Doxazosin mesylate sustained release tablet and preparation method thereof
A technology of doxazosin mesylate and sustained-release tablets, which is applied in the fields of pharmaceutical formulation, drug delivery, metabolic diseases, etc., can solve the problems of restricting large-scale production, complex production process, and high equipment requirements, so as to reduce side effects and overcome The production process is complicated and the effect of eliminating the peak and valley phenomenon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
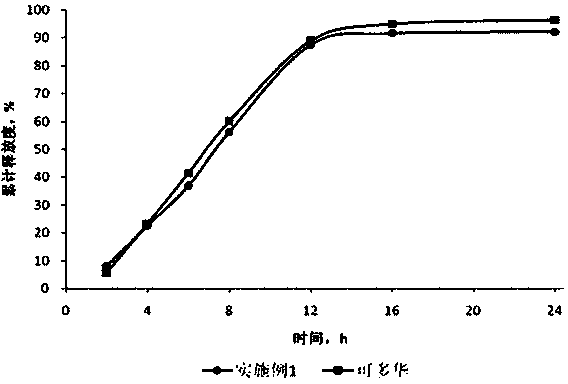
Examples
Embodiment 1
[0032] prescription composition
[0033]
[0034] Preparation:
[0035] The crude drug is crushed through a 100-mesh sieve, and the tablet core excipients are passed through an 80-mesh sieve. Weigh the excipients of the tablet core according to the prescription ratio, mix them uniformly, and then compress the tablet to obtain the doxazosin mesylate tablet, ie the tablet core.
[0036] Add PEG6000 and talcum powder into purified water, homogenize for 10 minutes with a high-shear homogenizer, slowly add the talc powder suspension into the polyacrylic acid resin aqueous dispersion, and at the same time stir with a mixer at a medium speed, and mix The suspension is filtered with a 40-mesh sieve to obtain a sustained-release coating solution. Coat the plain tablet with a coating pan, dry it, and increase the weight of the coating by 3.5% to 4.5%.
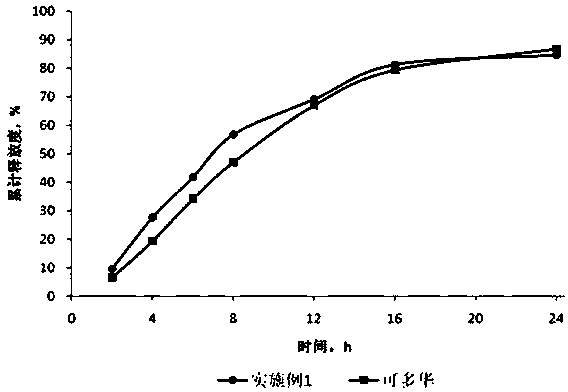
Embodiment 2
[0038] prescription composition
[0039]
[0040] Preparation:
[0041] The preparation method of doxazosin mesylate sustained-release tablets is the same as in Example 1.
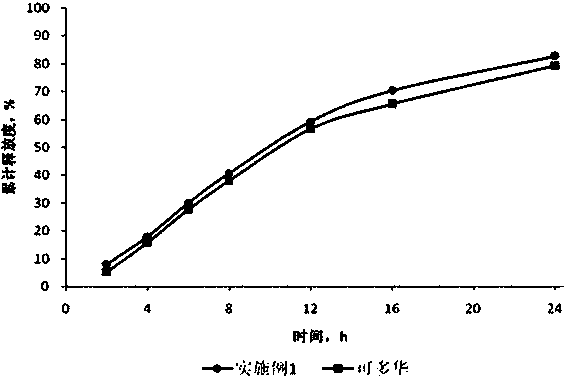
Embodiment 3
[0043] prescription composition
[0044]
[0045] Preparation:
[0046] The tablet core preparation method is the same as in Example 1.
[0047] Take 45% of the water in the formula and heat it to 70-80°C; add Tween 80, triethyl citrate and glyceryl monostearate to the hot water, and use a high-shear homogenizer to homogenize for 10 minutes. Add the remaining water to the emulsion and keep stirring to cool to room temperature; slowly pour the suspension into the polyacrylic resin water dispersion, and stir at a medium speed with an ordinary mixer at the same time; filter the suspension with a 40-mesh sieve, Sustained-release coating solution was prepared. Coat the plain tablets with a coating pan, dry, and increase the weight of the coating by 1.5% to 2.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com