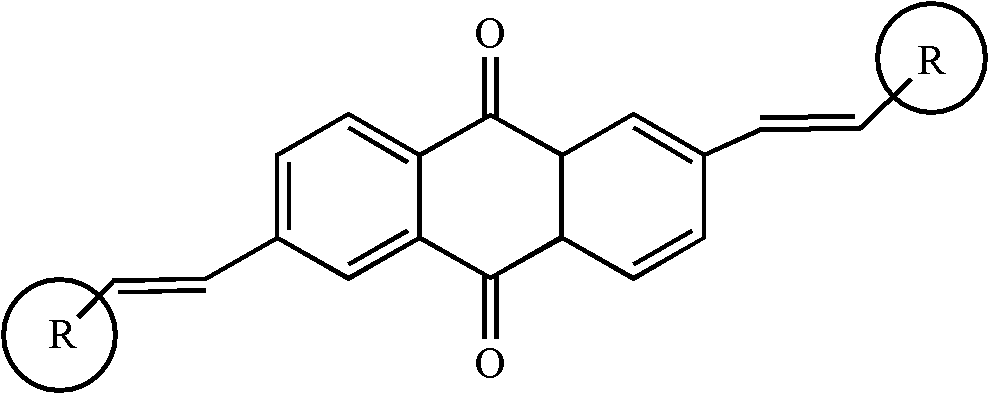
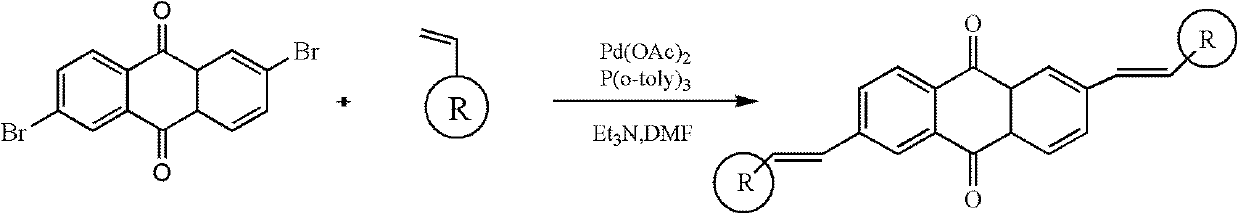
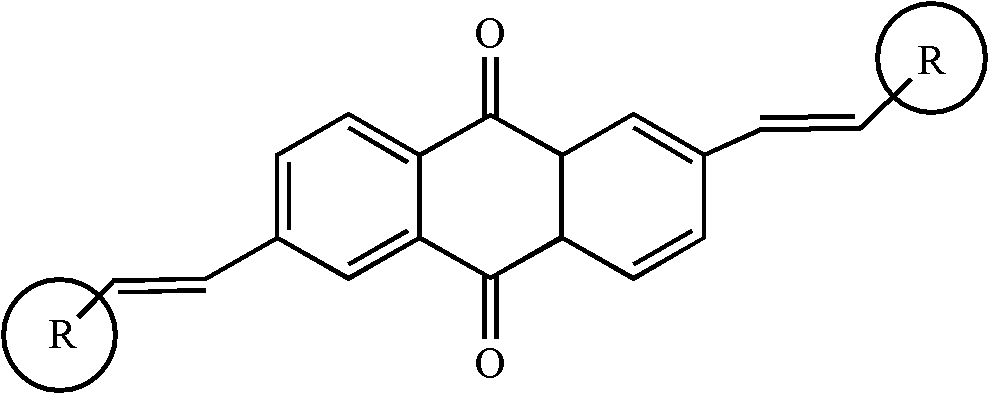
2, 6-di(aryl)-anthraquinone and preparation method thereof
An aromatic group and anthraquinone technology, which is applied in the field of symmetrical anthraquinone derivatives, can solve the problems of easy decomposition, weak photochemical stability, and restrictions on the application of thin films, and achieve good optical stability and thermal stability. Resistant to photodecomposition and long-lasting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 2, the preparation of 6-bis (4-methoxystyryl)-anthraquinone comprises the following steps:
[0025] With 2,6-dibromo-anthraquinone (15 grams), 4-methoxystyrene (8.6 grams), palladium acetate (0.9 grams), three-O-cresyl phosphorus (0.7 grams,) in a nitrogen atmosphere Under certain conditions, it was added to triethylamine solvent, stirred and heated at 75°C for 48 hours. Cool to room temperature after the reaction, distill under reduced pressure, separate and wash, dry and concentrate. The target product 2,6-bis(4-methoxystyryl)-anthraquinone was separated by chromatography column, 11.8 g, yield 73%.
Embodiment 2
[0027] Synthesis of 2,6-bis(4-aminostyryl)-anthraquinone
[0028] With 2,6-dibromo-anthraquinone (15 grams), 4-aminostyrene (9.2 grams), palladium acetate (1.2 grams), three-O-cresyl phosphorus (0.7 grams) under nitrogen protection atmosphere conditions, Add it to triethylamine, stir and heat at 90°C for 48 hours. Cool to room temperature after the reaction, distill under reduced pressure, separate and wash, dry and concentrate. The target product 2,6-bis(4-aminostyryl)-anthraquinone was separated by chromatography column, 13.3 g, yield 76%.
Embodiment 3
[0030] Synthesis of 2,6-bis(4-chlorostyryl)-anthraquinone
[0031] With 2,6-dibromo-anthraquinone (15 grams), 4-chlorostyrene (9.4 grams), palladium acetate (1 gram), three-O-cresyl phosphorus (0.7 grams) under nitrogen protection atmosphere conditions, Add it to triethylamine, stir and heat at 90°C for 24 hours. Cool to room temperature after the reaction, distill under reduced pressure, separate and wash, dry and concentrate. The target product 2,6-bis(4-chlorostyryl)-anthraquinone was separated by chromatography column, 9.4 g, yield 65%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com