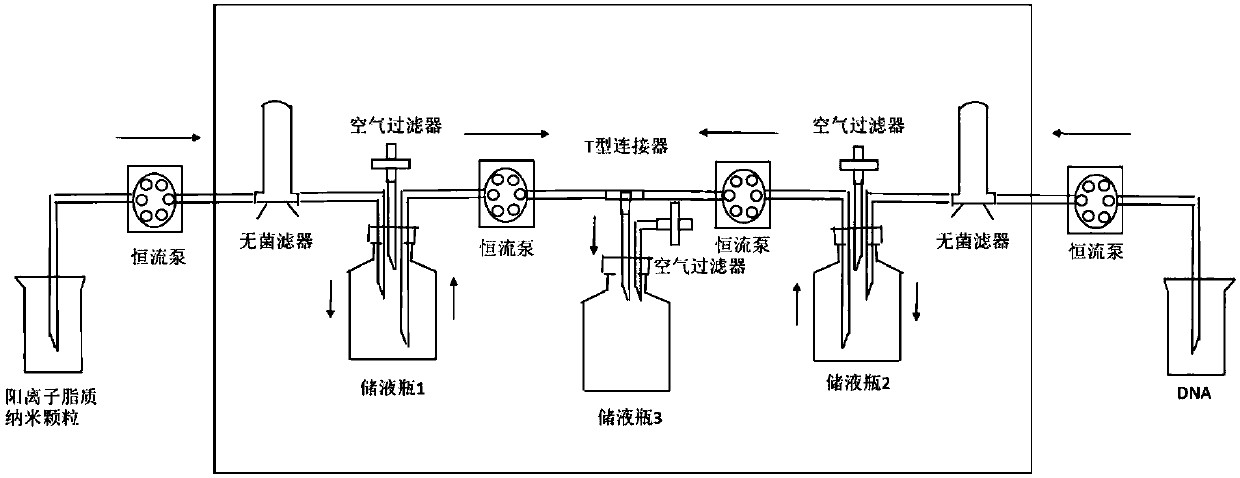
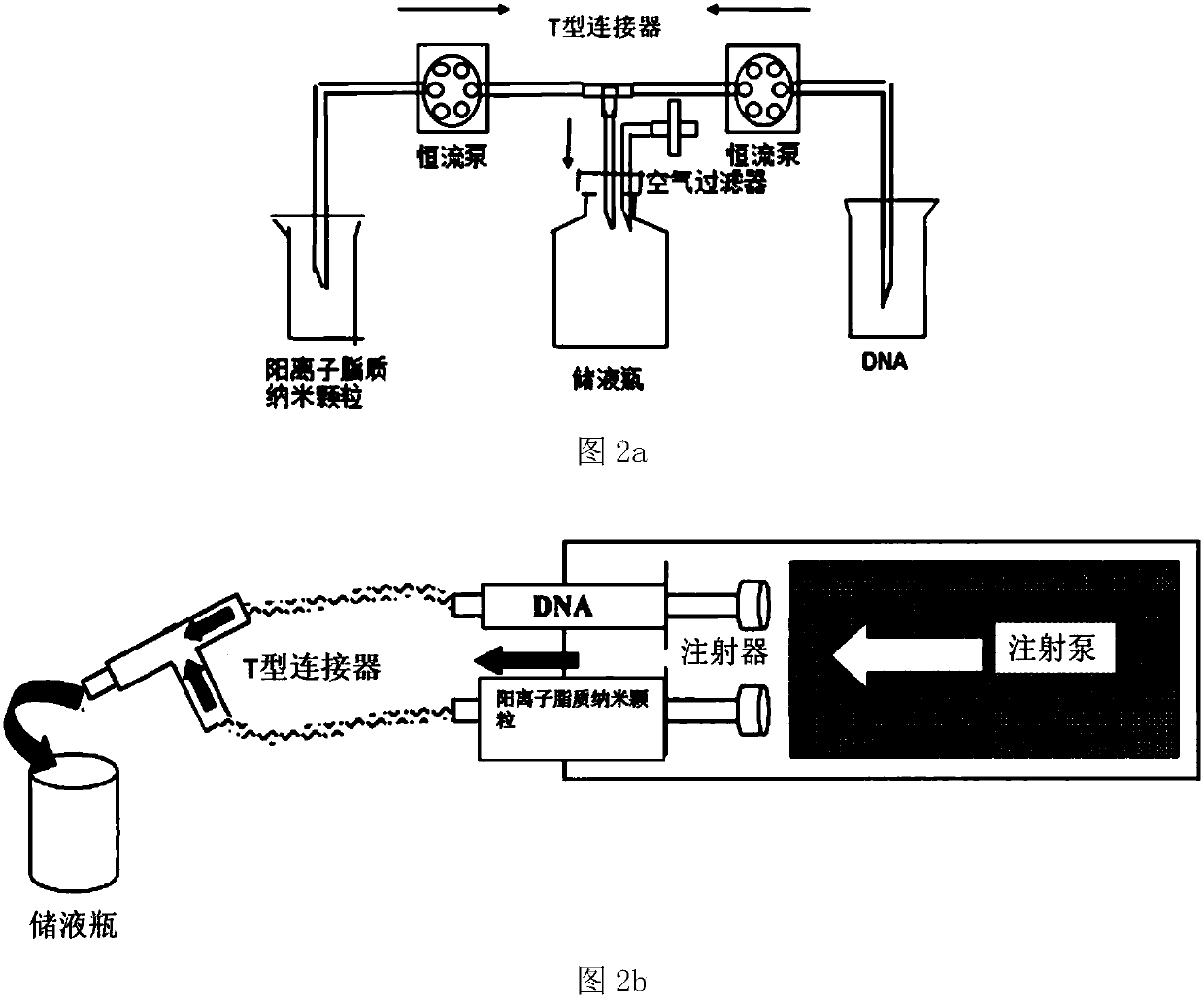
Positive ion lipid nanometer particle/DNA compound and preparation method thereof
A cationic lipid and nanoparticle technology, applied in the field of biomedicine, can solve the problems of long production time, poor uniformity, increased production cost and the like, and achieve the effects of simple structure, easy assembly and production cost saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Preparation and characterization of embodiment 1, DOTAP nanoparticles
[0079] 1. Preparation of DOTAP nanoparticles
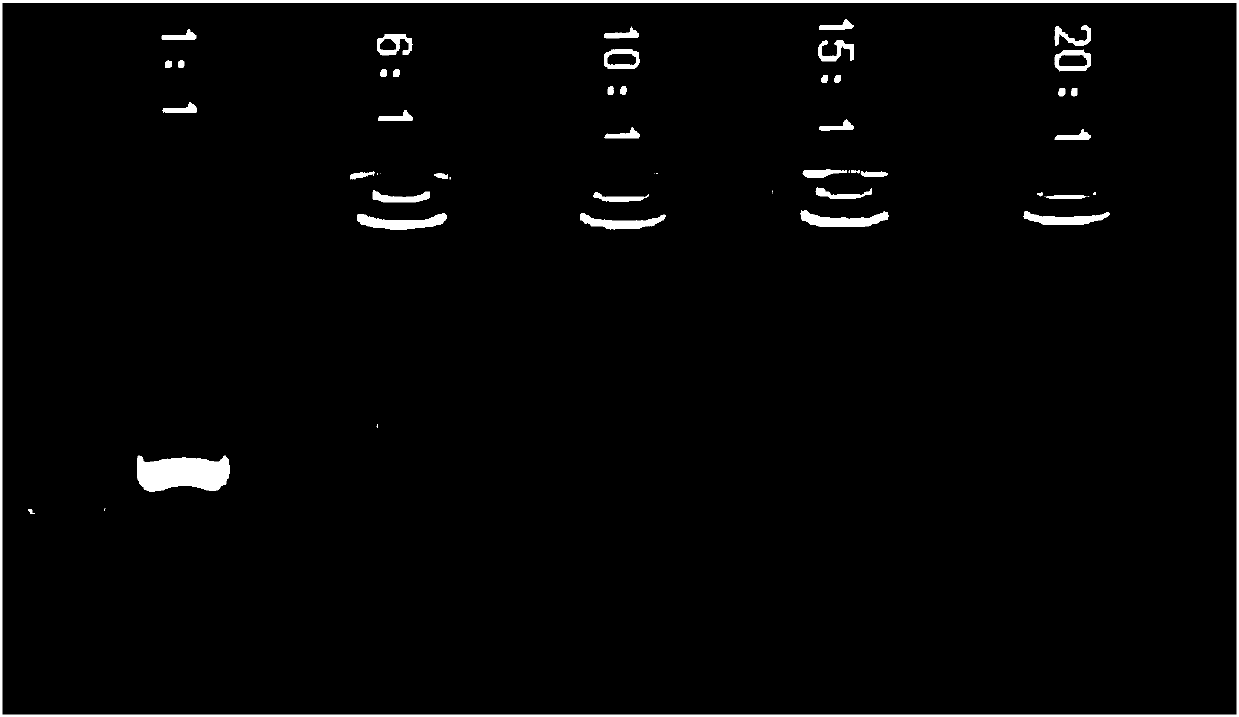
[0080] (1) As shown in Table 1, put DOTAP weighing different masses into a 250ml PETG (polyethylene terephthalate-1,4-cyclohexanedimethanol) bottle, add absolute ethanol solution , heated in a water bath at 50°C, and gently oscillated to accelerate the complete dissolution of DOTAP.
[0081] (2) Set up the peristaltic pump, set the rotating speed at 6-8rpm, add the ethanol solution containing DOTAP prepared in step (1) dropwise to the water phase solution at a rate of 30-120ml / min, the ethanol solution and the water phase The total volume of the solution is 60ml, and the volume ratio of the ethanol solution and the aqueous phase solution is shown in Table 1. The distance between the droplet outlet and the liquid surface is kept at about 5cm, and DOTAP can self-assemble in ethanol aqueous solution to form DOTAP nanoparticles.
[0082] (3) After the DO...
Embodiment 2
[0096] Embodiment 2, preparation and characterization of DOTAP / cholesterol (DOTAP / Chol) nanoparticles
[0097] 1. Preparation of DOTAP / Chol nanoparticles:
[0098] (1) Put 33.6mg DOTAP and 33.6mg cholesterol (mass ratio 1:1) weighed separately into 250ml PETG bottles, add absolute ethanol solution, heat in a water bath at 50°C, shake gently to accelerate DOTAP and complete dissolution of cholesterol.
[0099](2) set up the peristaltic pump, set the rotating speed to be 6-8rpm, add the ethanol solution containing DOTAP prepared in step (1) dropwise to the aqueous phase solution at a rate of 30-120ml / min, wherein the ethanol solution and The volume ratio of the aqueous solution is 1:3 or 1:4, and the distance between the droplet outlet and the liquid surface is kept at about 5cm. DOTAP and cholesterol can self-assemble in ethanol aqueous solution to form DOTAP / Chol nanoparticles.
[0100] (3) With embodiment 1 step (3).
[0101] (4) With embodiment 1 step (4).
[0102] 2. Ch...
Embodiment 3
[0105] Embodiment 3, preparation and characterization of DOTAP / DOPE nanoparticles
[0106] 1. Preparation of DOTAP / DOPE nanoparticles:
[0107] (1) Put 36.5mg DOTAP and 36.5mg DOPE (mass ratio 1:1) weighed separately into 250ml PETG bottles, add absolute ethanol solution, heat in a water bath at 50°C, shake gently to accelerate DOTAP and Complete dissolution of DOPE.
[0108] (2) set up the peristaltic pump, set the rotating speed to be 6-8rpm, add the ethanol solution containing DOTAP prepared in step (1) dropwise to the aqueous phase solution at a rate of 30-120ml / min, wherein the ethanol solution and The volume ratio of the aqueous phase solution is 1:3 or 1:4, and the droplet outlet is kept at about 5cm from the liquid surface. DOTAP and DOPE can self-assemble in ethanol aqueous solution to form DOTAP / DOPE nanoparticles.
[0109] (3) With embodiment 1 step (3).
[0110] (4) With embodiment 1 step (4).
[0111] 2. Characterization of DOTAP / DOPE nanoparticles:
[0112] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com