Benzothiazole-aniline compound used as pH fluorescent probe and preparation method thereof
A technology of aniline compound and benzothiazole, which is applied in the chemical field, can solve the problems of unstable luminophore, sensitive proton concentration, poor selectivity, etc., and achieve the effect of wide application prospect and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Synthesis of fluorescent probe 4-(4-benzothiazolylphenyl)ethynylaniline (compound I).
[0030]
[0031] (1) Synthesis of 2-(4-bromophenyl)benzothiazole
[0032] Add 0.46g (2.5mmol) p-bromobenzaldehyde and 0.31g (2.5mmol) o-aminothiophenol into 25mL triethyl phosphate, stir for 10min, add 2.5mL glacial acetic acid, stir rapidly and heat up to 60°C, add 1.8g (3.75mmol) lead tetraacetate, stirred for 30 minutes, cooled to room temperature, added 100mL water and 50mL dichloromethane, separated, collected the organic phase, extracted the aqueous layer with dichloromethane three times (50mL×3), collected and combined The organic phase was dried with anhydrous magnesium sulfate, the solvent was evaporated to dryness, and the resulting solid was separated and purified by 100-200 mesh silica gel column chromatography (dichloromethane: petroleum ether = 1:2) to obtain the product 2-(4-bromobenzene Base) benzothiazole 0.46g, yield 63%. Its melting point was measure...
Embodiment 2
[0043] Example 2: Response of fluorescence spectrum of fluorescent probe 4-(4-benzothiazolylphenyl)ethynylaniline (compound I) to pH.
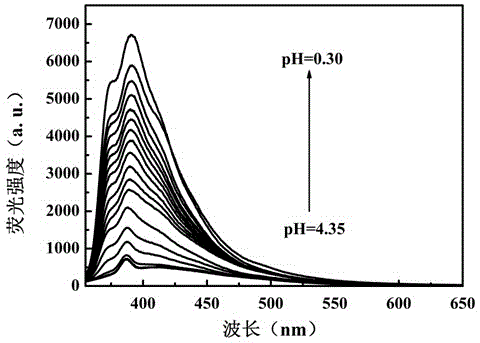
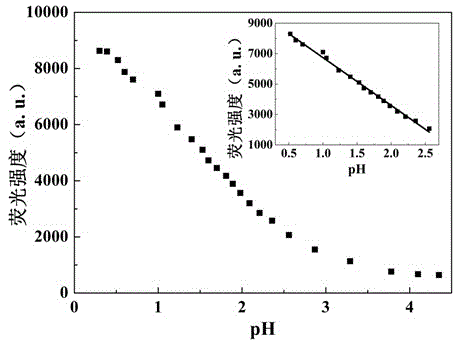
[0044] Britton-Robinson buffer is used to prepare a buffer solution with a pH value of 4.35-1.81, and a solution with a pH value of 0.3-1.81 is prepared with HCl and NaOH to obtain industrial wastewater with a simulated pH value in the range of 0.3-4.35. Each solution was 5mL, and the concentration was 1×10 -4 Mix 50 μL of DMF solution of compound I of M, and test its fluorescence spectrum after shaking evenly. The test conditions are: the excitation wavelength is 340nm, the slit width is 5nm / 5nm, and the voltage is 700V. Test results such as figure 1 and figure 2 shown.
[0045] From figure 1 It can be clearly found that when the pH value of the solution is lower than 4.35, the fluorescence emission peak intensity of the pH fluorescent probe 4-(4-benzothiazolylphenyl)ethynylaniline increases gradually with the decrease of the solution p...
Embodiment 3
[0047] Example 3: The response of the fluorescence spectrum of the fluorescent probe 4-(4-benzothiazolylphenyl)ethynylaniline (compound I) to pH in the presence of interfering ions.
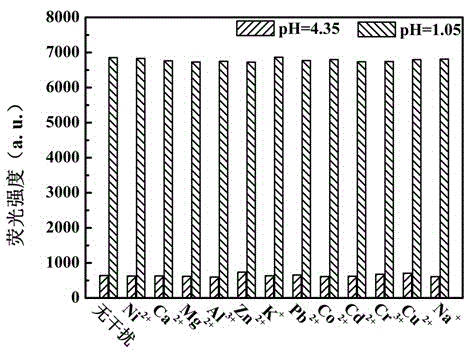
[0048] Use Britton-Robinson buffer solution to prepare a buffer solution with a pH value of 4.35, use HCl and NaOH to adjust and configure a solution with a pH value of 1.05, and use the above two solutions with different pH values to prepare Ni with a concentration of 1mM 2+ , Ca 2+ , Mg 2+ 、Al 3+ , Zn 2+ 、K + , Pb 2+ 、Co 2+ 、Cd 2+ 、Cr 2+ 、Cu 2+ and Na + Ionic solution, and 5mL were added to it with a concentration of 1×10 -4 Mix 50 μL of DMF solution of compound I of M, and test its fluorescence spectrum after shaking evenly. The test conditions are: the excitation wavelength is 340nm, the slit width is 5nm / 5nm, and the voltage is 700V. Test results such as image 3 shown.
[0049] From image 3 It can be seen from the figure that when the pH value of the solution is 4.35, the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com