Polydatin-containing composition and application thereof
The technology of polydipside and composition is applied in the field of compositions containing polydipside, which can solve the problems of no further description of the feasible preparation method of the solution, limited solubility, difficulty in reaching the drug concentration in conventional aqueous solutions, etc., and achieves good water solubility, low toxicity, Response to the effect of changing the temperature range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
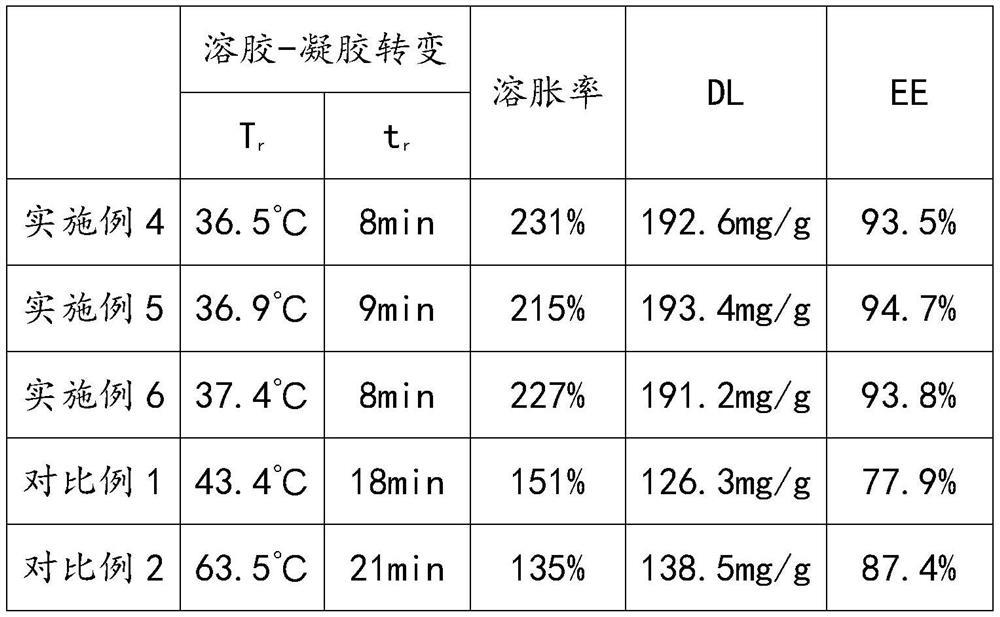
Examples
Embodiment 1
[0028] Composite hydrogels are made through the following steps:
[0029] Step 1, add 30wt% sodium hydroxide aqueous solution in chitosan, after stirring at 300r / min for 45min, the mixture is placed in a freezer, frozen at -23°C for 20h, then the frozen mixture is taken out, and placed in Thaw in the oven, then put the thawed mixture back into the three-necked flask, add isopropanol, stir at 300r / min for 30min, then add the isopropanol solution of chloroacetic acid drop by drop to keep the temperature of the reaction system below 8°C , after the dropwise addition, continue to react for 20h, then filter the reaction mixture, wash the filter cake with deionized water, centrifuge, take the filter cake, then fully dialyze with deionized water, freeze-dry the dialyzate, and obtain carboxymethyl Base chitosan, wherein, the amount ratio of chitosan, 30% sodium hydroxide aqueous solution, isopropanol, chloroacetic acid isopropanol solution is 1g: 4mL: 10mL: 4g, and in the isopropanol ...
Embodiment 2
[0032] Composite hydrogel is made through the following steps: with reference to each step in embodiment 1; In step one, the consumption ratio of the isopropanol solution of chitosan, 30% sodium hydroxide aqueous solution, isopropanol, chloroacetic acid is 1g: 6mL: 15mL: 5g, and the mass fraction of chloroacetic acid in the isopropanol solution of chloroacetic acid is 45%; in step 2, silk fibroin, poloxamer, deionized water, p-toluenesulfonic acid, carboxymethyl shell The dosage ratio of the polysan aqueous solution is 12mg: 100mg: 10mL: 2.5mL: 60mg, and the mass fraction of the carboxymethyl chitosan aqueous solution is 40%, and the poloxamer is P407.
Embodiment 3
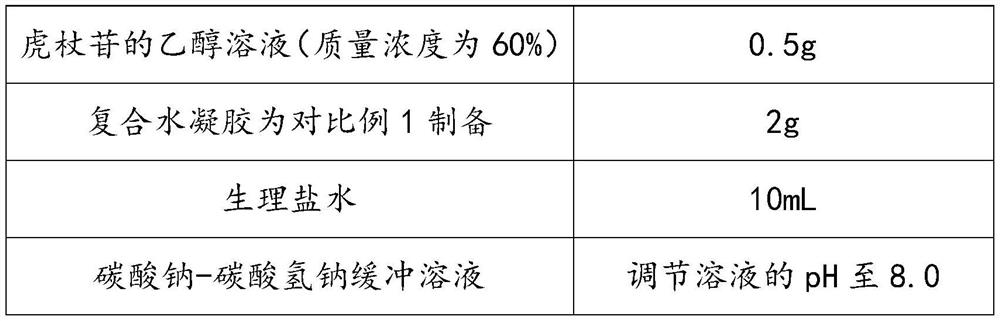
[0041] A composition containing polydatin, each raw material is weighed according to the formula in Table 1:
[0042] Table 1
[0043] raw material Dosage The ethanol solution of polydatin (mass concentration is 60%) 0.5g Composite hydrogel is prepared for embodiment 1 2g normal saline 10mL Sodium carbonate-sodium bicarbonate buffer solution Adjust the pH of the solution to 8.0
[0044] Preparation method: Use physiological saline as a solvent, immerse the composite hydrogel in the solvent, add polydatin ethanol solution, add sodium carbonate-sodium bicarbonate buffer solution, stir at 200r / min for 20min at 37°C, and then place in Stand at constant temperature in a fume hood for 12 hours, remove absolute ethanol, and obtain a polydatin composition.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com