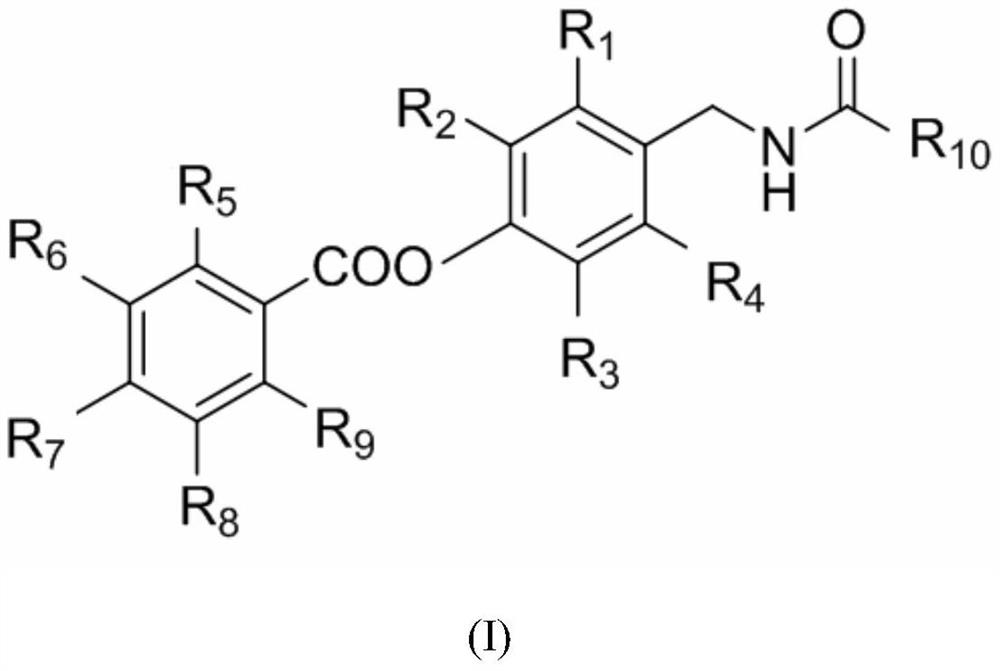
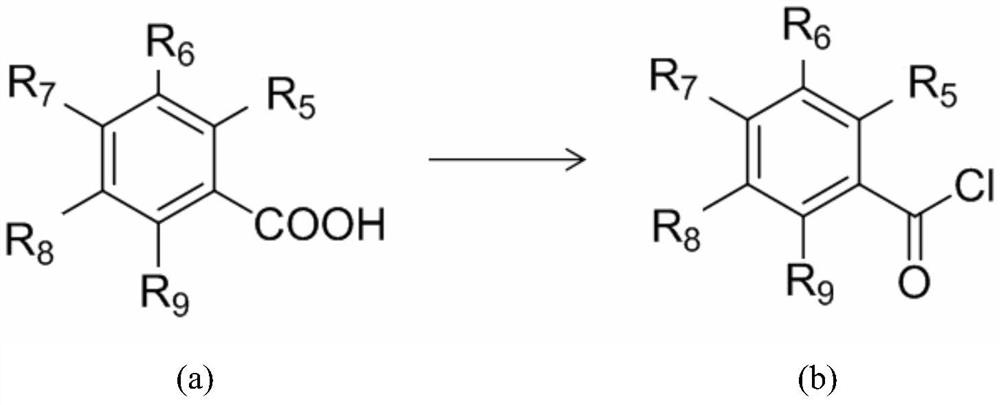
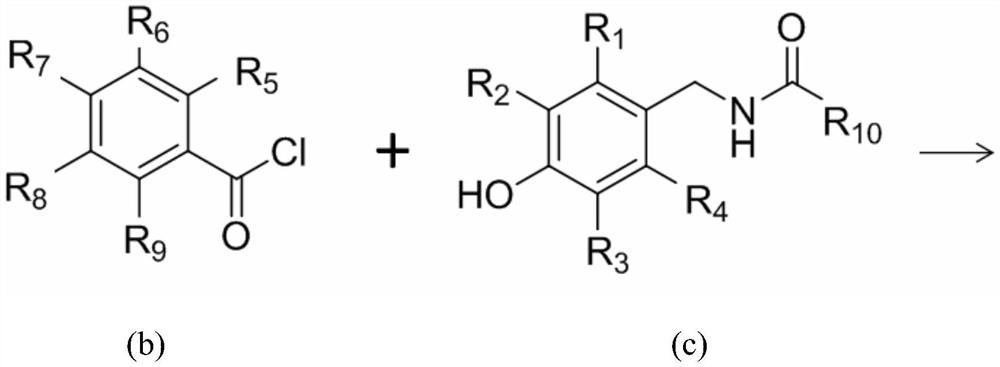
Derivatives containing capsaicin salicylate, preparation method and use
A compound, C1-C6 technology, applied in the field of capsaicin-containing salicylate derivatives and its preparation, can solve the problems of low bioavailability, low absorption, and insufficient capsaicin activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Preparation of (E)-8-methyl-N-(4-salicyloyloxy-3-methoxybenzyl)-6-nonenamide (compound 1, code 01)
[0063]
[0064] React 0.01mol salicylic acid with 3ml of thionyl chloride at 70°C, add 2 drops of catalyst pyridine, and react for 2 hours to remove excess thionyl chloride by suction filtration under reduced pressure, dissolve in 10ml of dichloromethane, and place on ice Stir in the bath. Dissolve 0.01mol capsaicin (trans-8-methyl-N-vanillyl-6-nonenamide) and pyridine in dichloromethane, and add it dropwise into the reaction flask through a constant pressure funnel (0.5h). Stir at 25°C for 7 hours. The target product was obtained by column chromatography with ethyl acetate:petroleum ether=1:3. The relevant data are as follows:
[0065] Code 01EI-MS m / z:425.2; Anal.Calcd.For C 25 h 31 NO 5 : C,70.57; H,7.34; N,3.29; Found C,70.57; H,7.32; N,3.28
Embodiment 2
[0067] Preparation of 8-methyl-N-(4-salicyloyloxy-3-methoxybenzyl)nonanamide (compound 2, code 02)
[0068]
[0069] React 0.01mol salicylic acid with 3ml of dichloromethane containing phosphorus pentachloride at 50°C, add 2 drops of catalyst pyridine, react for 5 hours and remove excess thionyl chloride by suction filtration under reduced pressure, and use 10ml of dichloromethane Dissolve and stir in an ice bath. Dissolve 0.01mol dihydrocapsaicin (N-(4-hydroxy-3-methoxybenzyl)-8-methylnonanamide) and pyridine in dichloromethane, and add it dropwise to the reaction flask through a constant pressure funnel (1h), and stirred at 35° C. for 10 hours after dropping. The target product was obtained by column chromatography with ethyl acetate:petroleum ether=1:3. The relevant data are as follows:
[0070] Code 02EI-MS m / z:427.2; Anal.Calcd.For C 25 h 33 NO 5 : C,70.23; H,7.78; N,3.28; Found C,70.25; H,7.75; N,3.26
Embodiment 3
[0072] Preparation of 7-methyl-N-(4-salicyloyloxy-3-methoxybenzyl) octanamide (compound 3, code 03)
[0073]
[0074] React 0.01mol salicylic acid with 3ml of thionyl chloride at 80°C, add 2 drops of catalyst triethylamine, and react for 1 hour to remove excess thionyl chloride by suction filtration under reduced pressure, dissolve in 10ml of acetone, and place on ice Stir in the bath. Dissolve 0.01mol nordihydrocapsaicin (7-Methyl-N-vanillyl-octamide) and triethylamine in acetone, add dropwise to the reaction flask through a constant pressure funnel (0.5h), and stir at 10°C for 5 Hour. The target product was obtained by column chromatography with ethyl acetate:petroleum ether=1:3. The relevant data are as follows:
[0075] Code 03EI-MS m / z:413.2; Anal.Calcd.For C 24 h 31 NO 5 : C,69.71; H,7.56; N,3.39; Found C,69.69; H,7.54; N,3.37
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com