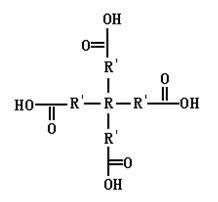
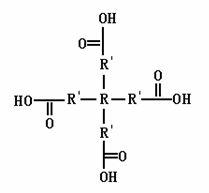
Tetra-branched rare earth complex and preparation method thereof
A technology of rare earth complexes and compounds, applied in the field of rare earth complexes and their preparation, can solve the problems of reduced luminous efficiency of complexes, fluorescence quenching, small conjugate plane, etc., to ensure efficient light conversion performance, enhanced fluorescence intensity, The effect of high light intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Step 1. Ligand M 1 Synthesis (molar ratio of pentaerythritol and oxalic acid is 1:4)
[0042] Put the above 12mmol of pentaerythritol and 48mmol of oxalic acid into 0.65mol of N,N-dimethylformamide solvent with a concentration of 99.1% by weight. At the same time, add dropwise 0.062mol of chemically pure pyridine liquid for reaction. The reaction temperature is 60°C, the reaction time is 10 hours, until no HCl is released; the reaction solution is cooled to room temperature and then poured into deionized water, stirring time is 15 minutes, to obtain white powder; the white powder is washed twice with deionized water and filtered After drying, the ligand product of the reaction between pentaerythritol and oxalic acid is obtained.
[0043] Step two, rare earth lanthanum complex [La(M 1 ) 2 M 2 ] Preparation
[0044] A. Dissolve the oxide of rare earth metal lanthanum in HCl, the molar ratio of the oxide of rare earth lanthanum to hydrochloric acid is 1:15, the dissolution temp...
Embodiment 2
[0050] Step 1. Ligand M 1 Synthesis of (1,2,4,5-pyromellitic acid and terephthalic acid molar ratio 1:6)
[0051] Put the above 12mmol of pyromellitic acid and 72mmol of terephthalic acid into
[0052] Into 0.8 mol of N,N-dimethylformamide solvent with a concentration of 99.3% by weight, at the same time, add dropwise 0.062 mol of chemically pure pyridine liquid for reaction. The reaction temperature is 95°C and the reaction time is 8
[0053] Hours, until no HCl is released; the reaction solution is cooled to room temperature and then poured into deionized water, stirring time is 18 minutes, to obtain white powder; the white powder is repeatedly washed with deionized water 3 times, filtered and dried to obtain 1, 2 ,4,5-Pyromellitic acid reacts with terephthalic acid as a ligand product.
[0054] Step two, rare earth cerium complex [Ce(M 1 ) 2 M 1 ] Preparation
[0055] A. Dissolve the rare earth metal cerium oxide in HCl with a molar ratio of rare earth cerium oxide and hydrochloric ...
Embodiment 3
[0061] Step 1. Ligand M 1 Synthesis of (the molar ratio of naphthalenetetracarboxylic acid and malonic acid is 1:10)
[0062] Put the above 12mmol of naphthalenetetracarboxylic acid and 120mmol of malonic acid into 1mol of N,N-dimethylformamide solvent with a concentration of 99.7% by weight, and add 0.124mol of chemically pure pyridine liquid to react. The temperature is 120℃, the reaction time is 10 hours, until no HCl is released; the reaction solution is cooled to room temperature and then poured into deionized water, stirring time is 20 minutes, to obtain white powder; the white powder is repeatedly washed with deionized water 5 times , Filter and dry to obtain the ligand product of naphthalenetetracarboxylic acid and malonic acid reaction.
[0063] Step two, rare earth europium complex [Eu(M 1 ) 2 M 1 ] Preparation
[0064] A. Dissolve the oxide of the rare earth metal europium in HCl, the molar ratio of the oxide of the rare earth europium to the hydrochloric acid is 1:20, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com