Carbinoxamine maleate oral slow-release suspension and preparation method thereof
A technology for carbinoxamine maleate and sustained-release suspensions, which is applied in pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., to improve taste pleasure and medication compliance , Obvious slow-release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
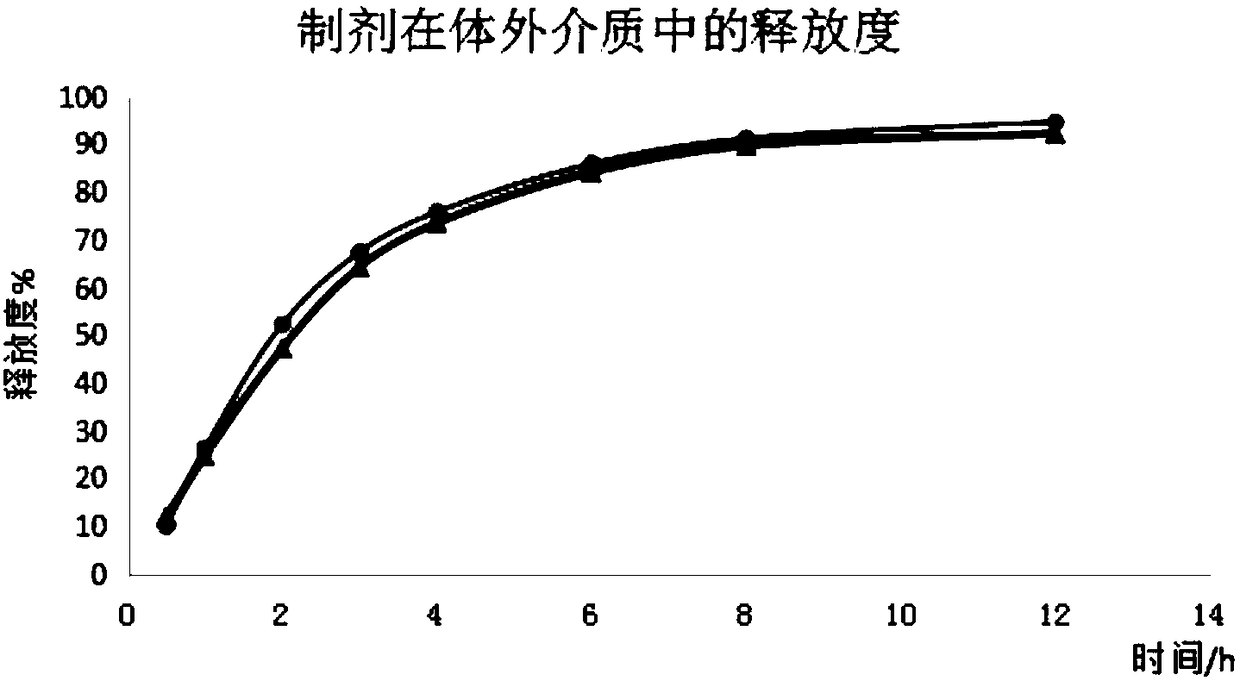
Image
Examples
Embodiment 1
[0062] Formula composition
[0063] Drug Resin Complex:
[0064] Carbinoxamine Maleate 24g
[0065] Sodium polystyrene sulfonate 24g
[0066] Deionized water 1 9kg
[0067] Impregnated Drug Resin Compound Granules
[0068] drug resin complex 2 48.2g
[0069] Kollicoat SR 30D 96.4g
[0070] 1,2-propanediol 11.2g
[0071] suspension
[0072]
[0073] Add deionized water to 30L
[0074] Note: 1. Deionized water is finally removed in this step, and its consumption is not included in the preparation.
[0075] 2. The prescription quantity of the drug resin compound is subject to the actual preparation.
[0076] 3. The prescription amount of impregnated drug resin composite particles is subject to the actual preparation.
[0077] Preparation Process:
[0078] 1. Preparation of carbinoxamine maleate-resin complex:
[0079] (1) Weigh 24 g of carbinoxamine maleate, add 3000 ml of deionized water in the prescribed amount, and stir at room temperature for 10 minutes to...
Embodiment 2
[0098] Formula composition
[0099] Drug Resin Complex:
[0100]Carbinoxamine Maleate 24g
[0101] Sodium polystyrene sulfonate 36g
[0102] Deionized water 1 11.3kg
[0103] Impregnated Drug Resin Compound Granules
[0104] drug resin complex 2 62.8g
[0105] Kollicoat SR 30D 157g
[0106] PEG-200 11.2g
[0107] suspension
[0108]
[0109] Add deionized water to 30L
[0110] Note: 1. Deionized water is finally removed in this step, and its consumption is not included in the preparation.
[0111] 2. The prescription quantity of the drug resin compound is subject to the actual preparation.
[0112] 3. The prescription amount of impregnated drug resin composite particles is subject to the actual preparation.
[0113] Preparation Process:
[0114] 1. Preparation of carbinoxamine maleate-resin complex:
[0115] (1) Weigh 24 g of carbinoxamine maleate, add 3000 ml of deionized water in the prescribed amount, and stir at room temperature for 10 minutes to disso...
Embodiment 3
[0134] Formula composition
[0135] Drug Resin Complex:
[0136] Carbinoxamine Maleate 24g
[0137] Sodium polystyrene sulfonate 48g
[0138] Deionized water 1 13.5kg
[0139] Impregnated Drug Resin Compound Granules
[0140] drug resin complex 2 74.8g
[0141] Kollicoat SR 30D 224.4g
[0142] PEG-400 11.2g
[0143] suspension
[0144]
[0145] Add deionized water to 30L
[0146] Note: 1. Deionized water is finally removed in this step, and its consumption is not included in the preparation.
[0147] 2. The prescription quantity of the drug resin compound is subject to the actual preparation
[0148] 3. The prescription amount of impregnated drug resin composite particles is subject to the actual preparation
[0149] Preparation Process:
[0150] 1. Preparation of carbinoxamine maleate-resin complex:
[0151] (1) Weigh 24 g of carbinoxamine maleate, add 3000 ml of deionized water in the prescribed amount, and stir at room temperature for 10 minutes to diss...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com