acetate solution
ActiveCN103622973BReduce dosageEasy to makeSalicyclic acid active ingredientsNervous disorderAqueous solutionTriamcinolone acetonide
The invention discloses a compound triamcinolone acetonide acetate solution preparation method. According to the method, a solution product with excellent stability is obtained by adding appropriate amount of a sodium chloride aqueous solution; in addition, with adoption of the method, the production cost is low, the safety and effectiveness of the product are not affected, the environmental pollution pressure is not worsened, and industrial production and application are suitable.
Owner:广东恒诚制药股份有限公司
Acetyl salicyl sulfonone oral cavity disintegration tablet and its preparation method
InactiveCN1296042CSolve the lack of hardnessGreat tasteOrganic active ingredientsPill deliveryAspirinAngina
An oral disintegrating tablet of flavone aspirin for treating coronary heart disease, angina pectoris, myocarditis, ischemic heart disease, hyperlipemia, etc is disclosed. Its preparing process is also disclosed.
Owner:COSCI MED TECH CO LTD
Astragaloside injection and preparation method thereof
ActiveCN100586420CImprove stabilityQuality is easy to controlOrganic active ingredientsPharmaceutical delivery mechanismGlycosidePharmaceutical medicine
The invention relates to an injection using astragalus root glycosides extract as the active ingredient, which also comprises pharmaceutically acceptable adjunct for injection, the content of the astragalus root glycosides in the total astragalus root extract is 80-100%, each unit of the preparation contains astragalus root glycosides 90-300mg.
Owner:CHENGDU DIAO JIUHONG PHARMA FAB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com