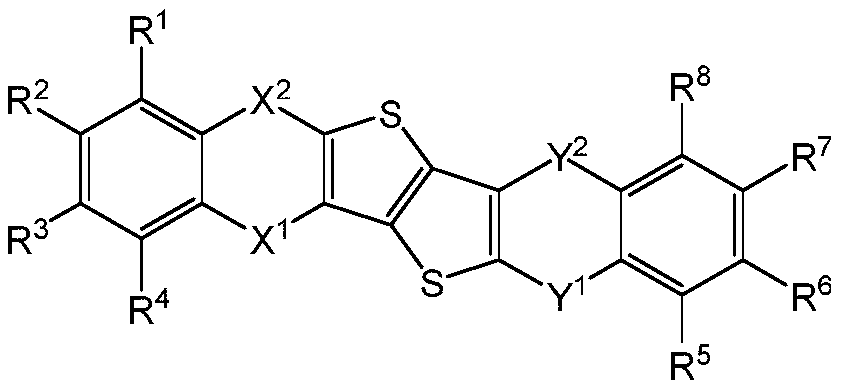
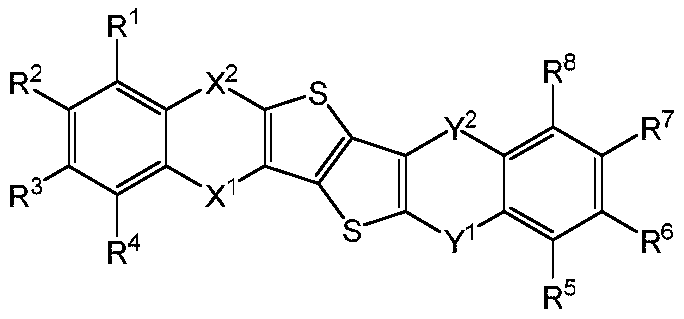
Thieno thiophene fused heterocyclic organic compound and application thereof
An organic compound, dithiophene technology, which is applied in the field of dithiophene condensed heterocyclic organic compounds, can solve the problems of electron and hole mismatch, efficiency roll-off, and shortened life, so as to reduce the turn-on voltage and improve The effect of life, good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
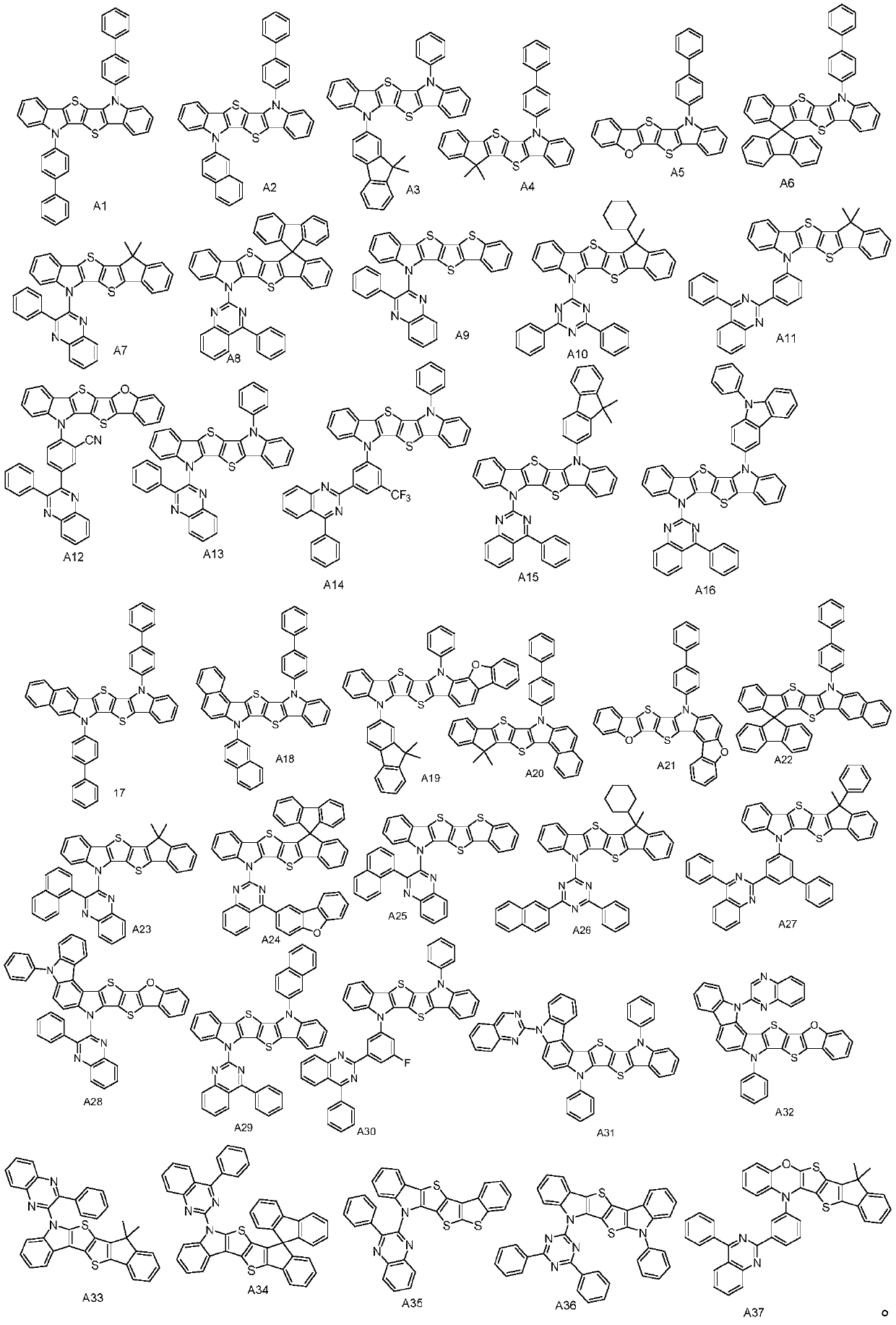
[0025] The synthesis of compound A1, the reaction equation is as follows:
[0026]
[0027] The synthesis method is as follows:
[0028] (1) Dissolve 100mmol of dithiophene in 500mL of dichloromethane, add to the reaction flask, add NBS in batches to a total of 200mmol, stir at room temperature, react for 8h, and the reaction is complete; add water to the reaction solution, solids are precipitated, filter to obtain a white solid M1;
[0029] (2) Add 100mmol M1, 220mmol o-nitrophenylboronic acid, (1%) Pd(PPh 3 ) 4 , sodium carbonate 40g (300mmol), toluene 800mL, ethanol 200mL and water 200mL, heated to reflux, reacted for 8h, and the reaction was completed; the reaction solution was extracted with ethyl acetate, and the organic phase was concentrated to obtain yellow solid M2;
[0030] (3) In the reaction flask, take 100mmol intermediate M2 and add it to 1000mL o-dichlorobenzene solution, then add 300mmol triphenylphosphine, heat to reflux, react for 12h, and the reaction...
Embodiment 2
[0034] The synthesis of compound A7, reaction equation is as follows:
[0035]
[0036] The synthesis method is as follows:
[0037] (1) Dissolve 100mmol of dithiophene in 500mL of dichloromethane, add to the reaction flask, add NBS in batches to 100mmol at 0°C, stir at room temperature, react for 8h, and the reaction is complete; add water to the reaction solution, solids precipitate out, filter , to obtain white solid M1;
[0038] (2) Add M1 (100mmol), o-nitrophenylboronic acid (110mmol), (1%) Pd(PPh 3 ) 4 , sodium carbonate 40g (300mmol), toluene 800mL, ethanol 200mL and water 200mL, heated to reflux, reacted for 8h, and the reaction was completed; the reaction solution was extracted with ethyl acetate, and the organic phase was concentrated to obtain yellow solid M2;
[0039] (3) Take 100mmol of intermediate M2 and add it to 1000mL o-dichlorobenzene solution, add (300mmol) triphenylphosphine, heat to reflux, react for 12h, and the reaction is complete; evaporate the so...
Embodiment 3
[0047] The synthesis of compound A13, the reaction equation is as follows:
[0048]
[0049] The synthesis method is as follows:
[0050] (1) Dissolve 100mmol of dithiophene in 500mL of dichloromethane, add to the reaction flask, add NBS in batches to 100mmol at 0°C, stir at room temperature, react for 8h, and the reaction is complete; add water to the reaction solution, solids precipitate out, filter , to obtain white solid M1;
[0051] (2) Add M1 (100mmol), o-nitrophenylboronic acid (110mmol), (1%) Pd(PPh 3 ) 4 , sodium carbonate 40g (300mmol), toluene 800mL, ethanol 200mL and water 200mL, heated to reflux, reacted for 8h, and the reaction was completed; the reaction solution was extracted with ethyl acetate, and the organic phase was concentrated to obtain yellow solid M2;
[0052] (3) Take 100mmol of intermediate M2 and add it to 1000mL o-dichlorobenzene solution, add (300mmol) triphenylphosphine, heat to reflux, react for 12h, and the reaction is complete; evaporate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com