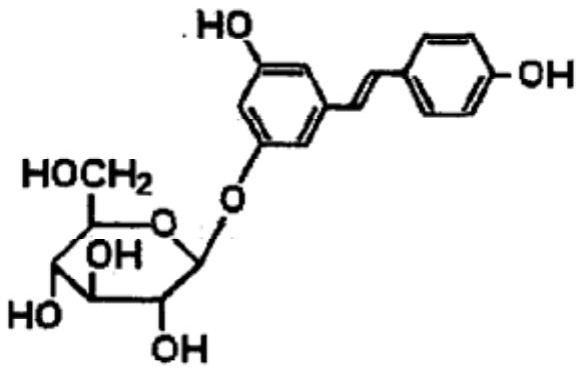
A kind of polydatin pharmaceutical composition without organic solvent and preparation method thereof
A technology of organic solvent and polydipside, applied in the field of medicine, can solve problems such as deepening and color of bubble head solution, and achieve the effect of increasing solubility, ensuring product quality, and meeting the needs of clinical medication.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
[0030] Preparation process: prepare sodium carbonate-sodium bicarbonate buffer solution for later use; weigh the prescribed amount of polyethylene glycol lauryl hydroxystearate and polydatin and add part of the prescribed amount to the sodium carbonate-sodium bicarbonate buffer solution. Stir to dissolve it completely, add sodium carbonate-sodium bicarbonate buffer solution to the full amount; remove the pyrogen by ultrafiltration; take the liquid medicine after ultrafiltration to check the content, pH value, bacterial endotoxin and other items; 0.22 μm sterilizing filter membrane for sterilizing filtration; potting; sterilizing; lamp inspection to obtain polydatin injection.
Embodiment 2
[0032]
[0033] Preparation process: prepare sodium carbonate-sodium bicarbonate buffer solution for later use; weigh the prescribed amount of polyethylene glycol lauryl hydroxystearate and polydatin and add part of the prescribed amount to the sodium carbonate-sodium bicarbonate buffer solution. Stir to dissolve it completely, add sodium carbonate-sodium bicarbonate buffer solution to the full amount; remove the pyrogen by ultrafiltration; take the liquid medicine after ultrafiltration to check the content, pH value, bacterial endotoxin and other items; 0.22 μm sterilizing filter membrane for sterilizing filtration; potting; sterilizing; lamp inspection to obtain polydatin injection.
Embodiment 3
[0035]
[0036] Preparation process: prepare sodium carbonate-sodium bicarbonate buffer solution for later use; weigh the prescribed amount of polyethylene glycol lauryl hydroxystearate and polydatin and add part of the prescribed amount to the sodium carbonate-sodium bicarbonate buffer solution. Stir to dissolve it completely, add sodium carbonate-sodium bicarbonate buffer solution to the full amount; remove the pyrogen by ultrafiltration; take the liquid medicine after ultrafiltration to check the content, pH value, bacterial endotoxin and other items; 0.22 μm sterile filter membrane for sterile filtration; filling and freeze-drying to obtain polydatin freeze-dried powder injection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com