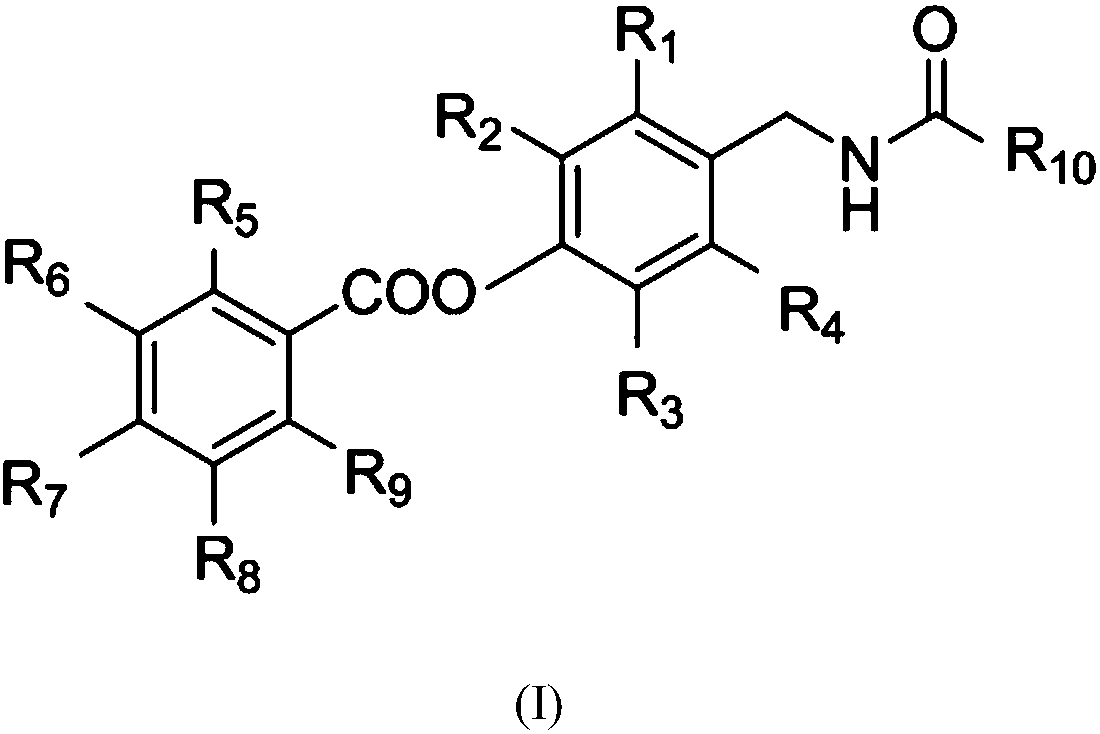
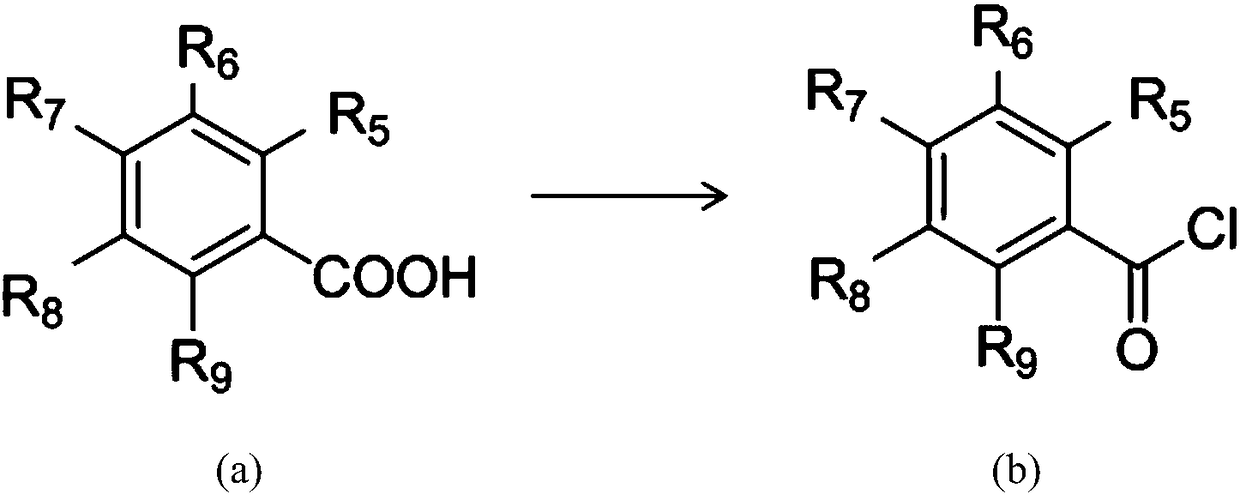
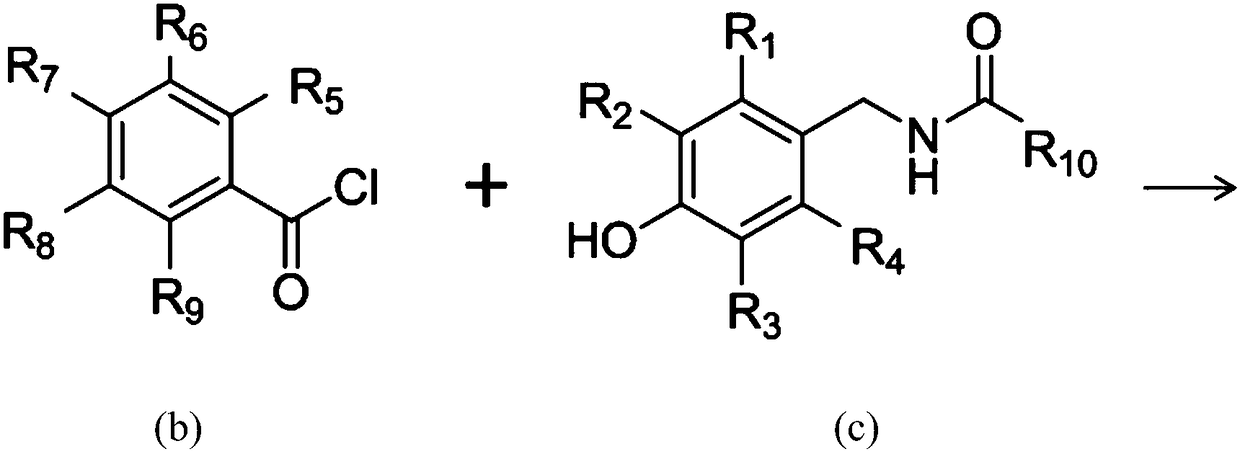
Salicylic acid-containing capsaicin ester derivative, preparation method and application
A hydrate and compound technology, applied in the field of capsaicin salicylate derivatives and their preparation, can solve the problems of low bioavailability, limited application, difficult to absorb irritation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Preparation of (E)-8-methyl-N-(4-salicyloyloxy-3-methoxybenzyl)-6-nonenamide (compound 1, code 01)
[0063]
[0064] React 0.01mol salicylic acid with 3ml of thionyl chloride at 70°C, add 2 drops of catalyst pyridine, and react for 2 hours to remove excess thionyl chloride by suction filtration under reduced pressure, dissolve in 10ml of dichloromethane, and place on ice Stir in the bath. Dissolve 0.01mol capsaicin (trans-8-methyl-N-vanillyl-6-nonenamide) and pyridine in dichloromethane, add dropwise to the reaction flask through a constant pressure funnel (0.5h), and drop Stir at 25°C for 7 hours. The target product was obtained by column chromatography with ethyl acetate:petroleum ether=1:3. The relevant data are as follows:
[0065] Code 01EI-MS m / z:425.2; Anal.Calcd.For C 25 h 31 NO 5 : C,70.57; H,7.34; N,3.29; Found C,70.57; H,7.32; N,3.28
Embodiment 2
[0067] Preparation of 8-methyl-N-(4-salicyloyloxy-3-methoxybenzyl)nonanamide (compound 2, code 02)
[0068]
[0069] React 0.01mol salicylic acid with 3ml of dichloromethane containing phosphorus pentachloride at 50°C, add 2 drops of catalyst pyridine, react for 5 hours and remove excess thionyl chloride by suction filtration under reduced pressure, and use 10ml of dichloromethane Dissolve and stir in an ice bath. Dissolve 0.01mol dihydrocapsaicin (N-(4-hydroxy-3-methoxybenzyl)-8-methylnonanamide) and pyridine in dichloromethane, and add it dropwise to the reaction flask through a constant pressure funnel (1h), and stirred at 35° C. for 10 hours after dropping. The target product was obtained by column chromatography with ethyl acetate:petroleum ether=1:3. The relevant data are as follows:
[0070] Code 02EI-MS m / z:427.2; Anal.Calcd.For C 25 h 33 NO 5 : C,70.23; H,7.78; N,3.28; Found C,70.25; H,7.75; N,3.26
Embodiment 3
[0072] Preparation of 7-methyl-N-(4-salicyloyloxy-3-methoxybenzyl) octanamide (compound 3, code 03)
[0073]
[0074] React 0.01mol salicylic acid with 3ml of thionyl chloride at 80°C, add 2 drops of catalyst triethylamine, and react for 1 hour to remove excess thionyl chloride by suction filtration under reduced pressure, dissolve in 10ml of acetone, and place on ice Stir in the bath. Dissolve 0.01mol nordihydrocapsaicin (7-Methyl-N-vanillyl-octamide) and triethylamine in acetone, add dropwise into the reaction flask through a constant pressure funnel (0.5h), and stir at 10°C for 5 Hour. The target product was obtained by column chromatography with ethyl acetate:petroleum ether=1:3. The relevant data are as follows:
[0075] Code 03EI-MS m / z:413.2; Anal.Calcd.For C 24 h 31 NO 5 : C,69.71; H,7.56; N,3.39; Found C,69.69; H,7.54; N,3.37
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com