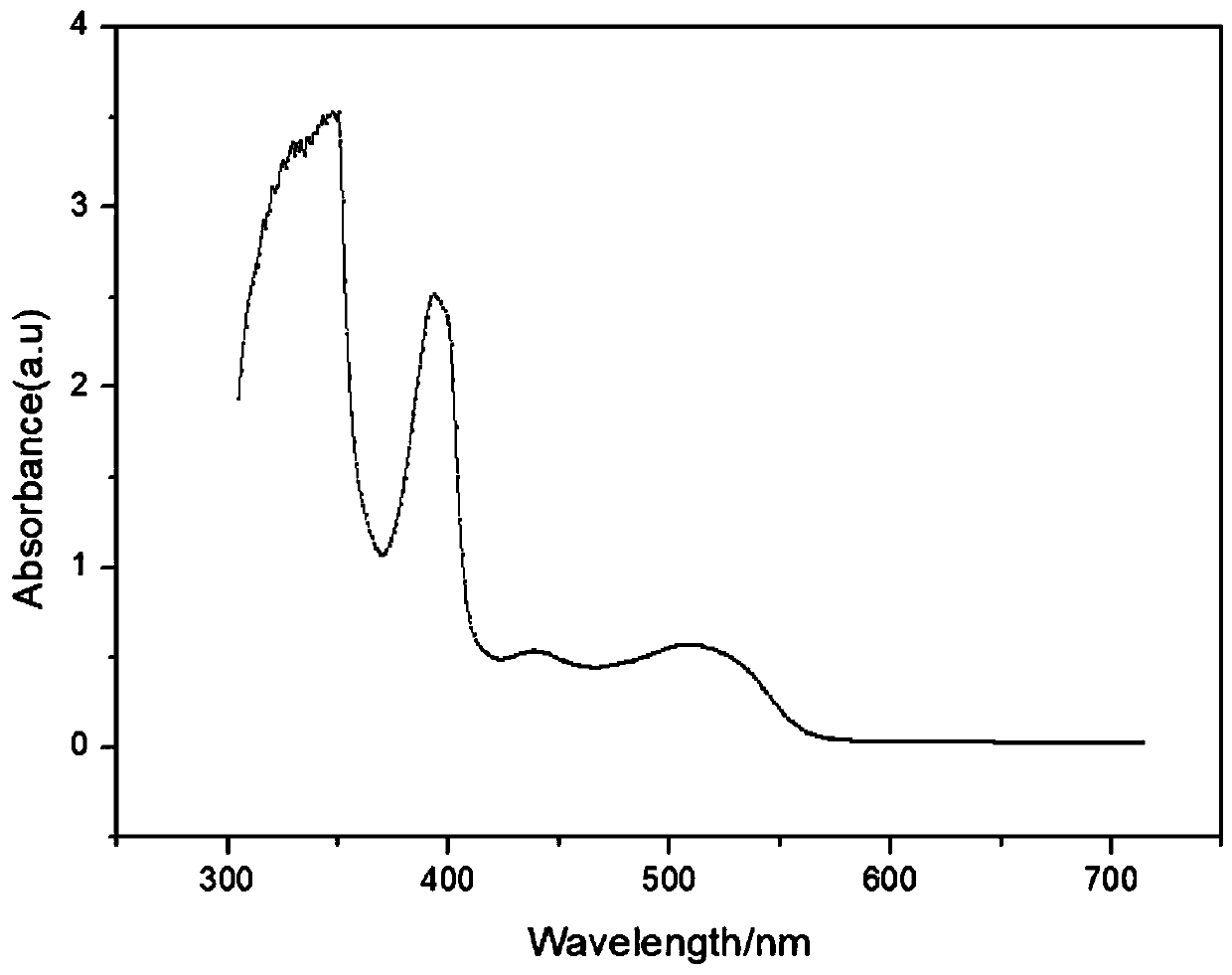
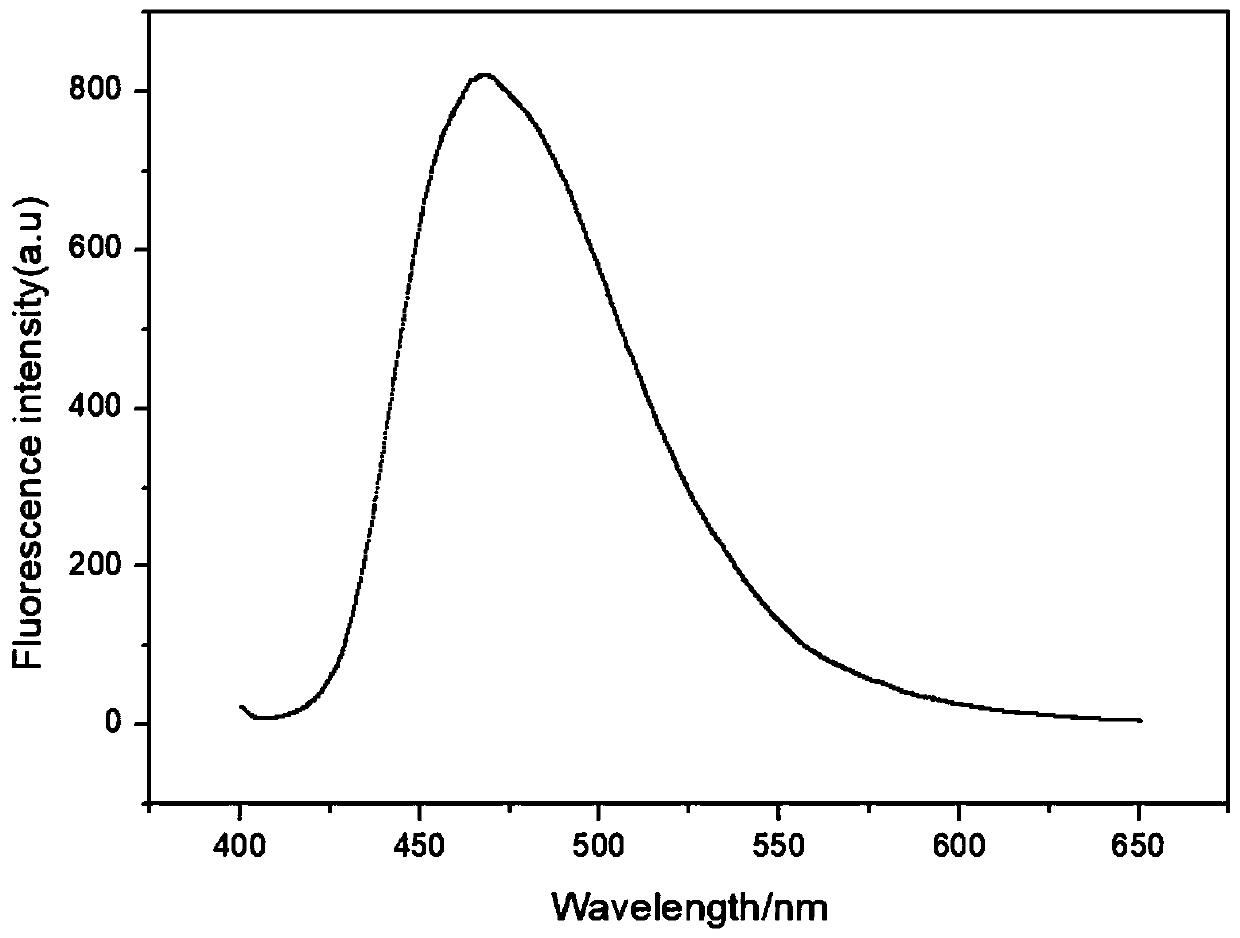
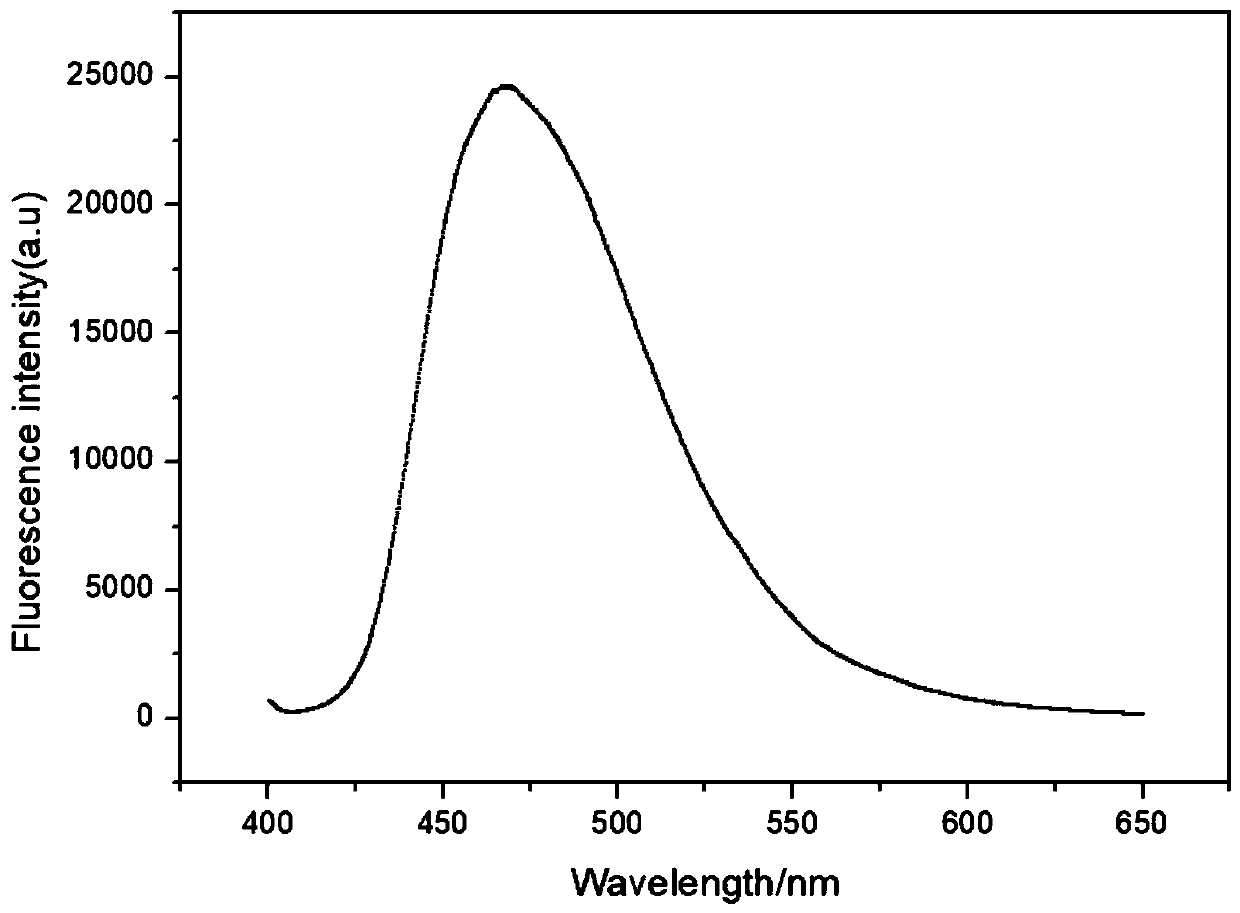
Fluorescent probe for detecting heavy metal ions in industrial wastewater, and preparation method thereof
A technology for heavy metal ions and industrial wastewater, applied in the fields of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of detection work, quenching of fluorescent probes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1) Dissolve indole-3-carbaldehyde in a solvent, put potassium carbonate into the above mixture, stir at room temperature for 10 minutes, then add 1,2,4,5-tetrafluorobenzene, and keep at 55°C, keep warm Reacted for 120min, and after the reaction was completed, compound I was obtained through purification;
[0030] 2) Take the compound I prepared in step 1), dissolve the compound I in THF, add glacial acetic acid, stir at room temperature for 15 minutes, then add p-phenylenediamine, reflux for 24 hours, remove the solvent by rotary evaporation, and purify by recrystallization Polymer II is obtained.
[0031] In the described step 1), the solvent is selected from one of methanol, ethanol or THF;
[0032] In the step 1), the dosage ratio of indole-3-carbaldehyde to 1,2,4,5-tetrafluorobenzene is 5:1;
[0033] In described step 1), the consumption ratio of potassium carbonate and indole-3-carbaldehyde is 0.01:1;
[0034] In the step 1), the purification treatment step is s...
Embodiment 2
[0042] 1) Dissolve indole-3-carbaldehyde in a solvent, put potassium carbonate into the above mixture, stir at room temperature for 20 minutes, then add 1,2,4,5-tetrafluorobenzene, and keep at 85°C, keep warm Reacted for 120min, and after the reaction was completed, compound I was obtained through purification;
[0043] 2) Take the compound I prepared in step 1), dissolve the compound I in THF, add glacial acetic acid, stir at room temperature for 10 min, then add p-phenylenediamine, reflux for 8 h, remove the solvent by rotary evaporation, and purify by recrystallization Polymer II is obtained.
[0044] In the described step 1), the solvent is selected from one of methanol, ethanol or THF;
[0045] In the step 1), the dosage ratio of indole-3-carbaldehyde to 1,2,4,5-tetrafluorobenzene is 8:1;
[0046] In described step 1), the consumption ratio of potassium carbonate and indole-3-carbaldehyde is 0.03:1;
[0047] In the step 1), the purification treatment step is specifical...
Embodiment 3
[0051] 1) Dissolve indole-3-carbaldehyde in the solvent, put potassium carbonate into the above mixture, stir at room temperature for 15 minutes, then add 1,2,4,5-tetrafluorobenzene, and keep at 65°C, keep warm After reacting for 100min, after the reaction was completed, compound I was obtained through purification;
[0052] 2) Take the compound I prepared in step 1), dissolve the compound I in THF, add glacial acetic acid, stir at room temperature for 12 minutes, then add p-phenylenediamine, reflux for 12 hours, remove the solvent by rotary evaporation, and purify by recrystallization Polymer II is obtained.
[0053] In the described step 1), the solvent is selected from one of methanol, ethanol or THF;
[0054] In the step 1), the dosage ratio of indole-3-carbaldehyde to 1,2,4,5-tetrafluorobenzene is 6:1;
[0055] In described step 1), the consumption ratio of potassium carbonate and indole-3-carbaldehyde is 0.02:1;
[0056] In the step 1), the purification treatment step...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com