Pharmaceutical preparation containing temozolomide, pharmaceutically acceptable salts or other derivatives thereof
The technology of a pharmaceutical preparation and temozolomide is applied in the field of freeze-dried preparations containing temozolomide, which can solve the problems of increasing the risk of medication and increasing the dissolution of the main drug, and achieves the effects of enhancing medication safety, enhancing solubilization effect and good redissolving effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
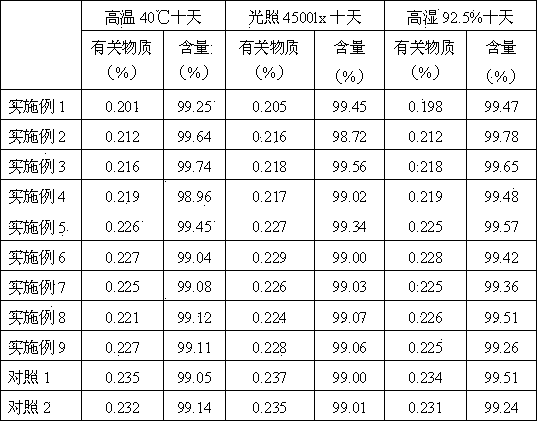
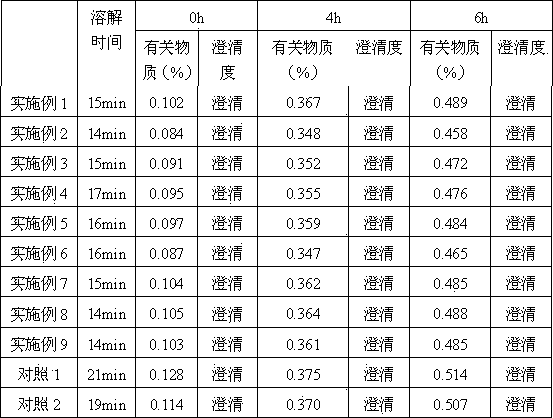
Examples
Embodiment example 1
[0038] prescription:
[0039] Raw materials Dosage Temozolomide 250mg Mannitol 1.5g Arginine hydrochloride 400mg Sodium citrate 587.5mg hydrochloric acid Appropriate amount Water for Injection Add water to make up to 100ml
[0040] Preparation:
[0041] Weigh the above-mentioned mannitol, arginine hydrochloride, and sodium citrate, add 80ml of water for injection, and stir until dissolved. Then add an appropriate amount of hydrochloric acid to adjust the pH value to 2.0-5.0, add temozolomide and stir until dissolved. Add water for injection to make up to 100ml. Add an appropriate amount of activated carbon, stir for 15-20 minutes, and decarbonize. Then it was sterilized and filtered through a 0.22 μm microporous membrane filter. Aliquoted, freeze-dried, filled with nitrogen.
[0042] Freeze-drying process:
[0043] Pre-freezing period: Put the plate loaded with the medicine bottle into the freeze dryer, the pre-free...
Embodiment example 2
[0047] prescription:
[0048] Raw materials Dosage Temozolomide 250mg lactose 2.0g Arginine hydrochloride 400mg Sodium citrate 600mg hydrochloric acid Appropriate amount Water for Injection Add water to make up to 100ml
[0049] Preparation:
[0050] Weigh the above-mentioned mannitol, arginine hydrochloride, and sodium citrate, add 80ml of water for injection, and stir until dissolved. Then add an appropriate amount of hydrochloric acid to adjust the pH value to 2.0-5.0, add temozolomide and stir until dissolved. Add water for injection to make up to 100ml. Add an appropriate amount of activated carbon, stir for 15-20 minutes, and decarbonize. Then it was sterilized and filtered through a 0.22 μm microporous membrane filter. Aliquoted, freeze-dried, filled with nitrogen.
[0051] The freeze-drying process is the same as the implementation case one
Embodiment example 3
[0053] prescription:
[0054] Raw materials Dosage Temozolomide 250mg Dextran 1.5g Lysine hydrochloride 400mg Potassium dihydrogen phosphate 500mg hydrochloric acid Appropriate amount Water for Injection Add water to make up to 100ml
[0055] Preparation:
[0056] Weigh the above-mentioned mannitol, lysine hydrochloride, and potassium dihydrogen phosphate, add 80ml of water for injection, and stir until dissolved. Then add an appropriate amount of hydrochloric acid to adjust the pH value to 2.0-5.0, add temozolomide and stir until dissolved. Add water for injection to make up to 100ml. Add an appropriate amount of activated carbon, stir for 15-20 minutes, and decarbonize. Then it was sterilized and filtered through a 0.22 μm microporous membrane filter. Aliquoted, freeze-dried, filled with nitrogen.
[0057] The freeze-drying process is the same as the implementation case one
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com