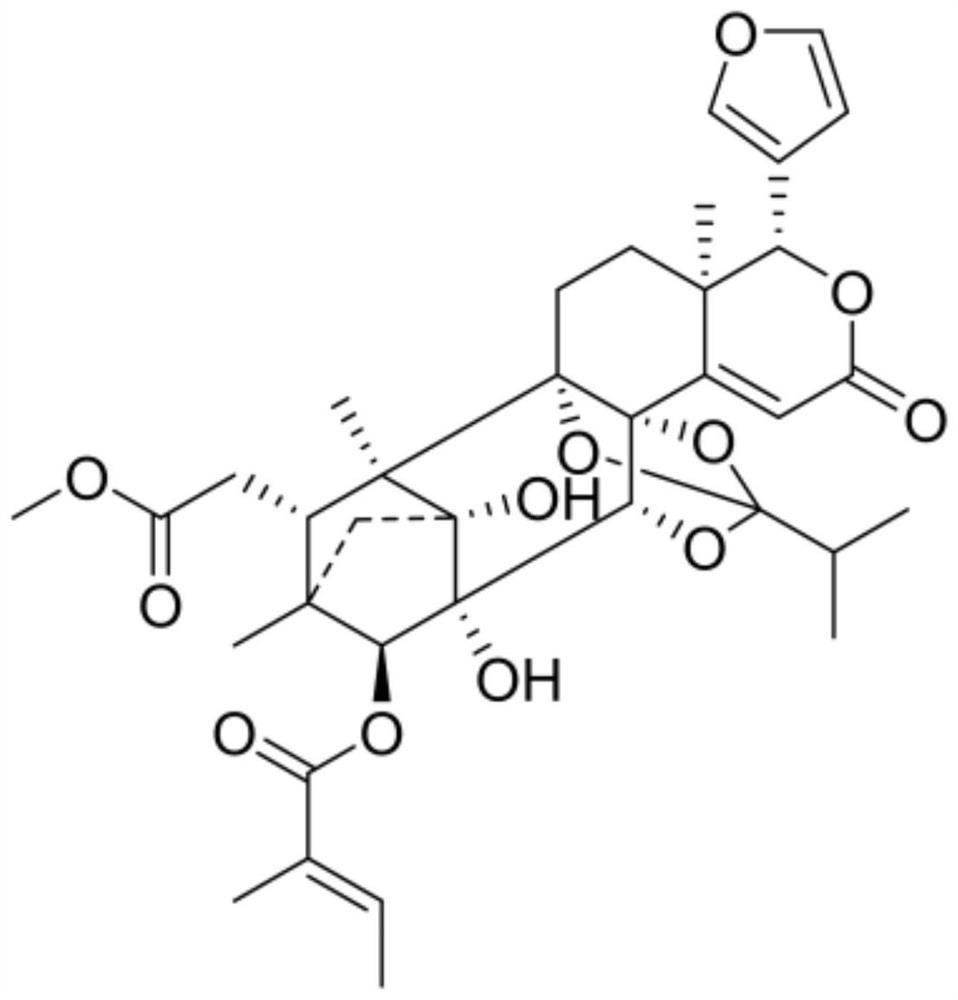
Application of terpenoid THXM 88-3-3 in peach blossom tree fruit as tumor multidrug resistance reversal agent
A multi-drug resistance, multi-drug resistance technology, applied in the direction of anti-tumor drugs, medical preparations containing active ingredients, drug combinations, etc., can solve the problem of limiting the effectiveness of cancer chemotherapy and reducing the effectiveness of intracellular anti-cancer drugs. concentration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
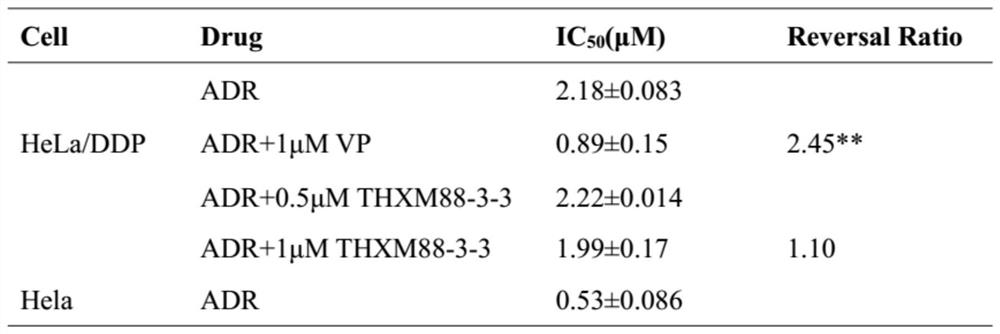
[0017] Example 1: THXM 88-3-3 affects the drug sensitivity of human cervical cancer drug-resistant cell line Hela / ddp to doxorubicin and mitoxantrone.
[0018] Hela cells in the logarithmic growth phase and its drug-resistant cell line Hela / ddp cells were inoculated in a 96-well plate at a seeding density of about 2,500 cells per well, and 36 wells around the periphery of the 96-well plate were not plated with cells. Only add PBS. Add 100 μL of DMEM medium containing 10% fetal bovine serum to each well, and place the 96-well plate at 37°C, 5% CO 2 and 100% humidity for 24 hours.
[0019] Three replicate wells were set up for each sample, and the experiment was repeated three times.
[0020] In this study, P-gP was mainly used as the research object, Verapamil was used as the standard reversal agent, and the chemotherapy drugs were doxorubicin and mitoxantrone.
[0021] Chemotherapy drugs are added. The chemotherapeutic drugs doxorubicin and mitoxantrone were prepared into ...
Embodiment 2
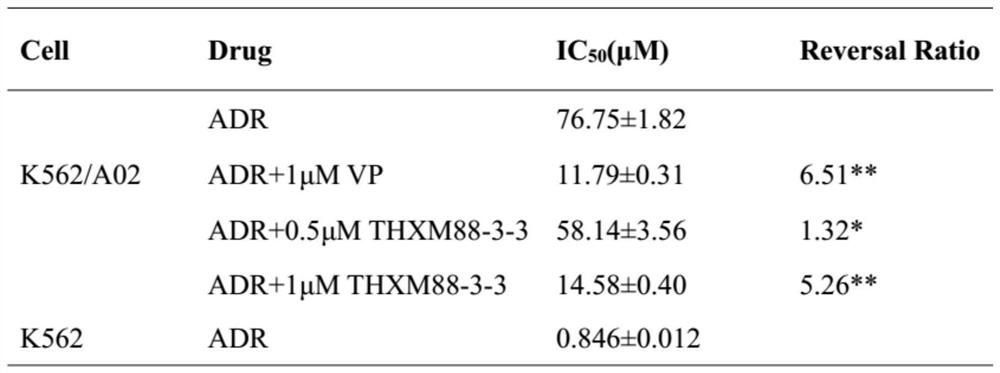
[0037] Example 2: THXM 88-3-3 affects the drug resistance of human chronic myeloid leukemia drug-resistant cell line K562 / A02 to doxorubicin.
[0038] K562 cells in the logarithmic growth phase and its drug-resistant cell line K562 / A02 cells were seeded in 96-well plates at a seeding density of about 1×10 per well. 4 cells, 36 wells around the periphery of the 96-well plate were not layered with cells, and only PBS was added. Add 100 μL of RPMI 1640 medium containing 10% fetal bovine serum to each well.
[0039] Three replicate wells were set up for each sample, and the experiment was repeated three times.
[0040] In this study, P-gP was mainly used as the research object, Verapamil was used as the standard reversal agent, and the chemotherapy drug was doxorubicin.
[0041] Chemotherapy drugs are added. The chemotherapeutic drug doxorubicin was prepared as a stock solution with different concentration gradients.
[0042] Add chemotherapy drugs into the 96-well plate accor...
Embodiment 3
[0054] Implementation Example 3: Effect on P-glycoprotein protein efflux function
[0055] The effect of BWB74-5-5 on the efflux function of MDR1 protein can change the fluorescence intensity of the corresponding fluorescent dye in the cell.
[0056] Hela and hela / dpp cells were cultured at a density of 1X10 4 Inoculate each well into a 96-well plate with a volume of 200 μL per well, set up 96 triplicates, and add sterilized distilled water to a circle around the 96-well plate. The 96-well plate was placed in a 37°C, 5% CO2 constant temperature incubator for 96 hours.
[0057] The reverse agent standard Verapamil was prepared to be 2mM, the dye Rhodamine123 was prepared to be 50ug / ml, and the test sample BWB74-5-5 was prepared to be 2.5mM.
[0058] After the standard substance and the sample BWB74-5-5 to be tested were added, they were placed in a 37°C, 5% CO2 constant temperature incubator and incubated for 15 minutes.
[0059] After 15 min, fluorescent dye was added and i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com