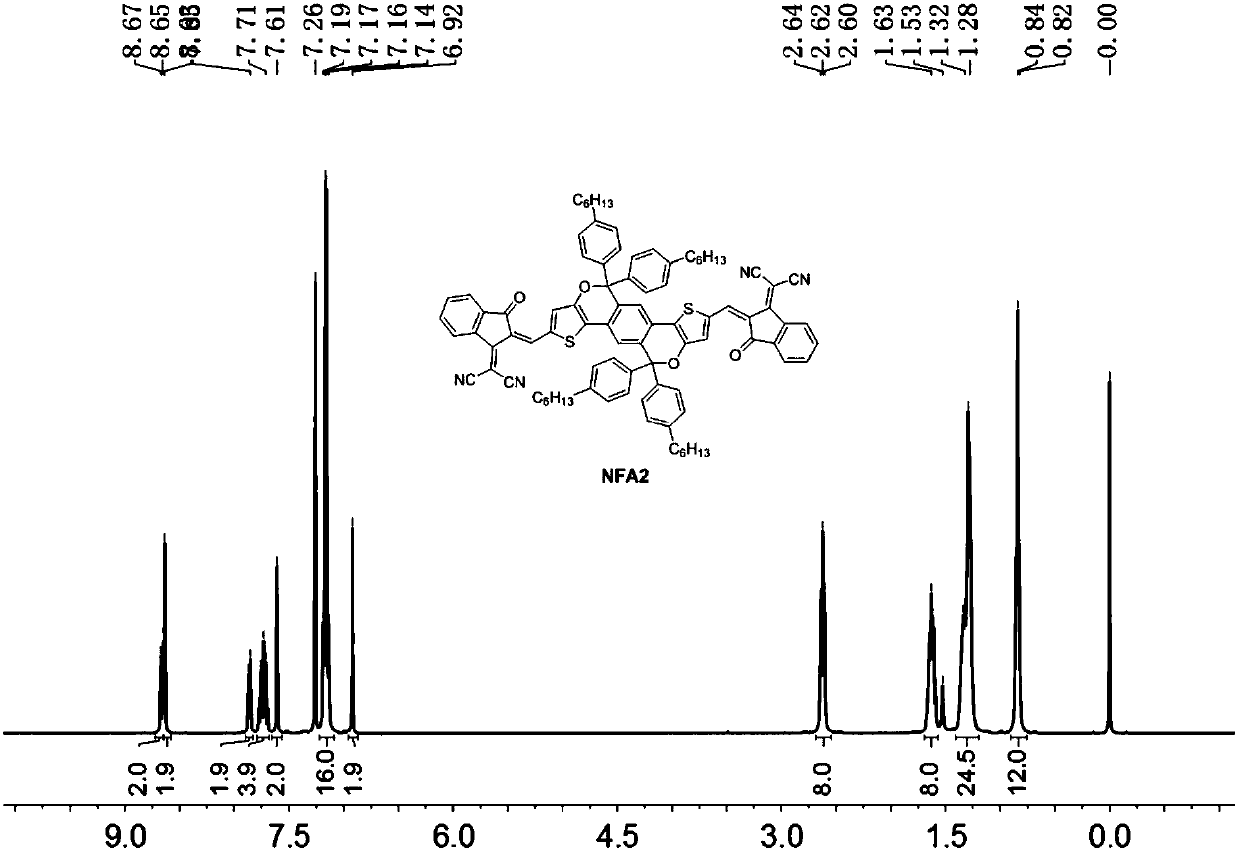
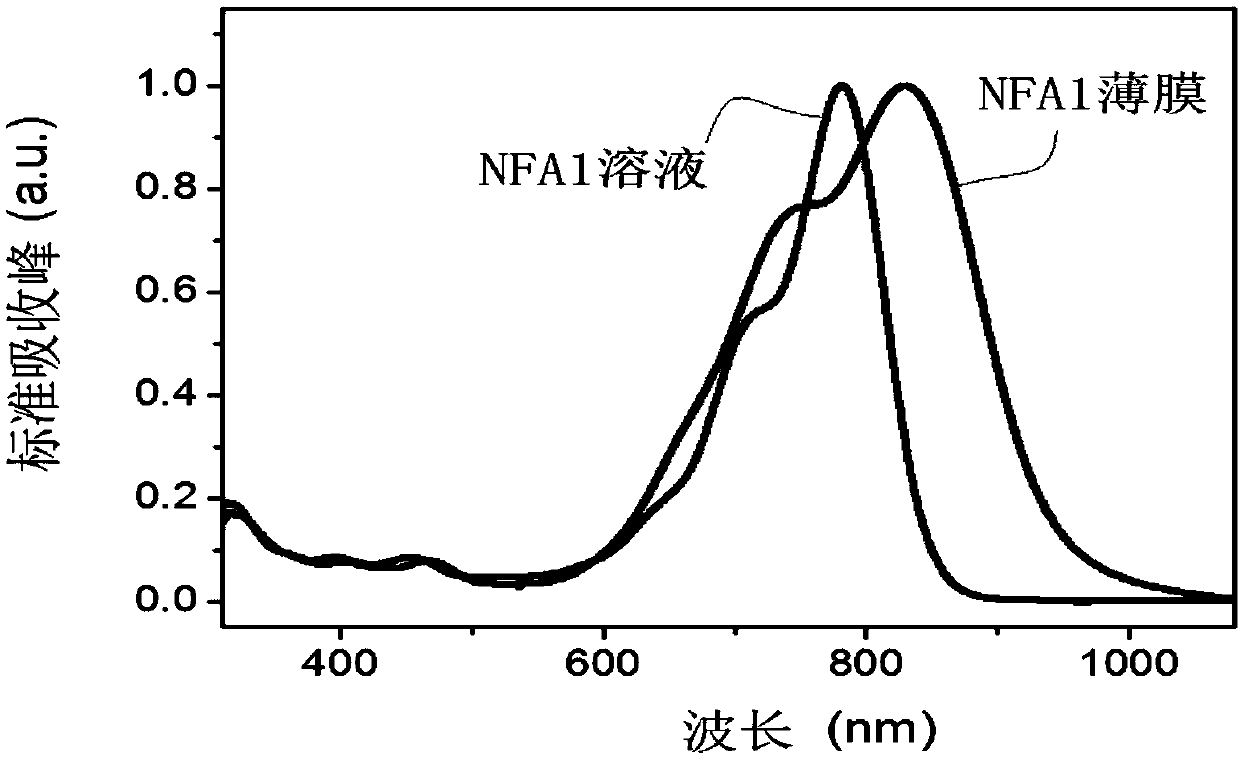
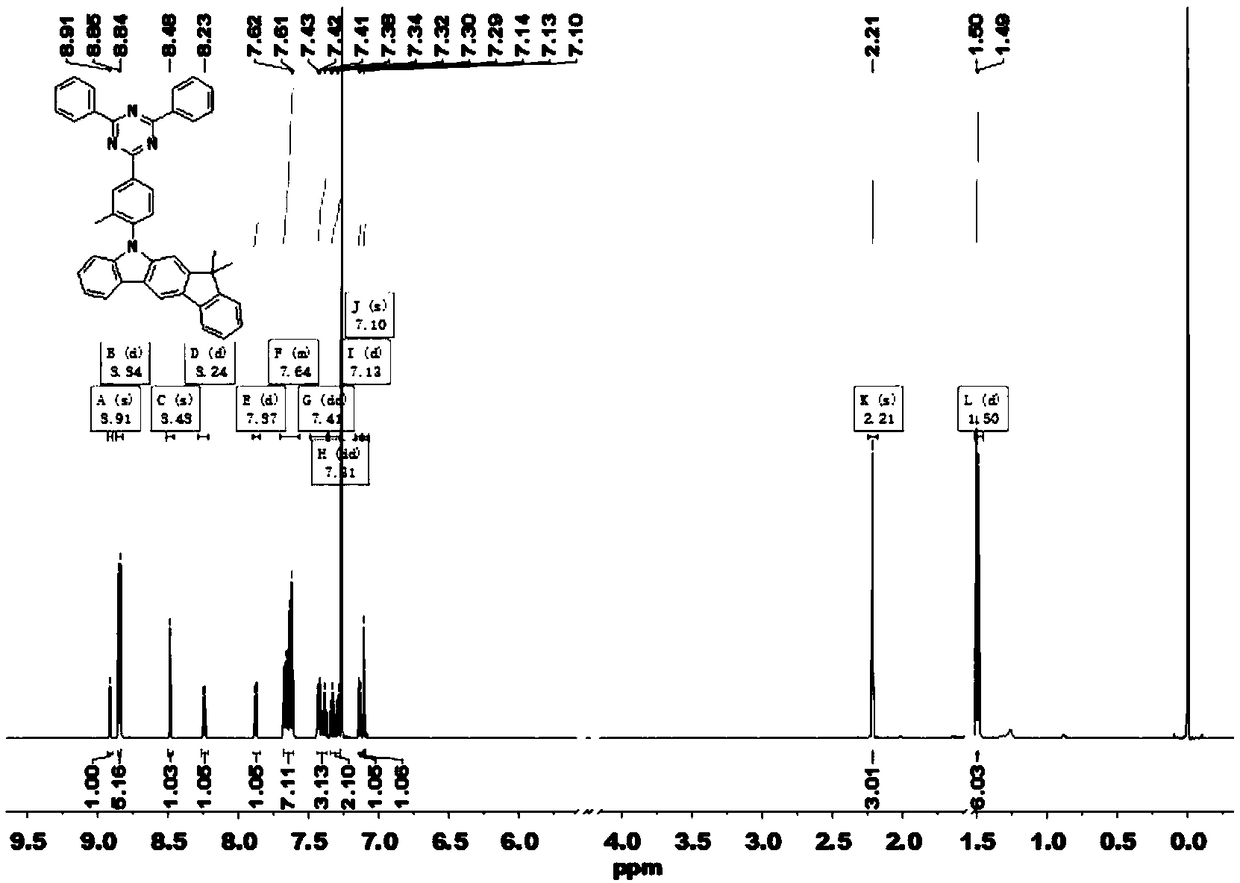
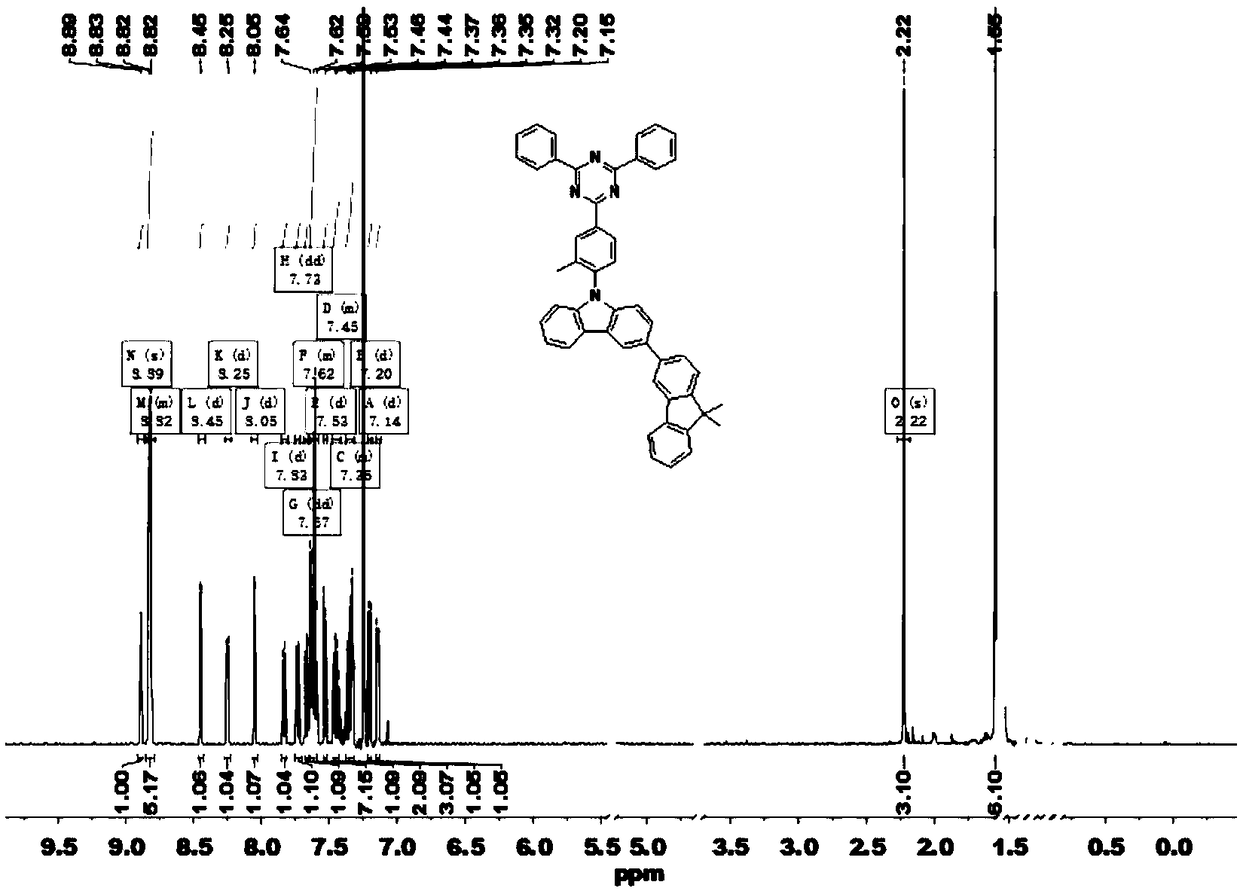
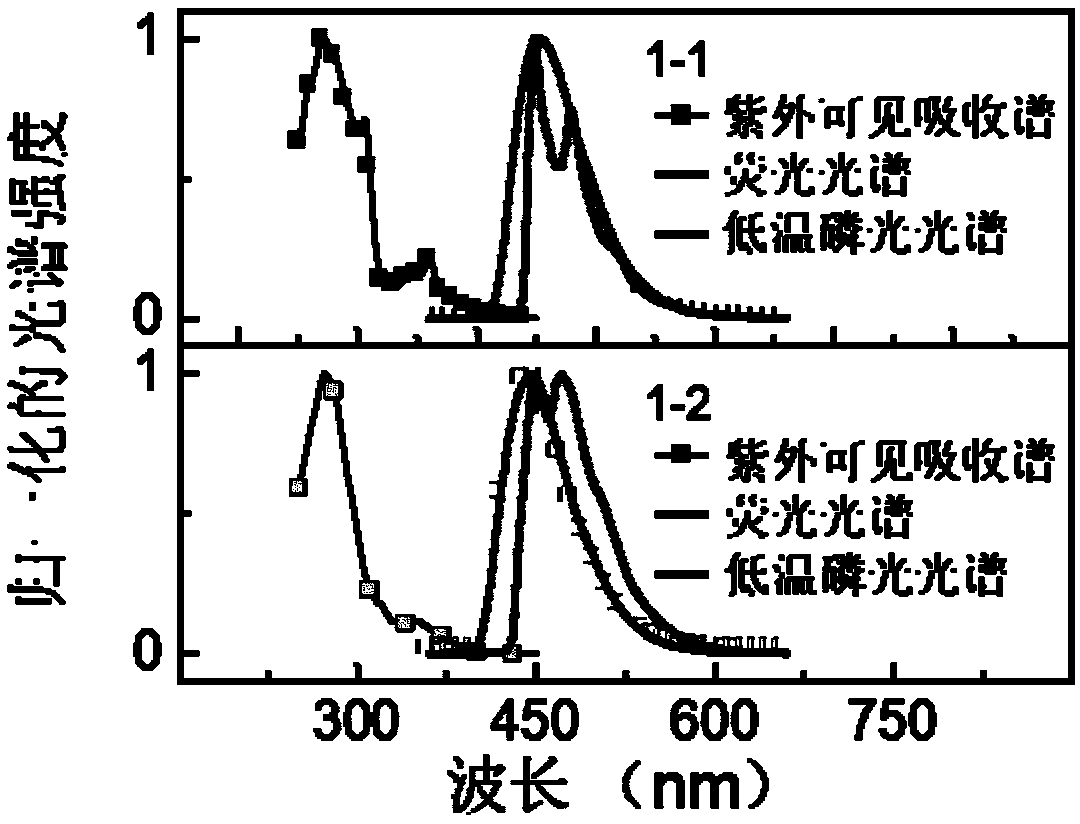
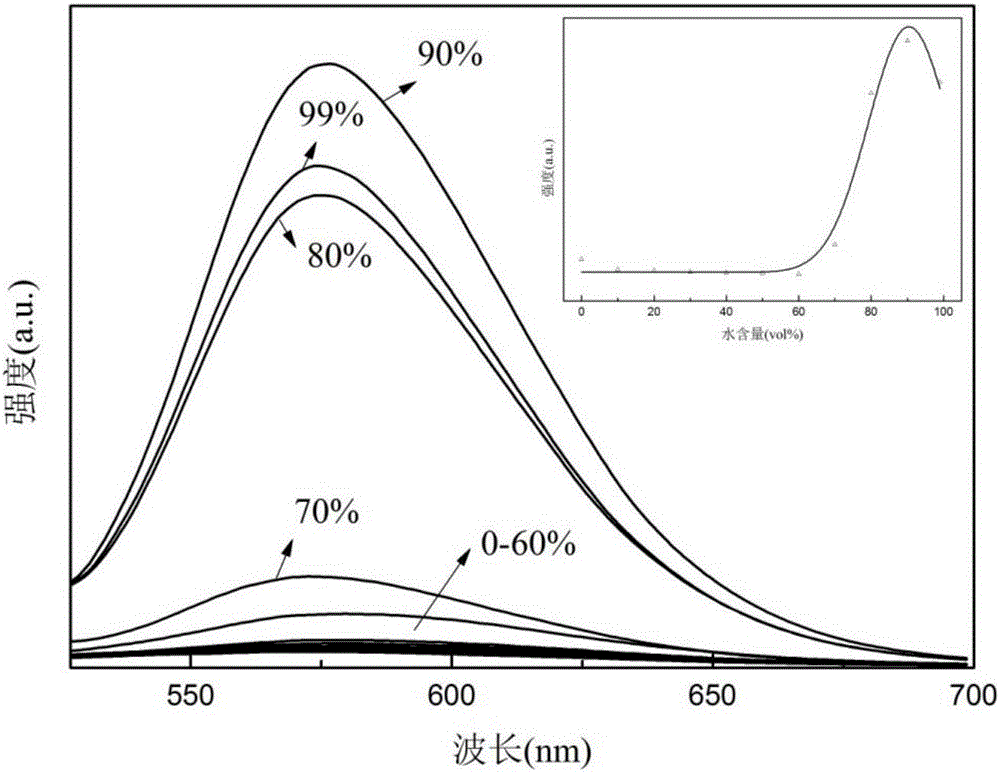
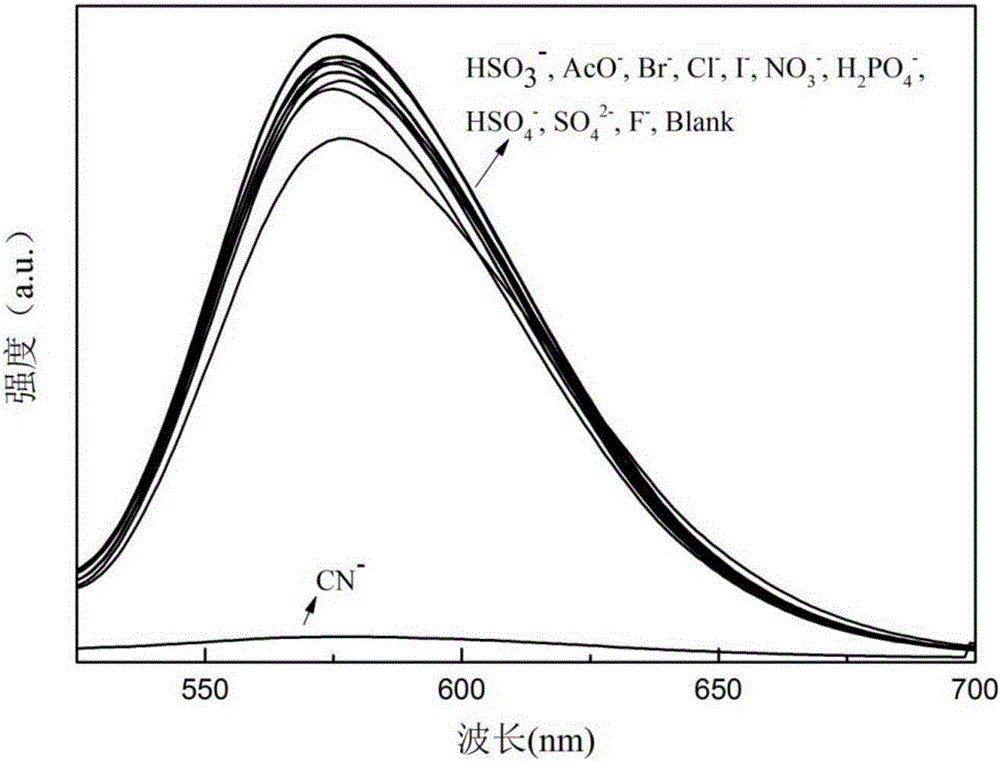
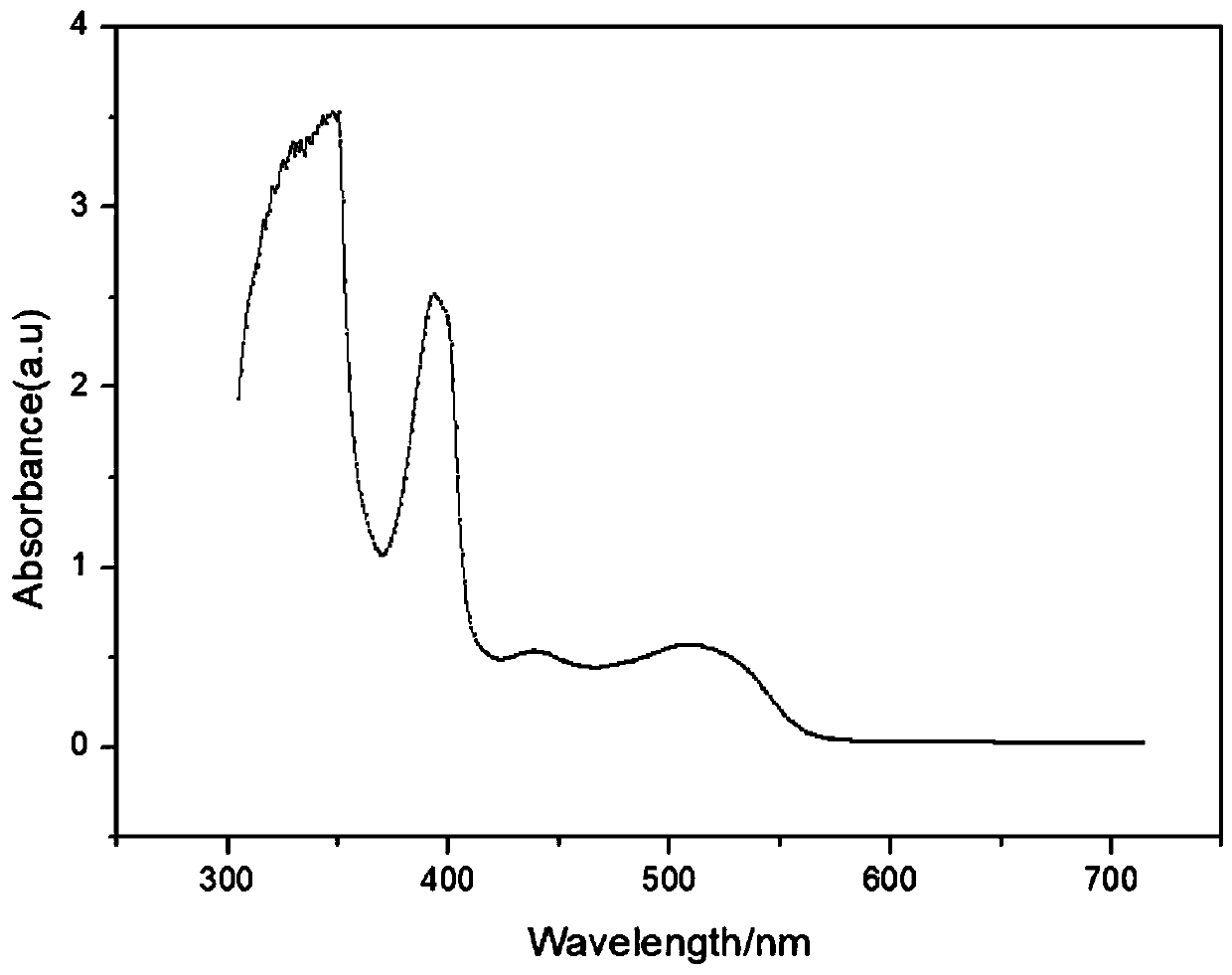
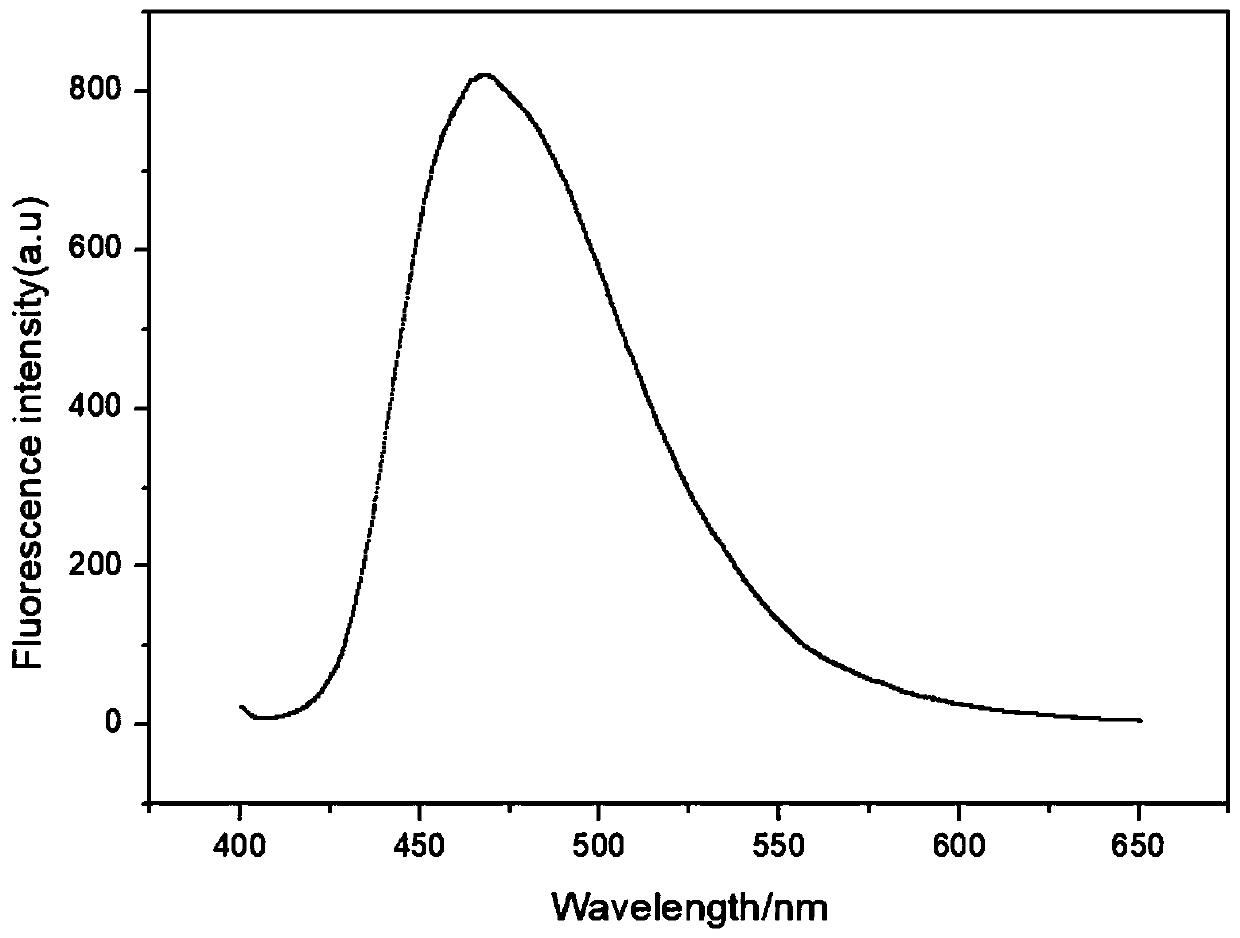
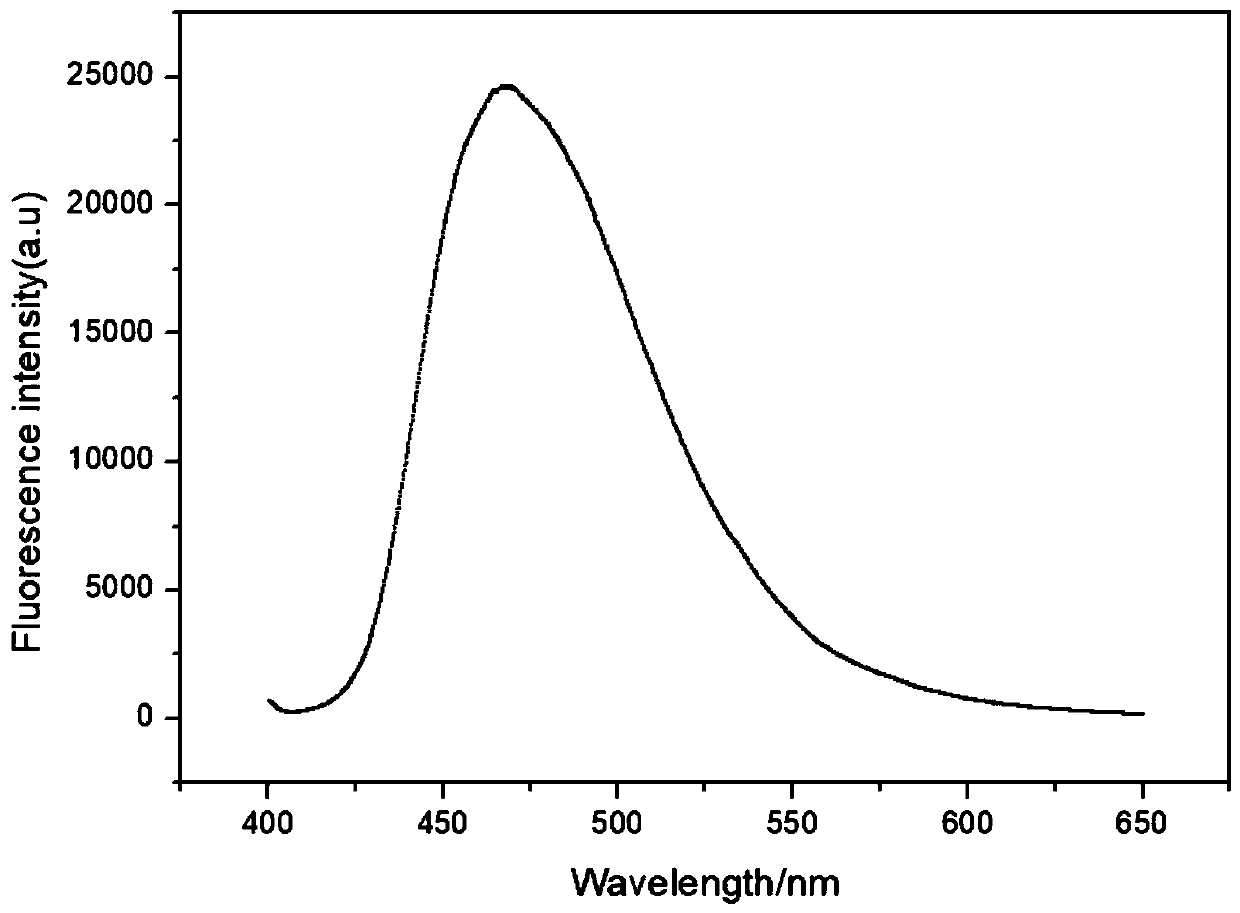
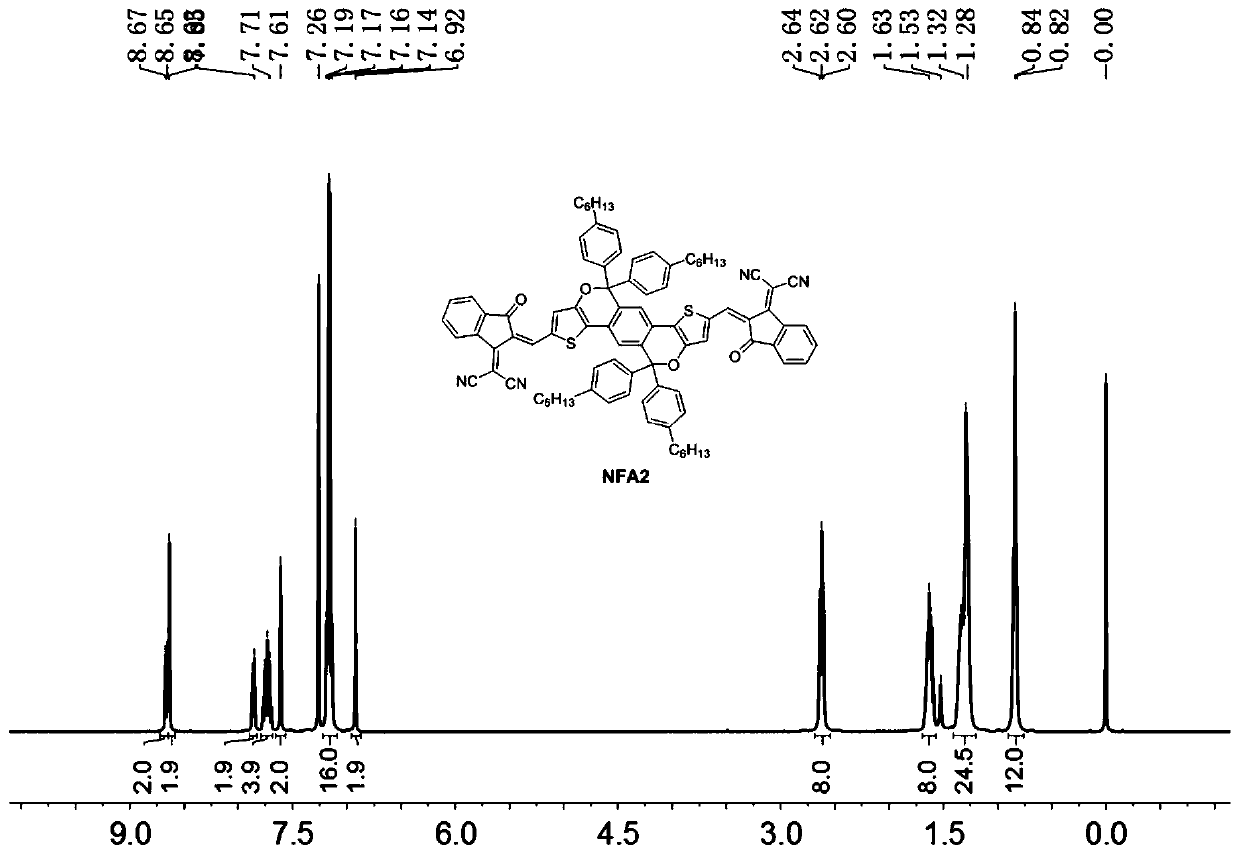
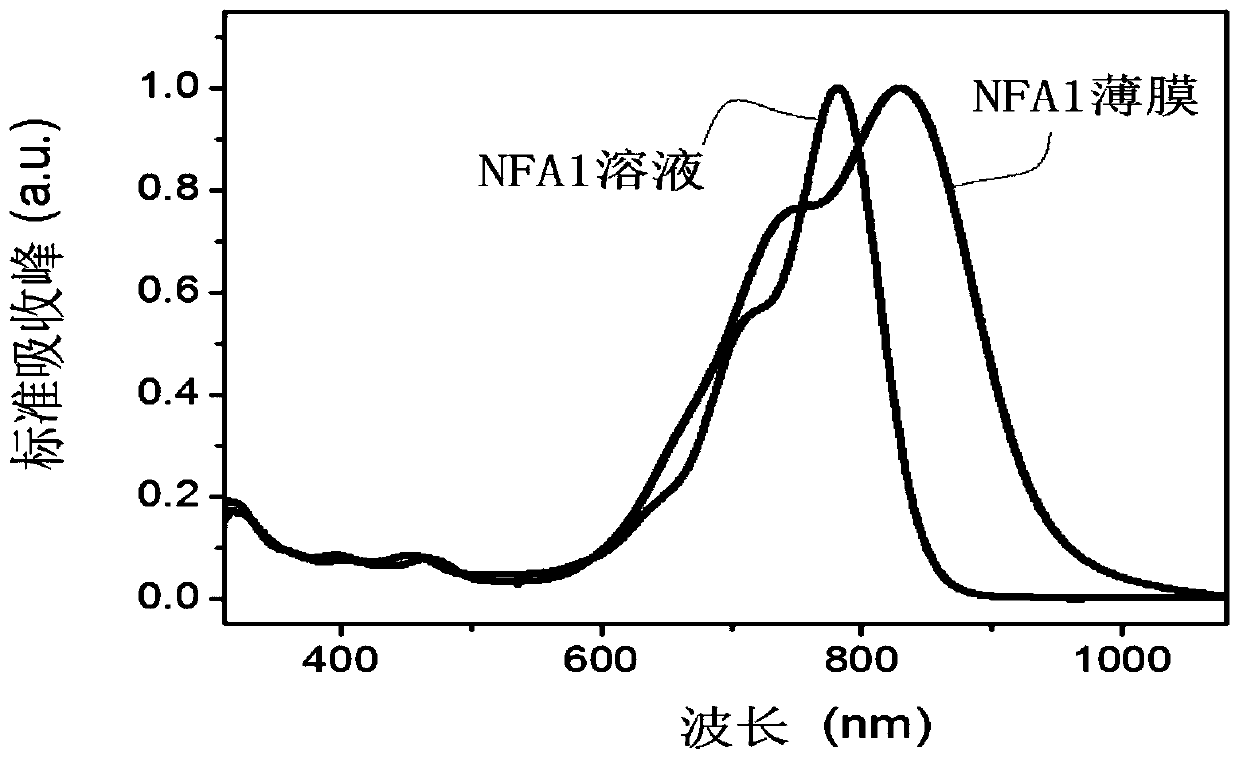
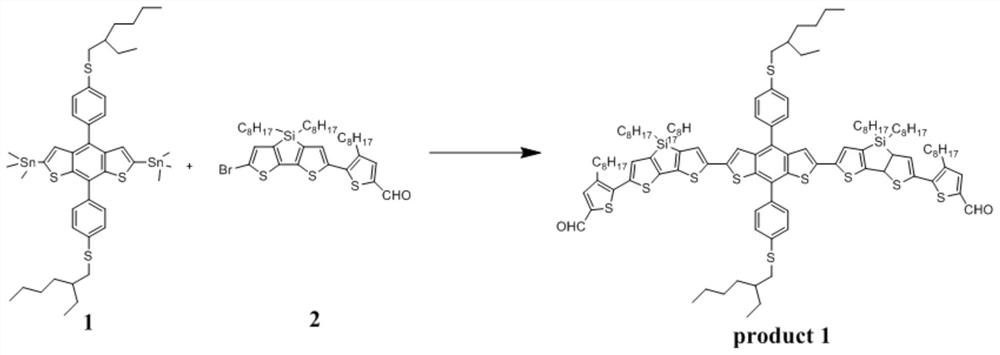
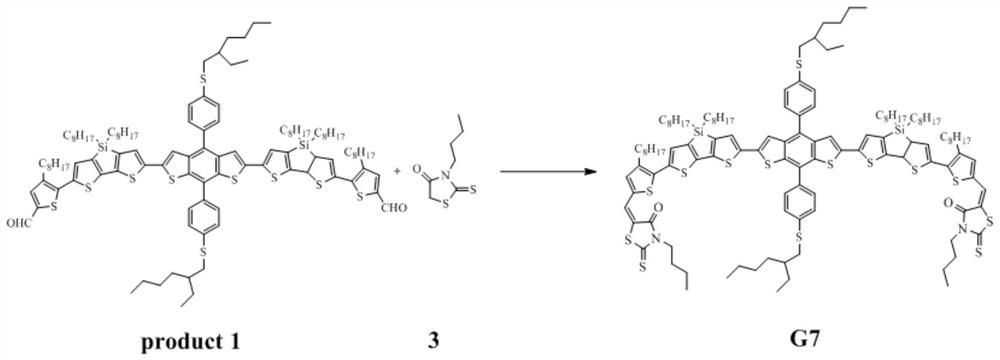
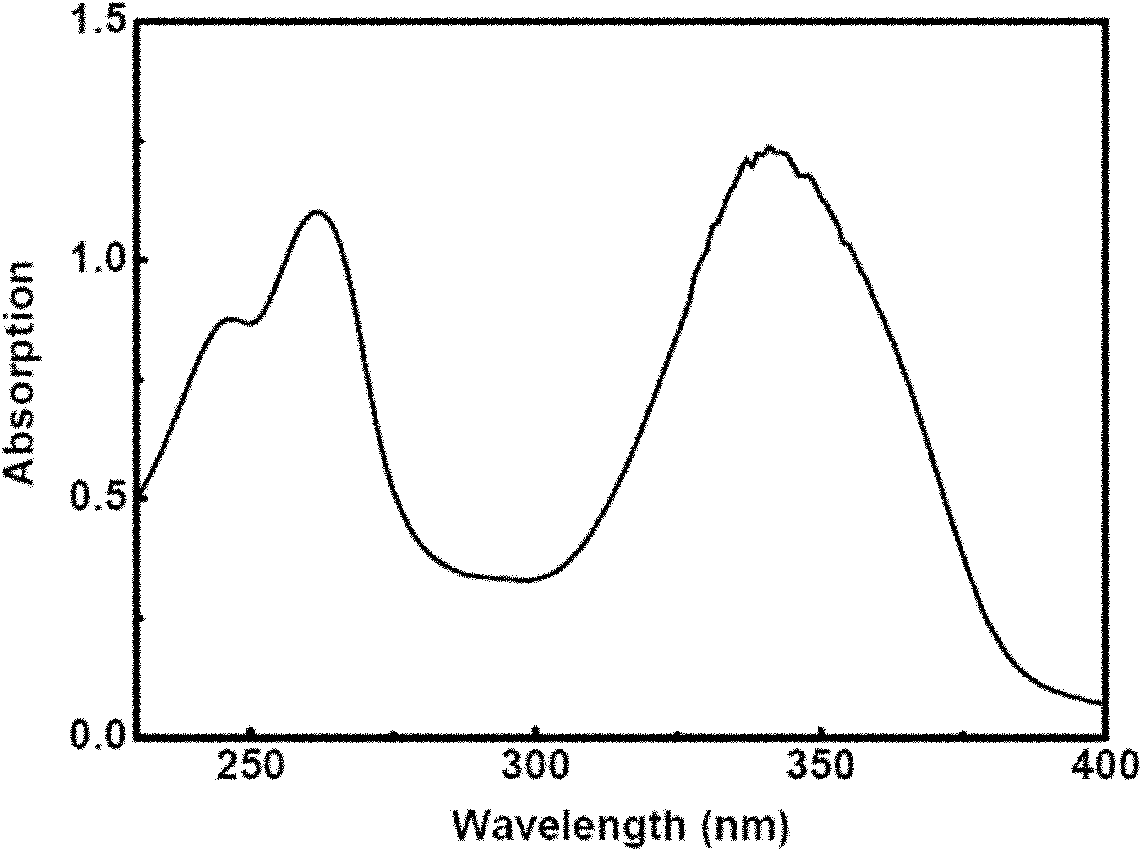
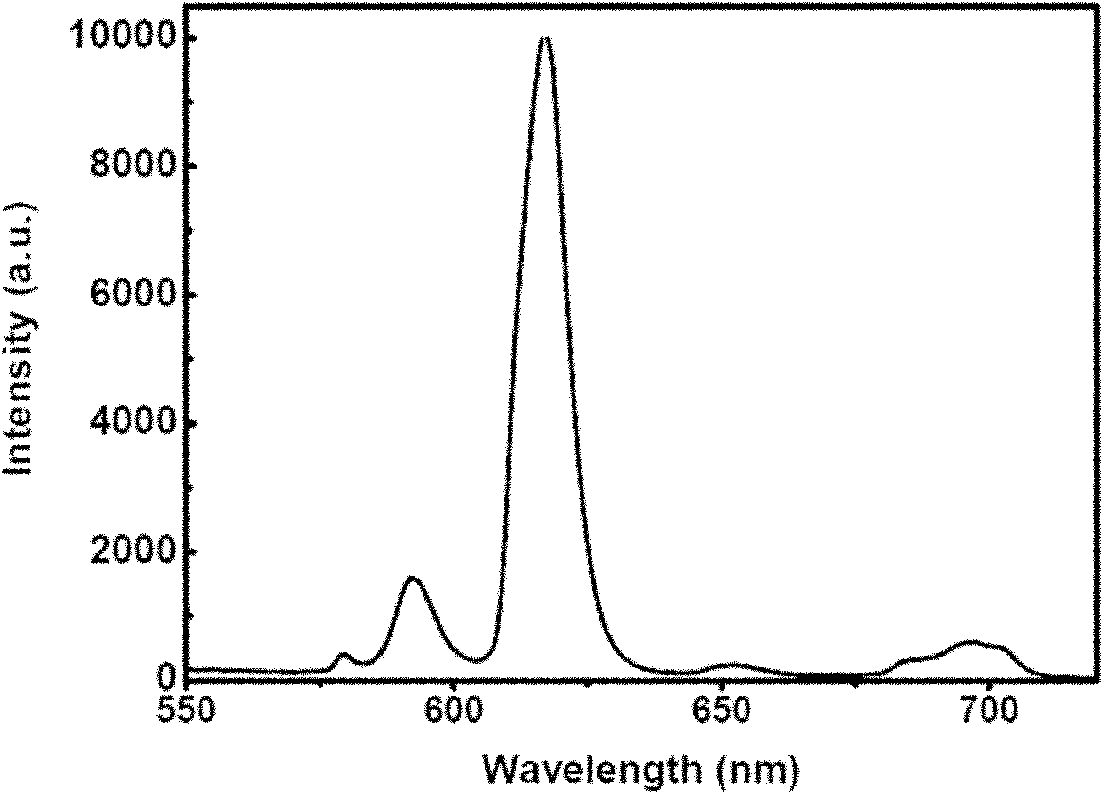
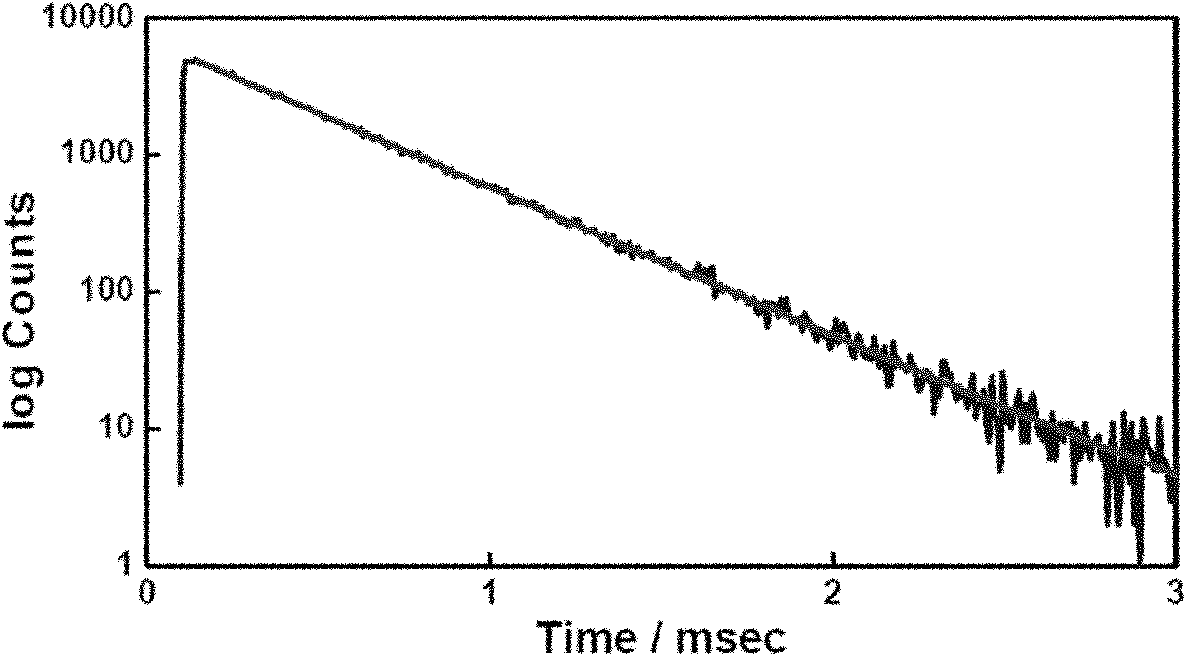
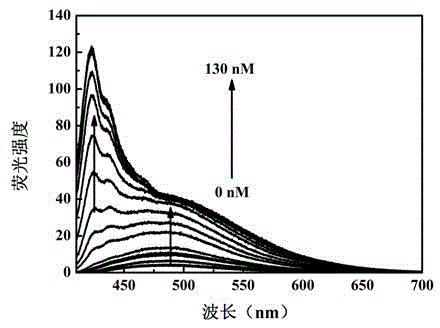
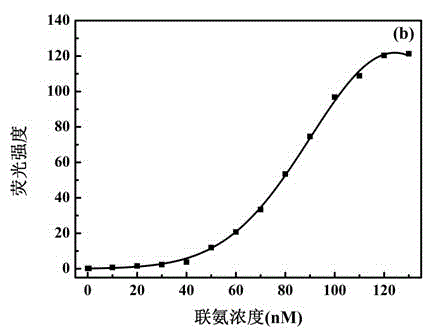
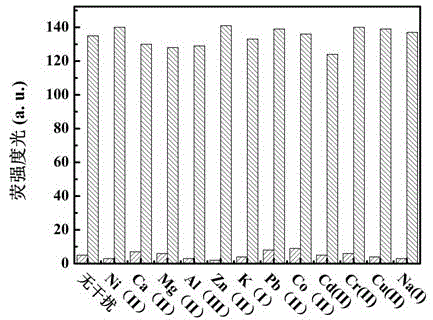
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
44results about How to "Conjugate plane large" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Benzothiazole-cyanophenyl compound serving as hydrazine fluorescence probe as well as preparation method and application method of benzothiazole-cyanophenyl compound
ActiveCN103214428ASimple preparation processConjugate plane largeOrganic chemistryFluorescence/phosphorescenceIndustrial waste waterStructural formula
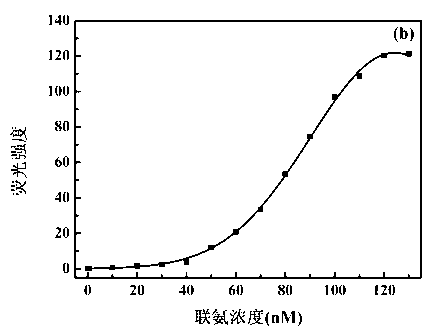
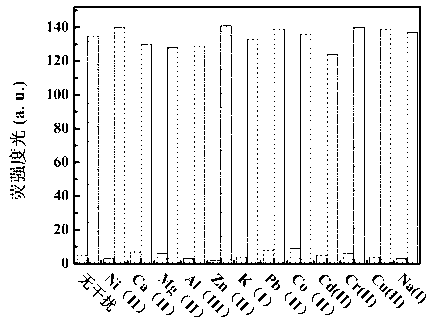
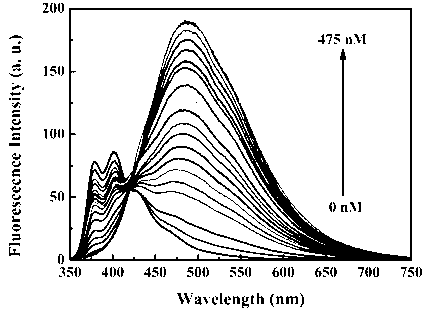
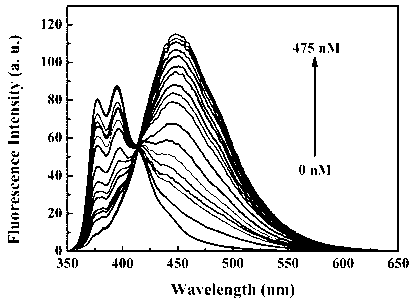
The invention discloses a benzothiazole-cyanophenyl compound serving as a hydrazine fluorescence probe. The benzothiazole-cyanophenyl compound has a structural formula as shown in (I); the compound is prepared by performing cyclodehydration with bromobenzaldehyde and 2-amino-4-chloro thiophenol serving as the raw materials, then performing coupled reaction in order to connect with a bromobenzaldehyde derivate, and finally performing Knoevenagel reaction with malononitrile. The benzothiazole-cyanophenyl compound has the advantages that the raw materials are low in price and easy to gain, the synthetic route is simple, and the yield is relatively high; rigid structures such as benzothiazole and phenylacetylene groups are introduced into such a fluorescence probe, thus high fluorescence quantum efficiency is realized, and relatively high thermal stability and dissolubility are brought. The probe adopts the photoinduced charge transfer mechanism and the conjugate passivation mechanism, therefore, a response range respect to hydrazine can be expanded; the probe has the characteristics of being fast in response, high in sensitivity and high in selectivity, is suitable for being applied to safety detection of foods as well as safety detection of a laboratory, in particular applied to industrial wastewater monitor; and the probe has a wide application prospect in environment monitoring, ecological protection, disease diagnosis, industrial production and pollution discharge inspection.
Owner:浙江富昇科技有限公司
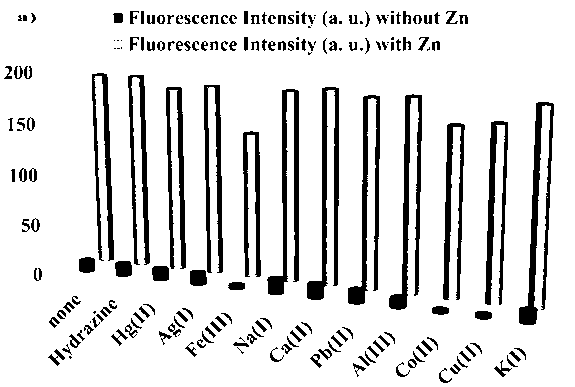
Fluorescent probe: benzothiazole-terpyridine compound used for distinguishing and detecting zinc ions and cadmium ions, preparation method and application method thereof
ActiveCN103265539ASimple preparation processConjugate plane largeOrganic chemistryFluorescence/phosphorescenceSolubilityDisease
The invention discloses a fluorescent probe: a benzothiazole-terpyridine compound used for distinguishing and detecting zinc ions and cadmium ions. The probe has a structural formula as shown in (I). The compound is prepared by using bromobenzaldehyde and O-aminothiophenol as raw materials, and performing a dehydration cyclization reaction, a coupling reaction and a terpyridine derivative reaction. According to the invention, the raw materials are cheap and easily available, a synthesis route is simple, a yield is relatively high, and the fluorescent probe is high in fluorescence quantum efficiency, and relatively high in heat stability and dissolvability. The probe uses a photoinduced charge transfer mechanism, has different luminescence response for the zinc ions and the cadmium ions, has characteristics of rapid response, high sensitivity and high selectivity, is suitable for food safety inspection, laboratory safety inspection, and especially industrial wastewater monitoring, and has a wide application prospect in environment monitoring, ecological protection, disease diagnosis, industrial production and sewage inspection.
Owner:浙江富昇科技有限公司
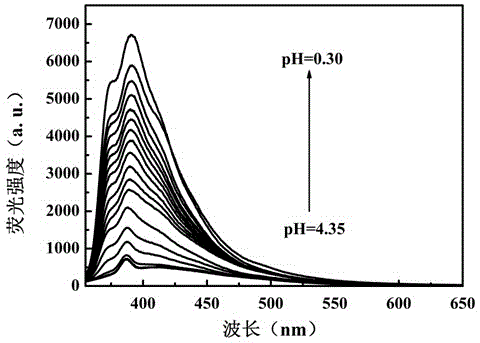
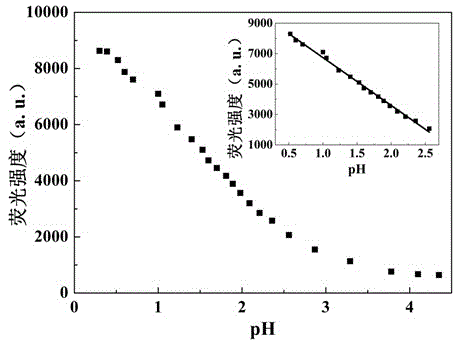
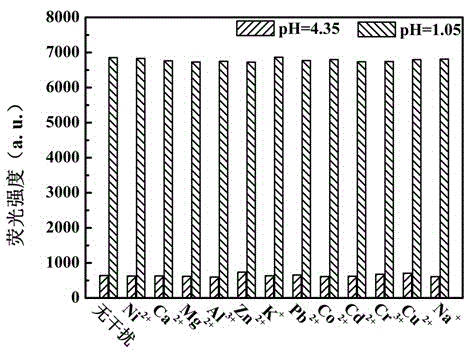
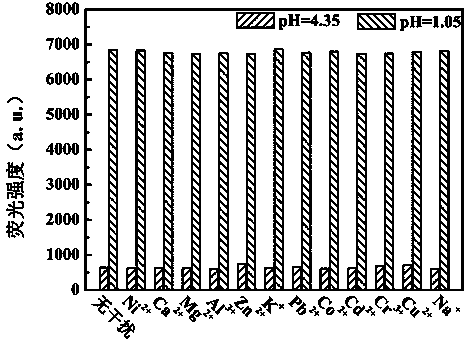
Benzothiazole-aniline compound used as pH fluorescent probe and preparation method thereof
ActiveCN102942537ASimple preparation processConjugate plane largeOrganic chemistryFluorescence/phosphorescenceAlkaneSolubility
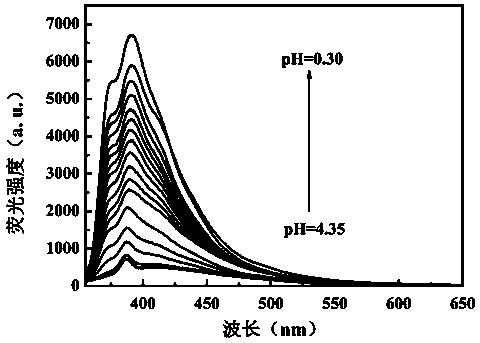
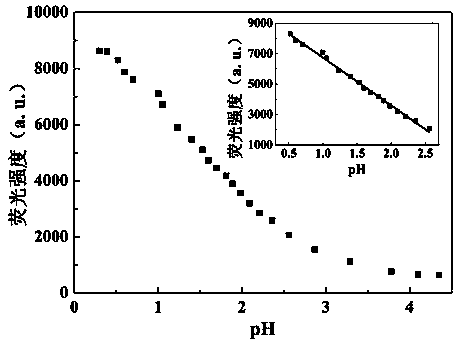
The invention discloses a benzothiazole-aniline compound used as a pH fluorescent probe and a preparation method thereof. The structural formula is shown as a chemical formula (shown in the description) (I), wherein in the formula, R1 is vinyl, acetenyl, styryl, phenylethynyl, biphenyl or perylene base; and R2 and R3 are both hydrogen, methyl or ethyl alkane. The preparation method comprises the following steps of: taking p-bromobenzaldehyde and 2-aminothiophenol as raw materials, and connecting with iodine-containing nitrobenzene derivative through dehydration cyclization reaction and coupling reaction; and generating the pH fluorescent probe benzothiazole-aniline derivative through reductive amination. Rigid structures, such as benzothiazole and phenylethynyl, are introduced into the fluorescent probe; the fluorescent probe is high in fluorescence quantum efficiency, and high in thermal stability and solubility. The probe can detect pH value under strong acid condition by adopting a photoinduced charge transfer mechanism and a conjugate passivation mechanism; and the probe has the characteristics of rapid response, high sensitivity and high selectivity, and has wide application prospect in environment monitoring, ecological protection, disease diagnosis, industrial production and sewage inspection.
Owner:浙江富昇科技有限公司
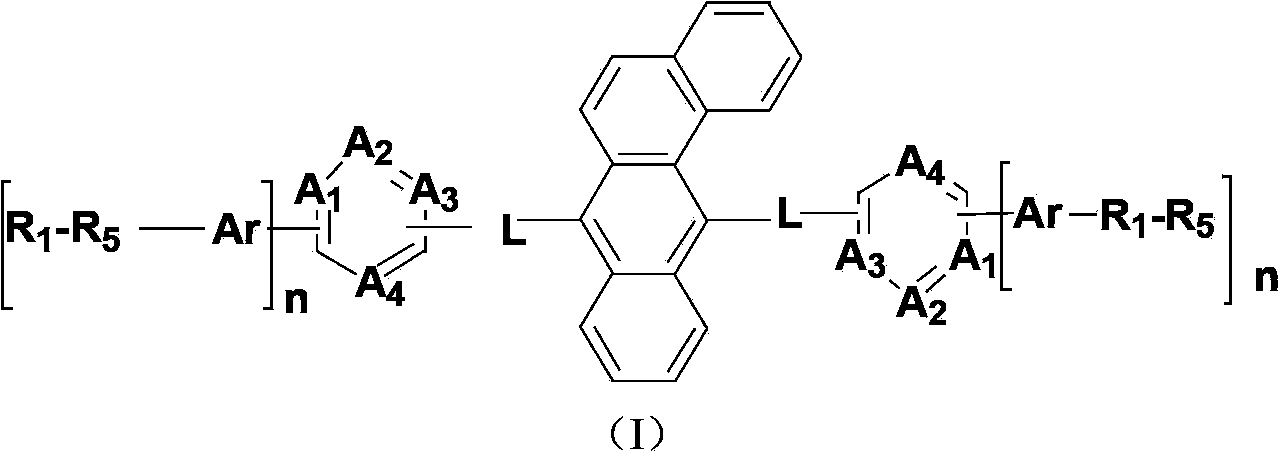
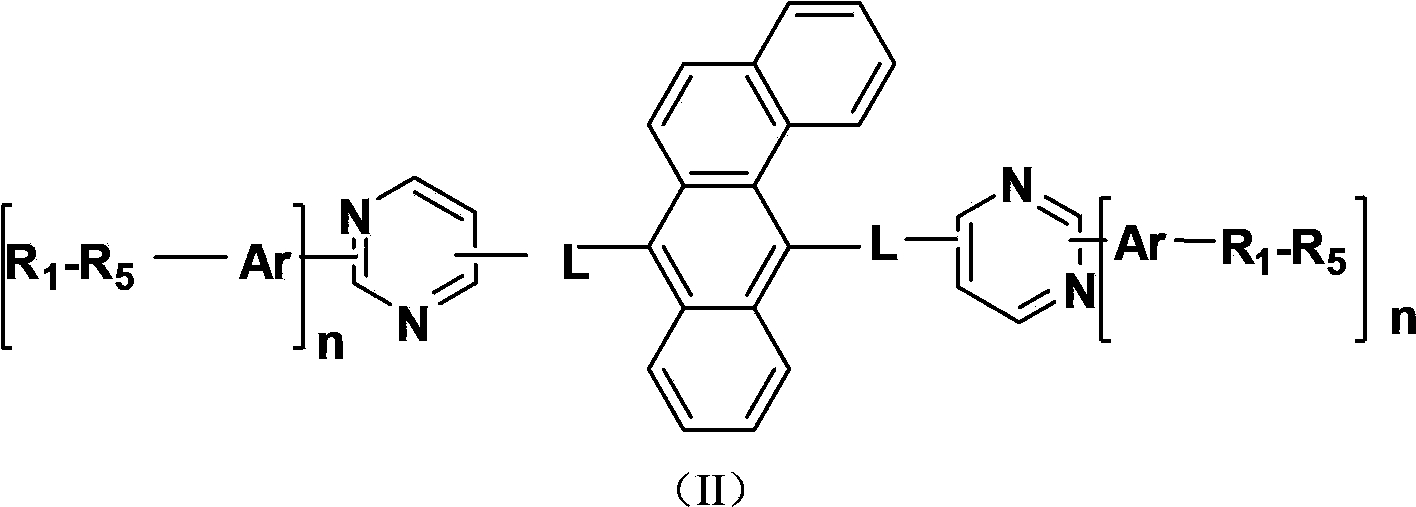
Benzanthracene derivatives containing pyrimidinyl, pyrazinyl or triazinyl groups and applications thereof
ActiveCN103570629AImprove film formationConjugate plane largeOrganic chemistrySolid-state devicesCrystallographyPyrazine
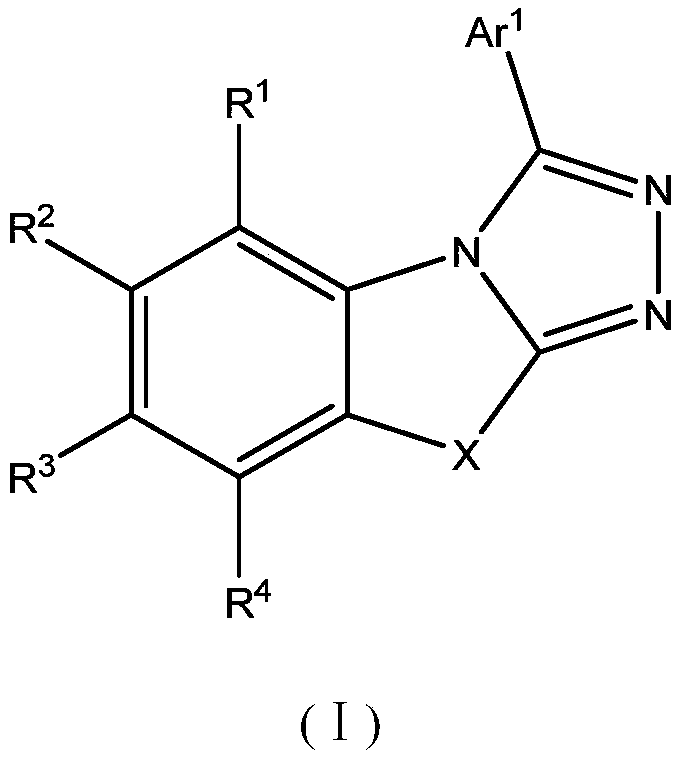
The invention provides a novel compound which is represented by the formula (I), wherein the R1 to R5 are independently selected from a C1-C20 aliphatic alkyl group or a C6-C20 aromatic group; the Ar is selected from: a C4-C30 aromatic ring, a C4-C30 N-containing heterocycle, a C4-C30 condensed heterocyclic aromatic hydrocarbon, a C4-C30 aromatic amino group, or a C4-C30 aromatic oxy group; n is 1 or 2; A1 to A4 represent N atoms or C atoms, when the A1 and A3 represent N atoms at the same time, the A2 and A4 represent C atoms; or when the A1 and A4 represent N atoms at the same time, the A2 and A3 represent C atoms; or when the A2 and A4 represent N atoms at the same time, the A1 and A3 represent C atoms; or when the A3 and A4 represent N atoms at the same time, the A1 and A2 represent C atoms; or when the A1, A3, and A 4 represent N atoms at the same time, the A2 represents a C atom; and L represents a single bond, or a C6-C10 aromatic ring, or a C4-C10 N-containing heterocycle. The compounds are used as an electron transmission layer material or a luminous main material in organic electroluminescence devices.
Owner:KUNSHAN VISIONOX DISPLAY TECH +2
Fused ring non-fullerene acceptor material, and preparation method and application thereof
ActiveCN107652304AIncreased electron cloud densityImprove electron donating abilityOrganic chemistrySolid-state devicesOrganic solventOxygen bridge
The invention provides a fused ring non-fullerene acceptor material, and a preparation method and an application thereof. The fused ring non-fullerene acceptor material comprises an electron donatingunit and electron withdrawing end groups, the electron donating unit is a carbon-oxygen bridge trapezoidal fused ring structure, the electron withdrawing end groups are connected to two ends of the electron donating unit, and the fused ring non-fullerene acceptor material has a structure represented by formula I or formula II. The prepared fused ring non-fullerene acceptor material prepared through the preparation method has excellent light absorption and carrier transport properties, can be easily dissolved in common organic solvents, has strong visible near-infrared absorption property and high electron mobility (being more than or equal to 10<-5> cm<2>.V<-1>.s<-1>), realizes a high short-circuit current and a high energy conversion efficiency in organic solar cells, and has a broad application prospect and high application values.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Organic light-emitting material and applications thereof
PendingCN110698458AHigh bond energyImprove thermal stabilityOrganic chemistrySolid-state devicesHeat stabilityMaterials science
The invention belongs to the technical field of organic photoelectric materials, and particularly relates to an organic light-emitting material and applications thereof. According to the invention, the organic light-emitting material has an indolocarbazole parent structure, has high interatomic bond energy and good thermal stability, and is beneficial to intermolecular solid-state accumulation; the preparation process of the derivative has characteristics of simpleness, easy performing and easily available raw materials, and is suitable for mass production and amplification; with the application of the arylamine-substituted indolo heterocyclic derivative in the light-emitting layer of an organic electroluminescent device, the proper energy level with the adjacent level is achieved, the holes and the electrons are easily injected, the turn-on voltage can be effectively reduced, the high exciton migration rate is achieved, and the device can achieve good light-emitting efficiency; and the compound has large conjugate plane, is beneficial to molecular accumulation, shows good thermodynamic stability, and shows long service life as the light-emitting layer material in an organic electroluminescent device.
Owner:YANTAI XIANHUA CHEM TECH CO LTD
Spirobifluorene fluorescence probe as well as preparation method and application thereof
InactiveCN102633726AConjugate plane largeHigh fluorescence quantum yieldOrganic chemistryFluorescence/phosphorescenceQuantum yieldGrignard reaction
The invention discloses a spirobifluorene fluorescence probe which is 2,7-di(benzimidazole) spirobifluorene. According to the invention, the structural formula of a product is shown in a formula (I). The preparation method of the spirobifluorene fluorescence probe comprises the following steps: carrying out coupling reaction, bromination, Grignard reaction, dehydration cyclization reaction and oxidization reaction on parabromotoluene which is used as a raw material so as to obtain a spirobifluorene dialdehyde compound; and reacting the spirobifluorene dialdehyde compound with o-phenylenediamine to obtain the compound (I). According to the invention, the raw material is cheap and available, the synthesis route is simple, the yield is high, and the preparation method is suitable for large-scale production. Because the three-dimensional rigid structure of spirobifluorene is introduced, the compound (I) has high fluorescence quantum yield and fluorescence intensity; the compound (I) has high sensitivity and good selectivity when being used for silver ion detection, detection limit of naked eye can reach 10<-5>M, and the detection limit of an instrument can reach 10<-6>M; the response time of the compound (I) on the silver ion is less than 2 seconds; and simultaneously, the compound (I) is a pH fluorescence probe having wide response range and has a good application prospect.
Owner:SHANGHAI NORMAL UNIVERSITY
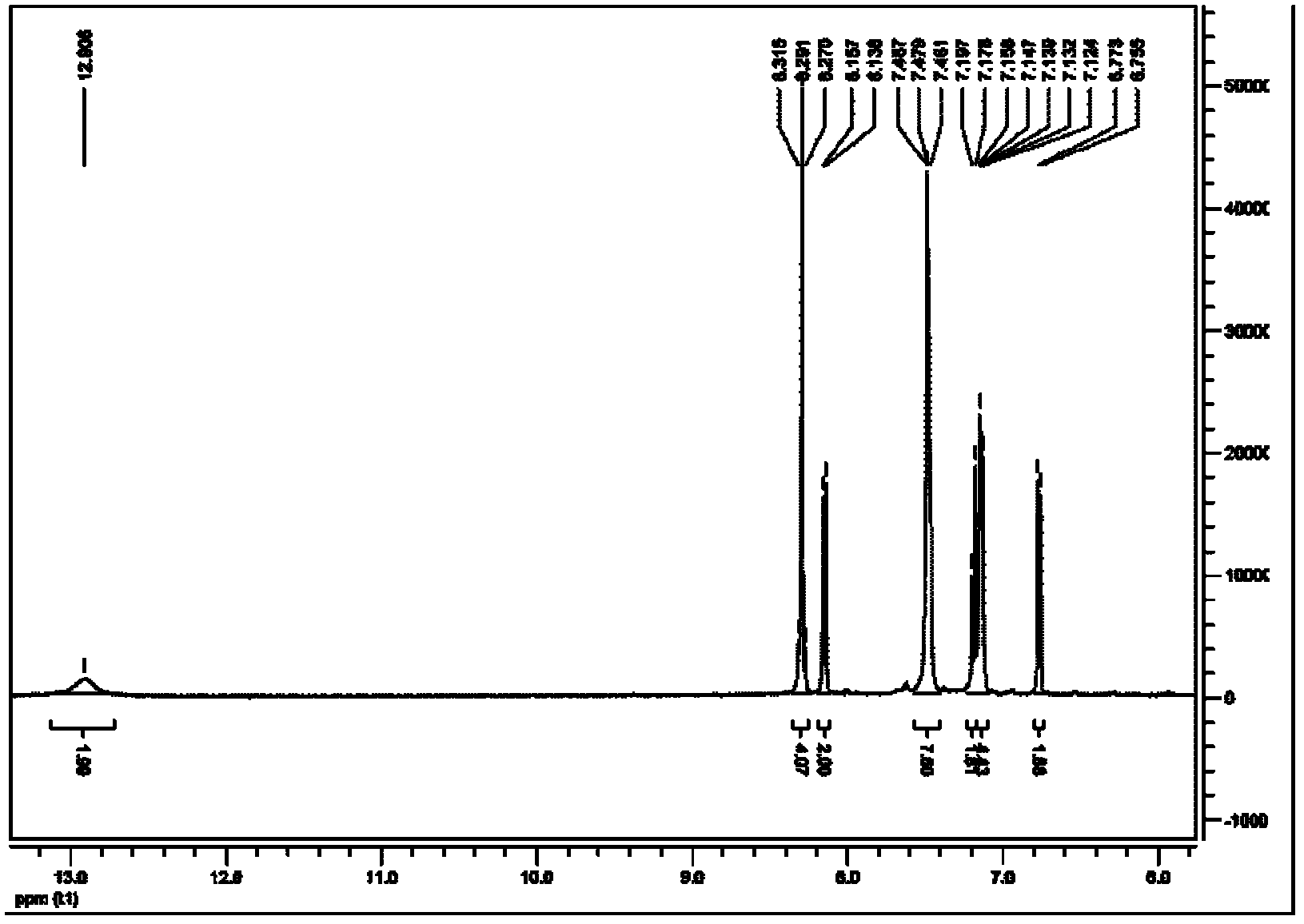
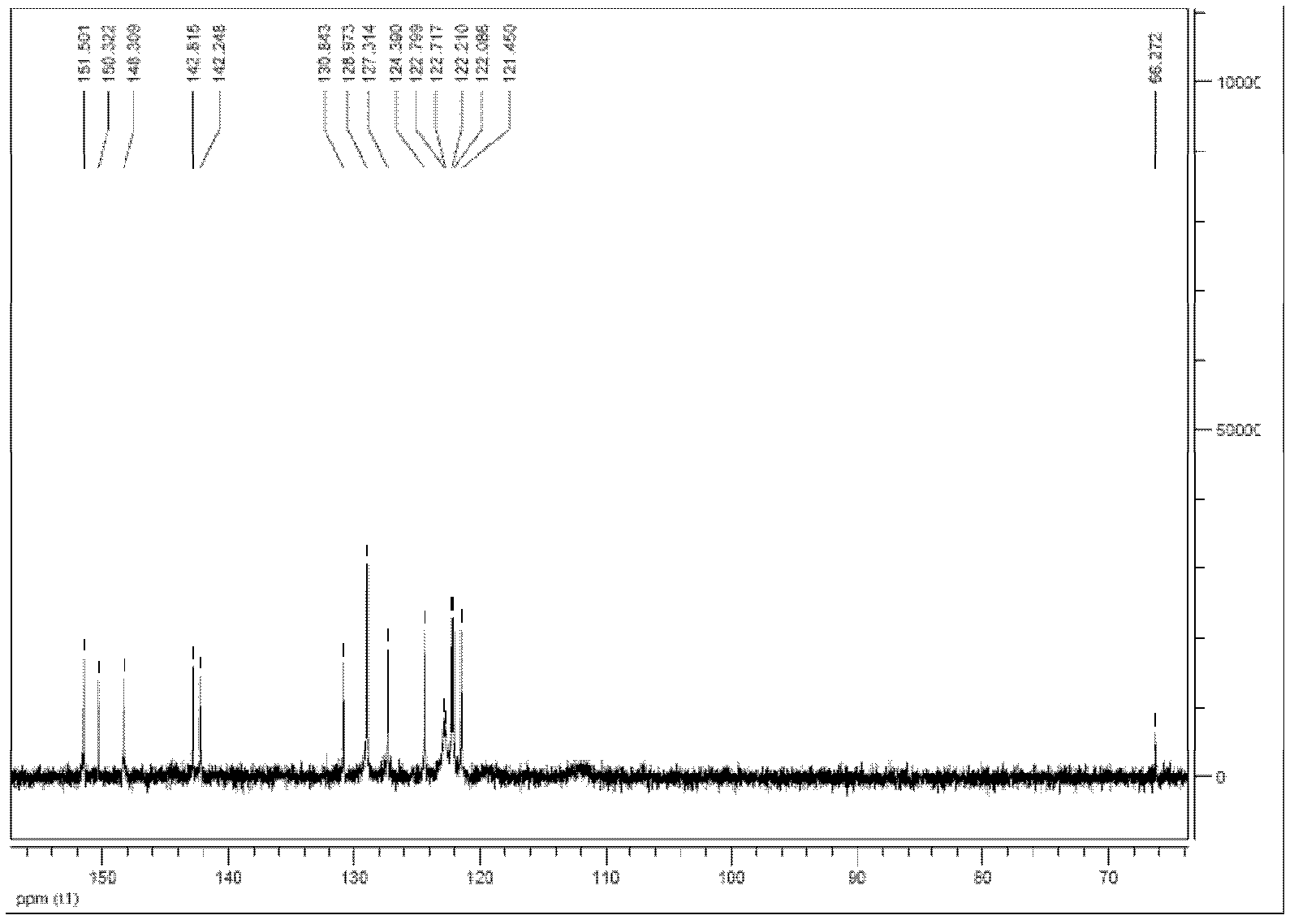
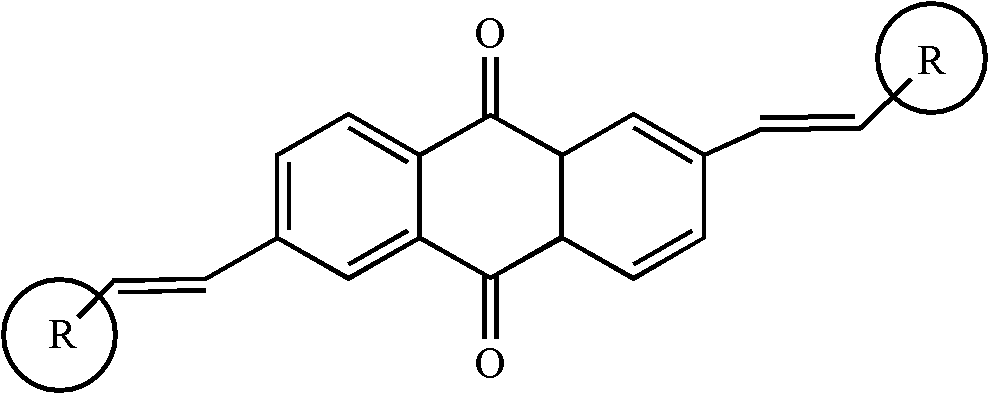
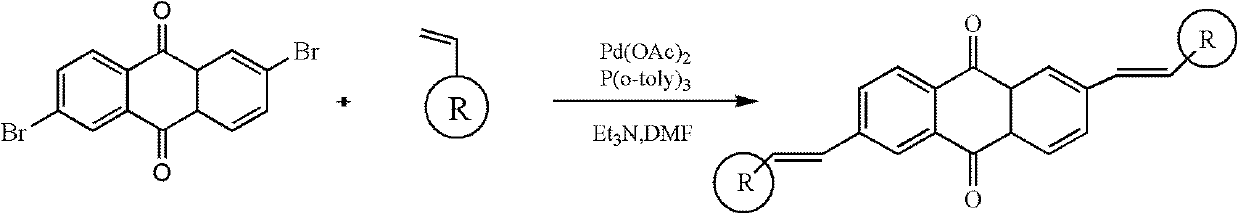
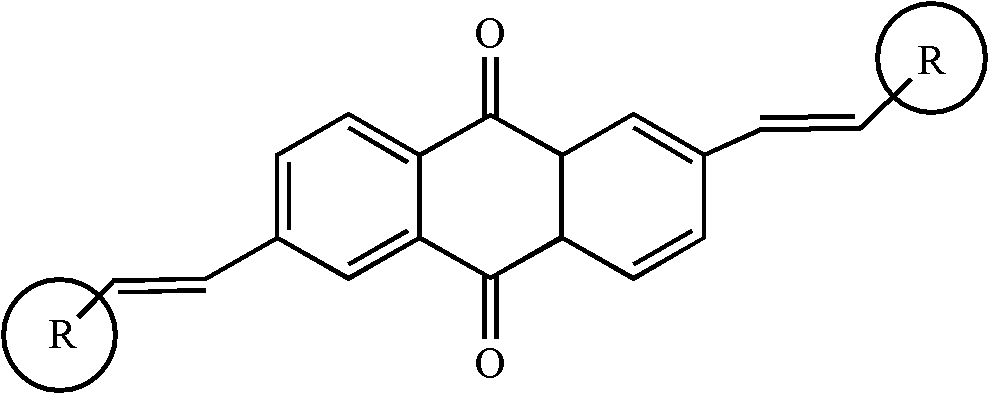
2, 6-di(aryl)-anthraquinone and preparation method thereof
InactiveCN102329210AImprove solubilityGood optical and thermal stabilityOrganic compound preparationCarboxylic acid esters preparationNonlinear absorptionSolubility
The invention relates to 2, 6-di(aryl)-anthraquinone and a preparation method thereof, the preparation method comprises the following steps: connecting electron donor or electron acceptor groups to the two sides of an anthraquinone ring in the 2 and 6 positions, and enabling a 2, 6-dibromo anthraquinone type derivative and a substituent group containing a vinyl group to generate an Heck reaction in alkaline conditions under the catalytic action of palladium acetate and tri-O-methoxy phosphorus for generating the 2, 6-di(aryl)-anthraquinone, and the general formula of the molecular structure of the 2, 6-di(aryl)-anthraquinone is shown in the specification, wherein R is the substituent group containing the vinyl group, the electron donor group or the electron acceptor group. As the compound has the larger conjugate plane and simultaneously has better photochemical stability, thermal stability and solubility, the compound can better capture multiple photons and excite the generation of multi-photon absorption and nonlinear absorption phenomena, and further meet the application requirements in the aspects of photoelectric conversion materials, nonlinear absorption materials and the like.
Owner:SHANGHAI INST OF OPTICS & FINE MECHANICS CHINESE ACAD OF SCI
Organic light-emitting material and organic light-emitting device
ActiveCN111269219AExtend your lifeHigh bond energyOrganic chemistrySolid-state devicesElectron transporting layerOrganic electroluminescence
The invention provides a novel organic light-emitting material. The novel organic light-emitting material is used for a light-emitting main body material and an electron transport layer material of anorganic light-emitting device. The invention also provides an organic light-emitting device comprising the novel organic light-emitting material.
Owner:YANTAI XIANHUA CHEM TECH CO LTD
D-A type conjugated polymer containing condensed ring lactone, and preparation method and application thereof
ActiveCN110408010AConjugate plane largePromotes π-π stackingSolid-state devicesSemiconductor/solid-state device manufacturingSolubilityOrganic solar cell
The invention provides a D-A type conjugated polymer containing a condensed ring lactone, and a preparation method and an application thereof. The structural formula of the D-A type conjugated polymercontaining the condensed ring lactone is represented by formula I. The condensed ring lactone group is introduced to the conjugated main chain of a polymer, so the molecular conjugate plane of the polymer is widened, the intermolecular pi-pi stacking interaction is promoted, and the intermolecular steric hindrance of the polymer is reduced. The D-A type conjugated polymer containing the condensedring lactone has the advantages of good solubility, good photoelectric properties, excellent light absorption and carrier transport performances, and realization of a high short circuit current and ahigh energy conversion efficiency in organic solar batteries.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Thermal activation delayed fluorescent material and application of thermal activation delayed fluorescent material to organic light emitting diodes
InactiveCN109400590AAppropriate energy levelImprove carrier transport performanceOrganic chemistrySolid-state devicesFlexible organic light-emitting diodeChemical structure
The invention provides a thermal activation delayed fluorescent material and application of the thermal activation delayed fluorescent material to organic light emitting diodes. The chemical structureof the thermal activation delayed fluorescent material is shown as a formula (1). A group R in the formula (1) is a chemical group, and triphenylamine derivatives and carbazole derivatives are linkedwith one another to form the chemical group; the R is selectively a type of the certain carbazole derivatives, and the numbers of annular carbon atoms of the certain carbazole derivatives are 18. Thethermal activation delayed fluorescent material and the application have the advantages that donor groups of organic light emitting materials are modified, accordingly, the current carrier transportperformance of the donor groups can be greatly improved, and the organic light emitting materials are good in device performance when applied to blue light, deep blue light or white light; the thermalactivation delayed fluorescent material not only can be used as blue light dye, but also can be used as a host material for yellow light or green light dye; the problem of efficiency roll-off under the condition of high brightness can be solved when the thermal activation delayed fluorescent material is applied to the organic light emitting diodes with single light emitting layers, the structuresof white organic light emitting diode devices can be simplified, and the industrial production cost can be reduced.
Owner:SUZHOU UNIV
Benzothiazole-triphenylamine dye with AIE effect and preparation method and application thereof
ActiveCN106220583AGood choiceReduce stepsOrganic chemistryLuminescent compositionsAlcoholBenzaldehyde
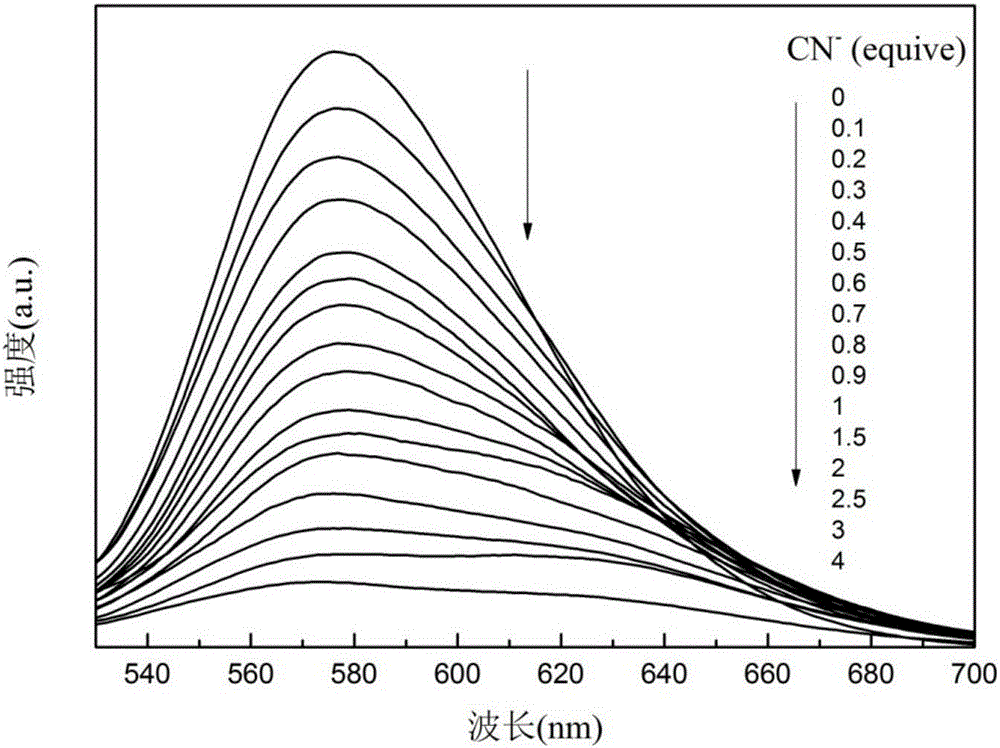
The invention discloses benzothiazole-triphenylamine dye with an AIE effect and a preparation method and application thereof. The preparation method of the benzothiazole-triphenylamine dye comprises the steps that benzothiazole-2-acetonitrile and diphenyl amino-4-benzaldehyde are dissolved into absolute ethyl alcohol, ammonium acetate is added, and reacting under stirring is conducted at room temperature; a coarse product is obtained through filtering, and the pure benzothiazole-triphenylamine dye is obtained through recrystallization. According to the benzothiazole-triphenylamine dye, a cyanide ion detection method which is high in selectivity and sensitivity, is not disturbed by other negative ions, can achieve the detection limit as low as the ppm level and is low in detection cost and convenient and fast to operate can be achieved through an aqueous solution, and the problems that in the cyanide ion detection field, the cost is high, operation is complex, and detection is insensitive can be solved.
Owner:SOUTH CHINA UNIV OF TECH
Naphtho-five-membered ring-benzofused heterocyclic organic compounds and application thereof
PendingCN111253374AConjugate plane largeHigh thermodynamic stabilityOrganic chemistrySolid-state devicesSimple Organic CompoundsElectron hole
The invention belongs to the technical field of organic photoelectric materials, and particularly relates to naphtha-five-membered ring-benzofused heterocycle organic compounds and application thereof. The organic compounds disclosed by the invention have a parent structure of naphtho-five-membered ring-benzofused heterocycle, are high in interatomic bond energy, present good thermal stability, are beneficial for intermolecular solid accumulation, and can be used as luminescent layer materials to effectively prolong the service life of materials. The compounds provided by the invention are derivatives of large conjugated fused heterocycles, are applied to light emitting layers, have proper energy levels matched with adjacent layers, facilitate injection of holes and electrons, can effectively reduce turn-on voltage, and can realize good light emitting efficiency in a device due to a relatively high exciton migration rate. The compounds disclosed by the invention have relatively large conjugate planes, are beneficial for molecular accumulation, show good thermodynamic stability and present long service life in devices. A preparation process of the derivatives is simple and feasible,raw materials are easy to obtain, and the derivatives are suitable for large-scale production.
Owner:YANTAI XIANHUA CHEM TECH CO LTD
Organic light-emitting material and organic light-emitting device
ActiveCN111303187AExtend your lifeHigh bond energyOrganic chemistrySolid-state devicesCarbazoleElectron transporting layer
The invention provides a novel organic light-emitting material which has a structure as shown in a general formula (I) and can be used for an organic light-emitting device and an electron transport layer material. The invention also provides the organic light-emitting device which comprises the novel organic light-emitting material provided by the invention. The organic light-emitting material disclosed by the invention has a parent structure of indolocarbazole, high interatomic bond energy and good thermal stability, is beneficial for intermolecular solid accumulation, and has effectively prolonged service life when used as a light-emitting layer material. Meanwhile, a preparation process of the material is simple and feasible, raw materials are easily available, and the process is suitable for industrial production.
Owner:YANTAI XIANHUA CHEM TECH CO LTD
Benzothiazole-aniline compound used as pH fluorescent probe and preparation method thereof
ActiveCN102942537BConjugate plane largeHigh fluorescence quantum efficiencyOrganic chemistryFluorescence/phosphorescenceAlkaneSolubility
The invention discloses a benzothiazole-aniline compound used as a pH fluorescent probe and a preparation method thereof. The structural formula is shown as a chemical formula (shown in the description) (I), wherein in the formula, R1 is vinyl, acetenyl, styryl, phenylethynyl, biphenyl or perylene base; and R2 and R3 are both hydrogen, methyl or ethyl alkane. The preparation method comprises the following steps of: taking p-bromobenzaldehyde and 2-aminothiophenol as raw materials, and connecting with iodine-containing nitrobenzene derivative through dehydration cyclization reaction and coupling reaction; and generating the pH fluorescent probe benzothiazole-aniline derivative through reductive amination. Rigid structures, such as benzothiazole and phenylethynyl, are introduced into the fluorescent probe; the fluorescent probe is high in fluorescence quantum efficiency, and high in thermal stability and solubility. The probe can detect pH value under strong acid condition by adopting a photoinduced charge transfer mechanism and a conjugate passivation mechanism; and the probe has the characteristics of rapid response, high sensitivity and high selectivity, and has wide application prospect in environment monitoring, ecological protection, disease diagnosis, industrial production and sewage inspection.
Owner:浙江富昇科技有限公司
Organic light-emitting material, and organic light-emitting device
PendingCN111303134AGood thermal stabilityExtend your lifeOrganic chemistrySolid-state devicesOrganic electroluminescenceElectron transporting layer
The invention provides a novel organic light-emitting material which is used as a light-emitting main body material and an electron transport layer material of an organic light-emitting device. The invention also provides the organic light-emitting device which comprises the novel organic light-emitting material provided by the invention.
Owner:YANTAI XIANHUA CHEM TECH CO LTD
Tetra-branched rare earth complex and preparation method thereof
InactiveCN101798321AConjugate plane largeExcellent Rigid StructureGroup 3/13 element organic compoundsIon clustersPhenanthroline
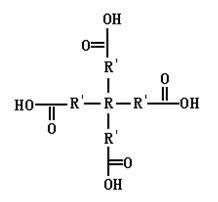
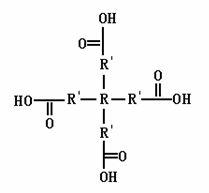
The invention relates to a tetra-branched rare earth complex and a preparation method thereof. The complex is prepared through complexing reaction of three ligands, namely two M1s and one M2 by taking a trivalent rare earth ion as a central ion. The chemical formula of the complex is Ln(M1)2M2, wherein Ln is the rare earth ion; M is the ligands consisting of M1 and M2; M1 is a monocarboxylic compound synthesized by a tetratomic alcohol compound and an aliphatic or aromatic dicarboxylic compound; M2 is 1,10-phenanthroline; and a ligand solvent is dimethyl sulfoxide or N,N-dimethyl formamide. The rare earth complex of the invention has a network pore structure, bigger conjugate plane, better rigid structure and structural stability, and more coordinative rare earth ions. A rare earth polymer luminescent material synthesized by the tetra-branched rare earth complex has no phenomenon that ions are accumulated to form ion clusters so as to cause fluorescent quenching, and can effectively perform high-efficiency photon switching to enhance the luminescent efficiency of the complex.
Owner:JIANGSU XINGYE PLASTIC
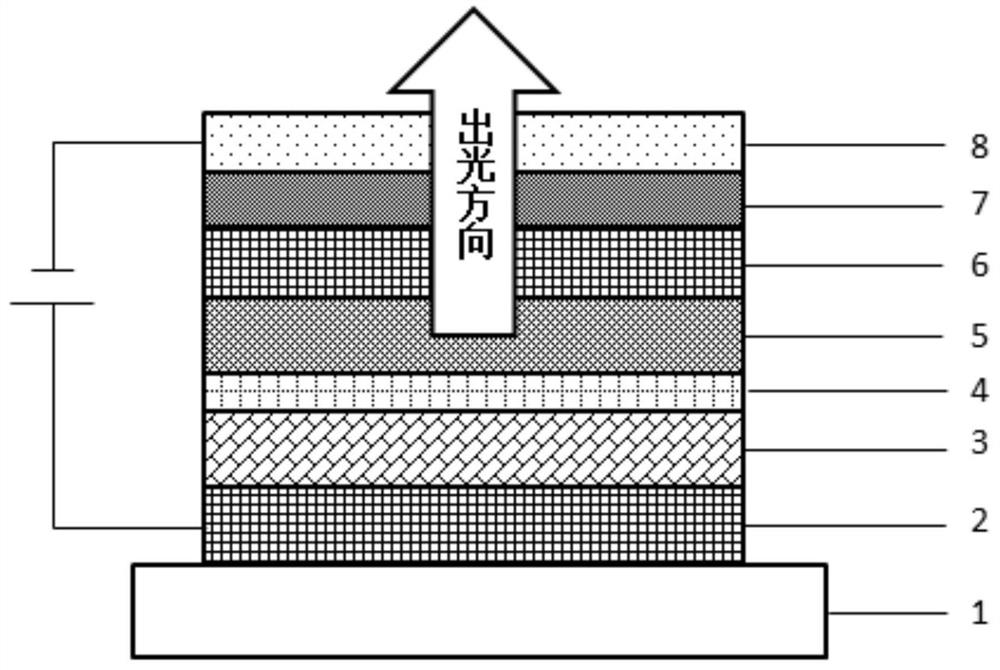
Novel HIT and EB material matched organic light-emitting device
ActiveCN112490376AImprove stabilityConjugate plane largeOrganic chemistrySolid-state devicesPhysicsOrganic electroluminescence
The invention discloses a novel HIT and EB material matched organic light-emitting device, which is sequentially provided with a substrate, a first electrode, an organic functional material layer anda second electrode from bottom to top, and is characterized in that the organic functional material layer comprises: a hole transport region located on the first electrode; a light-emitting layer overthe hole transport region, the light-emitting layer including a host material and a guest material; and an electron transmission region which is located on the light-emitting layer. The hole transport region sequentially comprises a hole injection layer, a hole transport layer and an electron blocking layer from bottom to top, the hole injection layer comprises a hole transport layer material anda P-type doped material, and the hole transport layer comprises an organic compound containing dimethyl fluorenyl in the structure. The electron blocking layer comprises an organic electroluminescentdevice having improved luminous efficiency and lifespan, and a display comprising the same, and more particularly, to an organic electroluminescent device having improved luminous efficiency and lifespan, and an electronic device comprising the same.
Owner:JIANGSU SUNERA TECH CO LTD
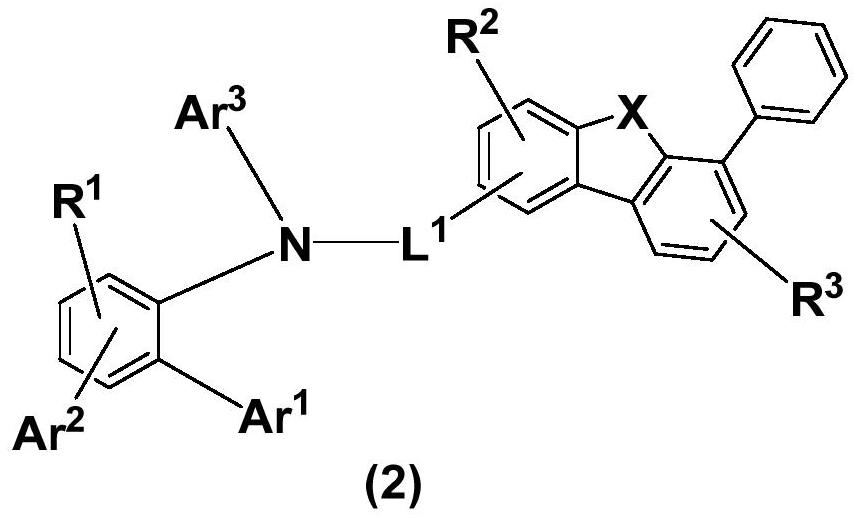
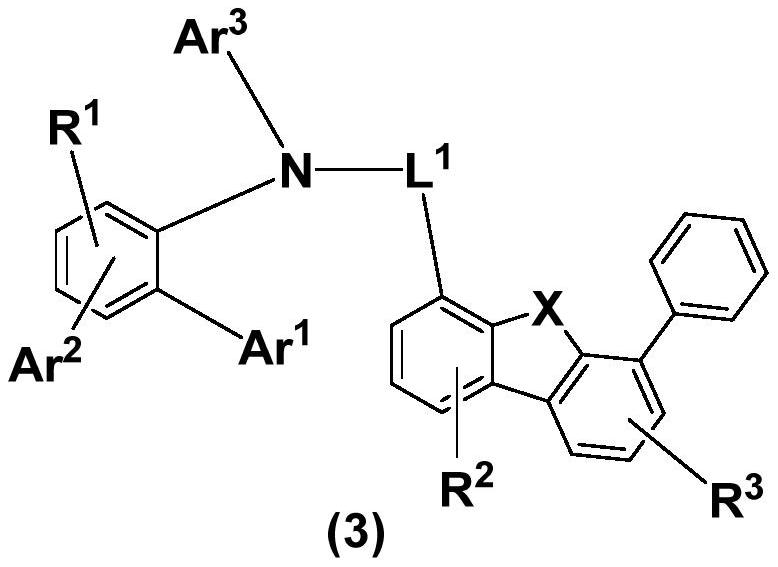
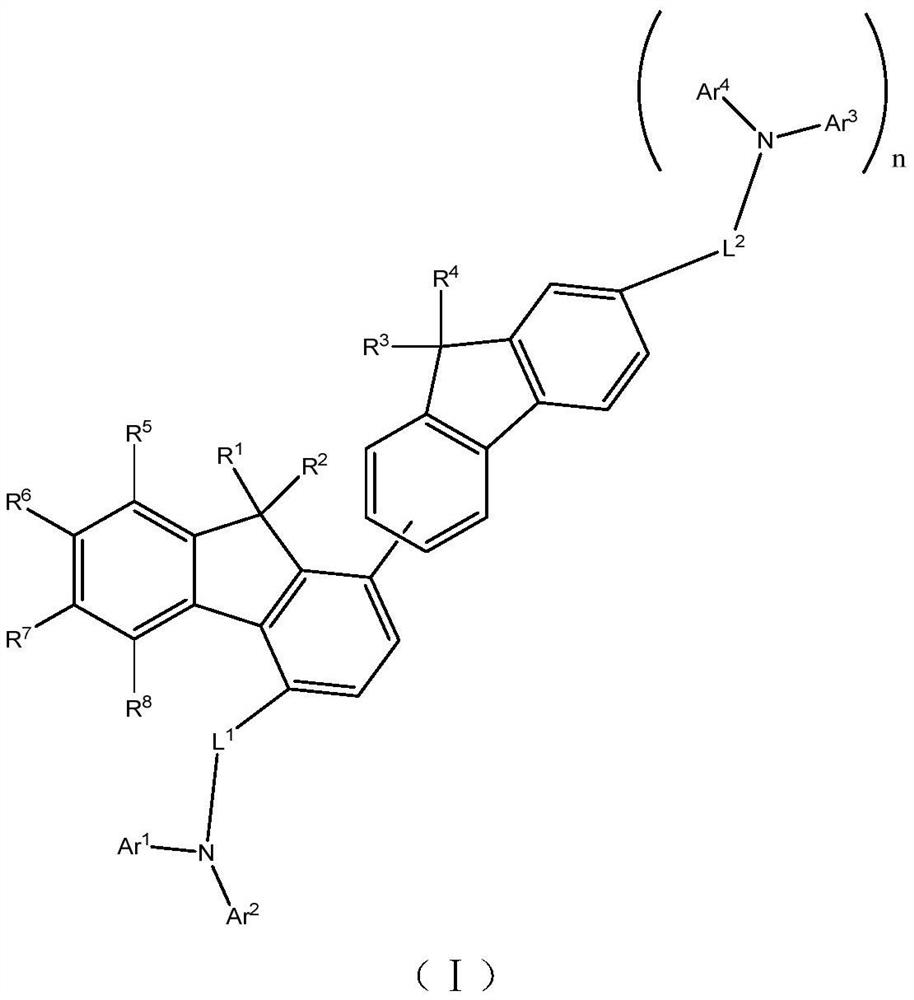
Condensed ring aromatic amine compound and organic electroluminescent display device comprising same
PendingCN113292480AConjugate plane largeGood chemical stabilityOrganic chemistrySolid-state devicesHole transport layerOrganic electroluminescence
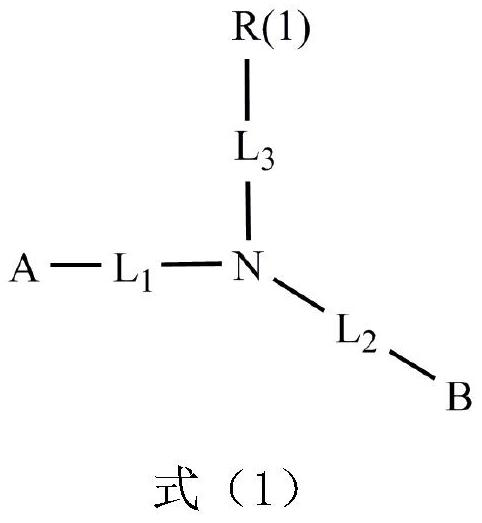
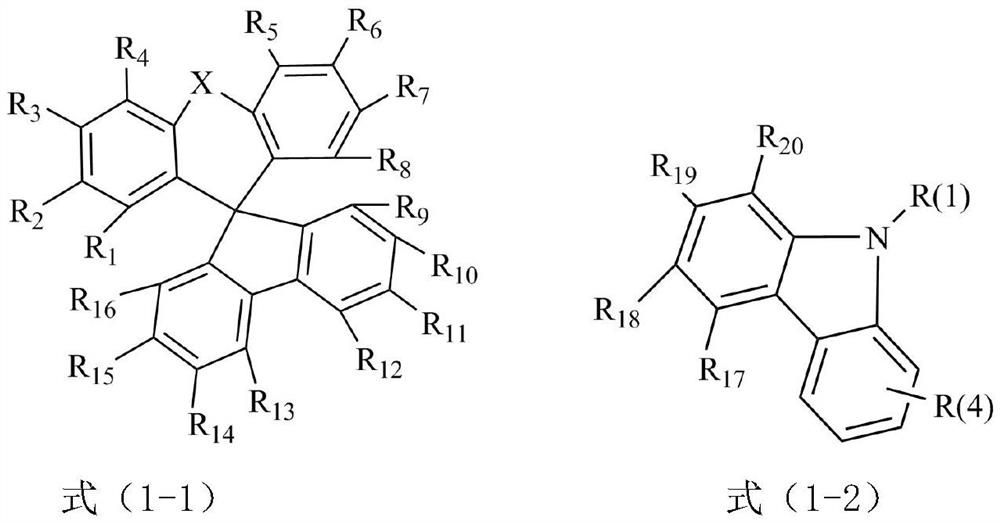
The invention discloses a condensed ring aromatic amine compound and an organic electroluminescent display device comprising the same, and belongs to the technical field of organic electroluminescent materials. The condensed ring aromatic amine compound has a structure represented by the formula, wherein A and B respectively have structures represented by the formulas defined in the specification, L1, L2 and L3 are respectively and independently selected from a single bond, substituted or unsubstituted arylene with the ring-forming carbon atom number of 5-60 and substituted or unsubstituted heteroarylene with the ring-forming carbon atom number of 5-60, and X is independently selected from O, C, N or S atoms; and at least any two adjacent groups of R1 to R16 and R17 to R20 are bonded to each other to form a ring structure. The condensed ring aromatic amine compound has a large conjugate plane, the stability of the compound can be improved, an organic electroluminescent display device obtained by adopting the compound as a hole transport layer material can reduce the problem of crosstalk of light emitting display, the driving voltage is low, and the service life is long.
Owner:上海钥熠电子科技有限公司
Compound, application thereof and organic light-emitting device comprising compound
PendingCN112125880AImprove thermal stabilityExtend your lifeOrganic chemistrySolid-state devicesOrganic electroluminescenceElectron blocking layer
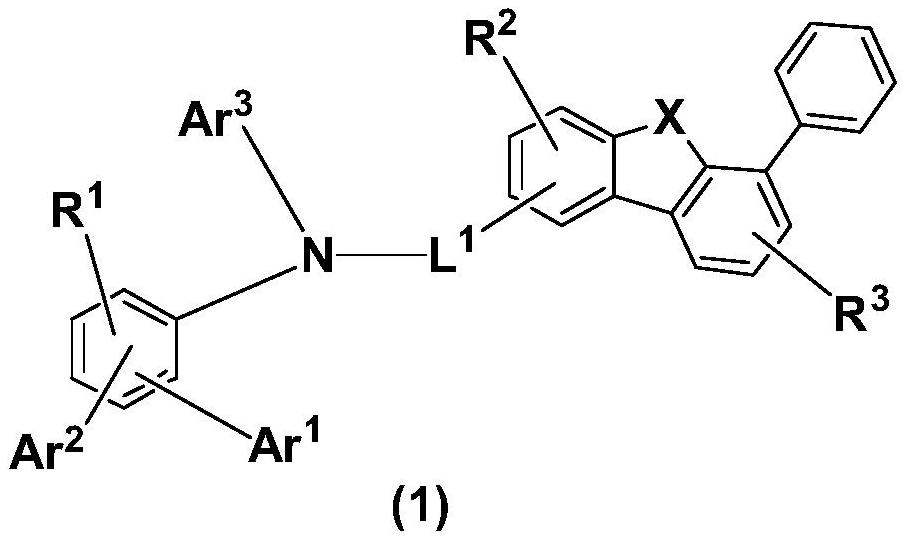
The invention relates to a compound, application thereof and an organic light-emitting device comprising the compound. The compound has a structure as shown in the following formula (1), wherein X isselected from O, S or Se; L1 is selected from substituted or unsubstituted arylene groups of C6-C30; Ar1-Ar3 are each independently selected from one of a substituted or unsubstituted C6-C30 aryl group and a substituted or unsubstituted C3-C30 heteroaryl group, and R1 to R3 each independently represent a monosubstituted group to a maximum permissible substituent group, and each is independently selected from one of hydrogen, C1-C30 alkyl, C1-C30 alkoxy, halogen, cyano, nitro, hydroxyl, silyl, amino, substituted or unsubstituted C6-C30 arylamino, substituted or unsubstituted C3-C30 heteroarylamino, substituted or unsubstituted C6-C30 aryl and substituted or unsubstituted C3-C30 heteroaryl. When the compound provided by the invention is used as a hole transport layer material or an electronblocking layer material in an OLED device, excellent device performance and stability are shown. The invention also discloses an organic light-emitting device adopting the compound with the general formula.
Owner:BEIJING ETERNAL MATERIAL TECH
Compound, hole transport material and organic electroluminescent device
ActiveCN112125813AHigh bond energyImprove thermal stabilityOrganic chemistrySolid-state devicesDisplay deviceHeat stability
The invention provides a compound shown as a general formula (I), which can be used for a hole transport material. The compound has a parent structure of difluorene substituted arylamine, is high in bond energy between atoms, has good thermal stability, is beneficial to intermolecular solid accumulation, is strong in hole transition capability, and can effectively reduce device voltage and prolongthe service life of the material when being used as a hole transport material. The invention further provides an organic light-emitting device and a display device containing the compound shown in the general formula (I).
Owner:YANTAI XIANHUA CHEM TECH CO LTD
Thieno thiophene fused heterocyclic organic compound and application thereof
PendingCN111423455AConjugate plane largeHigh thermodynamic stabilityOrganic chemistrySolid-state devicesElectron holeSimple Organic Compounds
The invention belongs to the technical field of organic photoelectric materials, and particularly relates to a thieno thiophene fused heterocyclic organic compound and application thereof. The organiccompound provided by the invention has a thieno thiophene fused heterocyclic parent structure, has high bond energy between atoms, has good thermal stability, is beneficial to intermolecular solid accumulation, and can effectively prolong material service life when being used as a luminescent layer material. The compound provided by the invention is a derivative of a large conjugated fused heterocycle, is applied to a light emitting layer, has a proper energy level with adjacent levels, is beneficial to injection of holes and electrons, can effectively reduce turn-on voltage, and can realizegood light emitting efficiency in a device due to a relatively high exciton migration rate. The compound disclosed by the invention has a relatively large conjugate plane, is beneficial to molecular accumulation, shows good thermodynamic stability and shows long service life in a device. The preparation process of the derivative is simple and feasible, raw materials are easy to obtain, and the derivative is suitable for large-scale production.
Owner:YANTAI XIANHUA CHEM TECH CO LTD
Fluorescent probe for detecting heavy metal ions in industrial wastewater, and preparation method thereof
ActiveCN110256644AConjugate plane largeFluorescence enhancementFluorescence/phosphorescenceLuminescent compositionsChemical industryWastewater
The present invention belongs to the field of chemical industry, and particularly relates to a fluorescent probe for detecting heavy metal ions in industrial wastewater, and a preparation method thereof. The dispersed "branched structure" of 1,2,4,5-tetrafluorobenzene is used to make a polymer II have a hyperbranched structure, so the self-quenching phenomenon caused by pi-pi stacking is effectively avoided, and the fluorescent probe can efficiently emit fluorescence after being combined with the heavy metal ions, thereby the fluorescent probe has the basic conditions for being used as a heavy metal ion fluorescent probe. In addition, the special C=N structure of a Schiff base makes fluorescent probe complex with positively charged metal ions, so complexation further enlarges the conjugate plane and increases the fluorescence intensity, thereby the presence of heavy metal ions is detected.
Owner:徐从燕
A kind of fused ring non-fullerene acceptor material and its preparation method and application
ActiveCN107652304BImprove electron donating abilityHigh densityOrganic chemistrySolid-state devicesOrganic solar cellVisible near infrared
The invention provides a fused-ring non-fullerene acceptor material and its preparation method and application. The fused-ring non-fullerene acceptor material includes an electron-donating unit and an electron-withdrawing terminal group, and the electron-donating unit is a carbon-oxygen Bridge-ladder fused ring structure, the electron-withdrawing end group is connected to both ends of the electron-donating unit, and the fused-ring non-fullerene acceptor material has a structure shown in formula I or formula II, through the preparation method of the present invention The prepared fused-ring non-fullerene acceptor material has excellent light absorption and carrier transport properties, is easily soluble in common organic solvents, has strong visible and near-infrared light absorption properties, and high electron mobility (≥10 ‑5 cm 2 ·V ‑1 ·s ‑1 ), which can achieve high short-circuit current and energy conversion efficiency in organic solar cells, and has broad application prospects and high application value.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
A d-a type conjugated polymer containing fused ring lactone and its preparation method and application
ActiveCN110408010BConjugate plane largePromotes π-π stackingSolid-state devicesSemiconductor/solid-state device manufacturingOrganic solar cellPolymer science
The invention provides a D-A type conjugated polymer containing fused ring lactone, its preparation method and application. The structural formula of the D-A type conjugated polymer containing fused ring lactone provided by the present invention is shown in formula I. The present invention introduces the fused-ring lactone group into the conjugated main chain of the polymer, thereby broadening the conjugation plane of the polymer molecule, promoting the π-π stacking between the molecules, and reducing the intermolecular π-π stacking of the polymer. Steric hindrance. The D-A type conjugated polymer containing fused ring lactone provided by the present invention has good solubility and photoelectric properties, as well as excellent light absorption and carrier transport properties, and can achieve higher performance in organic solar cells. Short circuit current and energy conversion efficiency.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Spirobifluorene fluorescence probe as well as preparation method and application thereof
InactiveCN102633726BConjugate plane largeHigh fluorescence quantum yieldOrganic chemistryFluorescence/phosphorescenceQuantum yieldGrignard reaction
The invention discloses a spirobifluorene fluorescence probe which is 2,7-di(benzimidazole) spirobifluorene. According to the invention, the structural formula of a product is shown in a formula (I). The preparation method of the spirobifluorene fluorescence probe comprises the following steps: carrying out coupling reaction, bromination, Grignard reaction, dehydration cyclization reaction and oxidization reaction on parabromotoluene which is used as a raw material so as to obtain a spirobifluorene dialdehyde compound; and reacting the spirobifluorene dialdehyde compound with o-phenylenediamine to obtain the compound (I). According to the invention, the raw material is cheap and available, the synthesis route is simple, the yield is high, and the preparation method is suitable for large-scale production. Because the three-dimensional rigid structure of spirobifluorene is introduced, the compound (I) has high fluorescence quantum yield and fluorescence intensity; the compound (I) has high sensitivity and good selectivity when being used for silver ion detection, detection limit of naked eye can reach 10<-5>M, and the detection limit of an instrument can reach 10<-6>M; the response time of the compound (I) on the silver ion is less than 2 seconds; and simultaneously, the compound (I) is a pH fluorescence probe having wide response range and has a good application prospect.
Owner:SHANGHAI NORMAL UNIVERSITY
Undoped small organic molecule hole transport material as well as preparation method and application thereof
PendingCN114716469AEffective control of crystallinityStrong cavitation powerSilicon organic compoundsFinal product manufactureOrganic moleculesSide chain
The invention provides an undoped small organic molecule hole transport material as well as a preparation method and application thereof, and relates to the technical field of photoelectric materials. According to the undoped small organic molecule hole transport material, benzene ring substituted benzodithiophene is used as a central core, thiophene bithienosilole is used as a pi bridge, rhodanine is used as an end group, benzene rings are added on side chains of the central core, the conjugacy of molecules is improved, formation of an intermolecular pi-pi stacking structure is facilitated, and the hole transport efficiency is improved. Thienyl thienosilole contains a long C-Si bond, so that steric hindrance is reduced, intermolecular pi-pi accumulation is further promoted, and more ordered orientation is generated. The undoped small organic molecule hole transport material has relatively strong hole capability and relatively strong capability of absorbing light in a green light wavelength range, can remarkably improve the hole mobility, and can obtain relatively high photoelectric conversion efficiency and relatively strong stability when being applied to a perovskite solar cell.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
Rare-earth compound olefin monomer and preparation method and application thereof
InactiveCN101962389BImprove solubilityImprove thermal stabilityGroup 3/13 element organic compoundsLuminescent compositionsSolubilityPhotoluminescence
Owner:HANGZHOU NORMAL UNIVERSITY
Benzothiazole-cyanophenyl compound serving as hydrazine fluorescence probe as well as preparation method and application method of benzothiazole-cyanophenyl compound
ActiveCN103214428BSimple preparation processConjugate plane largeOrganic chemistryFluorescence/phosphorescenceIndustrial waste waterStructural formula
The invention discloses a benzothiazole-cyanophenyl compound serving as a hydrazine fluorescence probe. The benzothiazole-cyanophenyl compound has a structural formula as shown in (I); the compound is prepared by performing cyclodehydration with bromobenzaldehyde and 2-amino-4-chloro thiophenol serving as the raw materials, then performing coupled reaction in order to connect with a bromobenzaldehyde derivate, and finally performing Knoevenagel reaction with malononitrile. The benzothiazole-cyanophenyl compound has the advantages that the raw materials are low in price and easy to gain, the synthetic route is simple, and the yield is relatively high; rigid structures such as benzothiazole and phenylacetylene groups are introduced into such a fluorescence probe, thus high fluorescence quantum efficiency is realized, and relatively high thermal stability and dissolubility are brought. The probe adopts the photoinduced charge transfer mechanism and the conjugate passivation mechanism, therefore, a response range respect to hydrazine can be expanded; the probe has the characteristics of being fast in response, high in sensitivity and high in selectivity, is suitable for being applied to safety detection of foods as well as safety detection of a laboratory, in particular applied to industrial wastewater monitor; and the probe has a wide application prospect in environment monitoring, ecological protection, disease diagnosis, industrial production and pollution discharge inspection.
Owner:浙江富昇科技有限公司
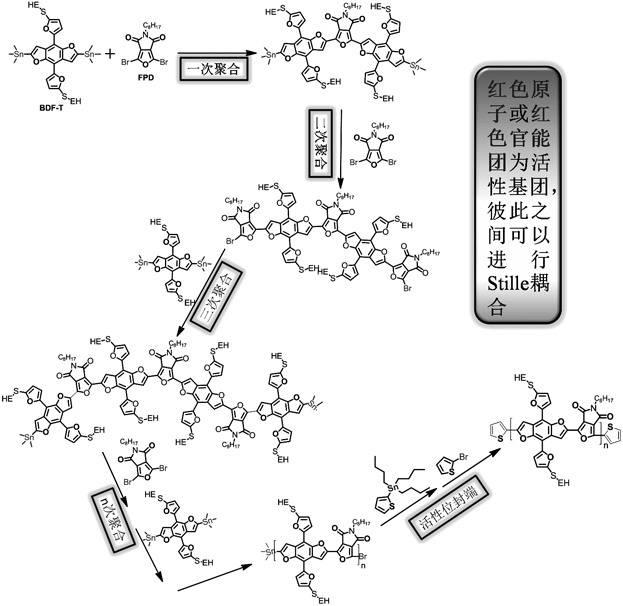
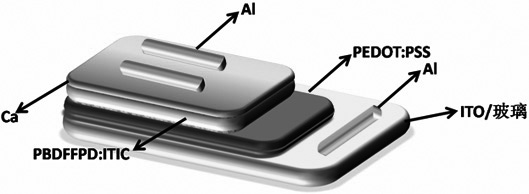
A kind of whole furan skeleton donor-acceptor conjugated polymer, its preparation method and the organic solar cell prepared by using it
ActiveCN109776765BConjugate plane largeStrong π−π stackingSolid-state devicesSemiconductor/solid-state device manufacturingOrganic solar cellFuran
The invention provides a per-furan framework donor-receptor conjugated polymer and a preparation method thereof as well as an organic solar cell made of the per-furan framework donor-receptor conjugated polymer. The structural formula of the polymer is shown in the description, wherein EH is shown in the description, and n is 24-25. The prepared polymer PBDFFPD is high in conjugate plane, high inin-molecule and inter-molecule mutual action, and low in HOMO energy level; a PBDFTD:ITIC based organic solar cell has 9.30% of photoelectric conversion efficiency under the collaborative processing of chloronaphthalene and methyl alcohol, and FF is up to 70.3%, so that the top record of the photoelectric conversion efficiency of an FTD based polymer organic solar cell is reached. In addition, thedeviation between the average efficiency and the maximum efficiency of a 20 battery under the optimal condition of the PBDFTD:ITIC cell is kept at 0.64%, so that the PBDFFTD is high in potential to prepare an efficient organic solar cell, and the operation is free from strict requirements; the polymer is suitable for popularization.
Owner:HENAN UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com