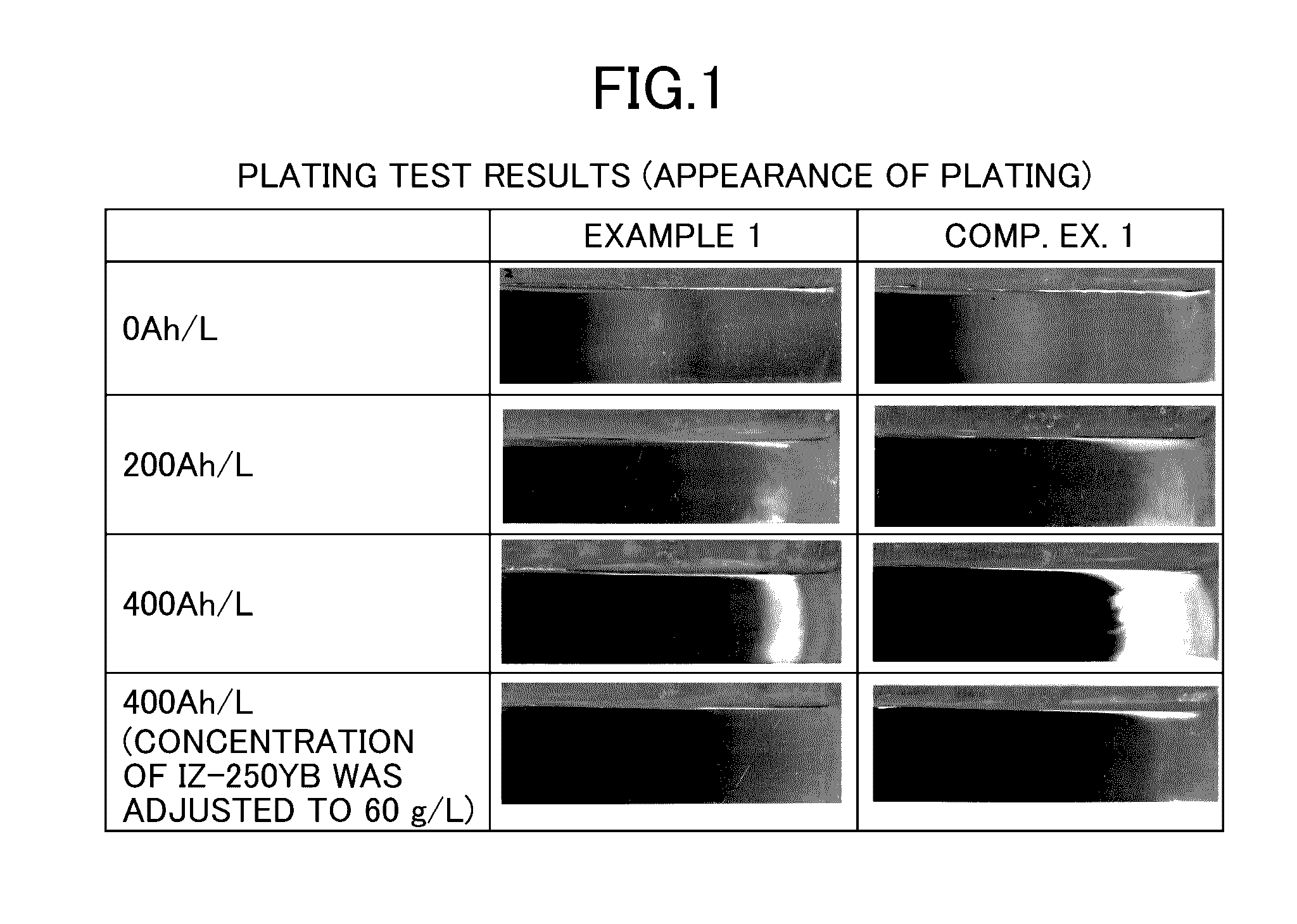
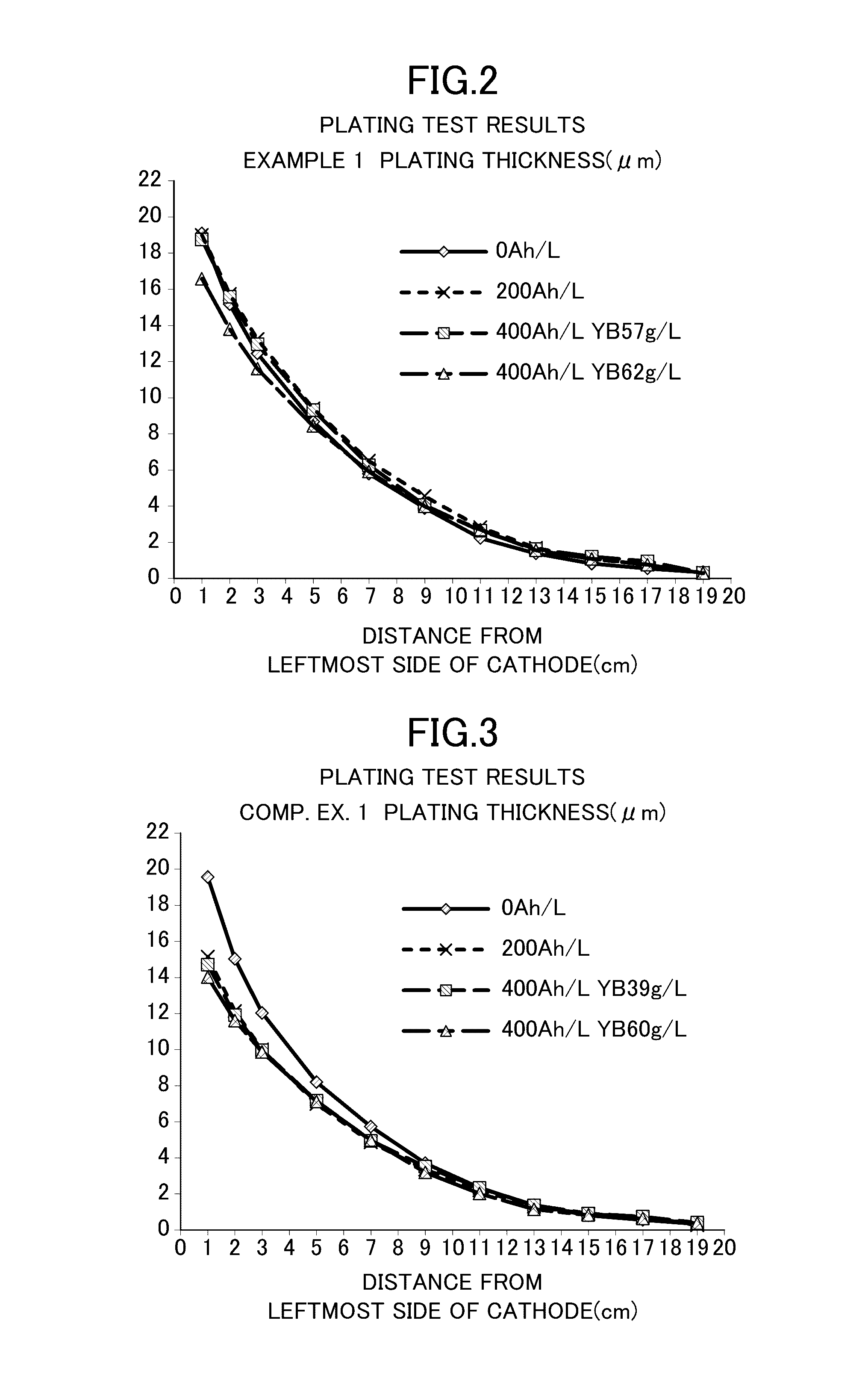
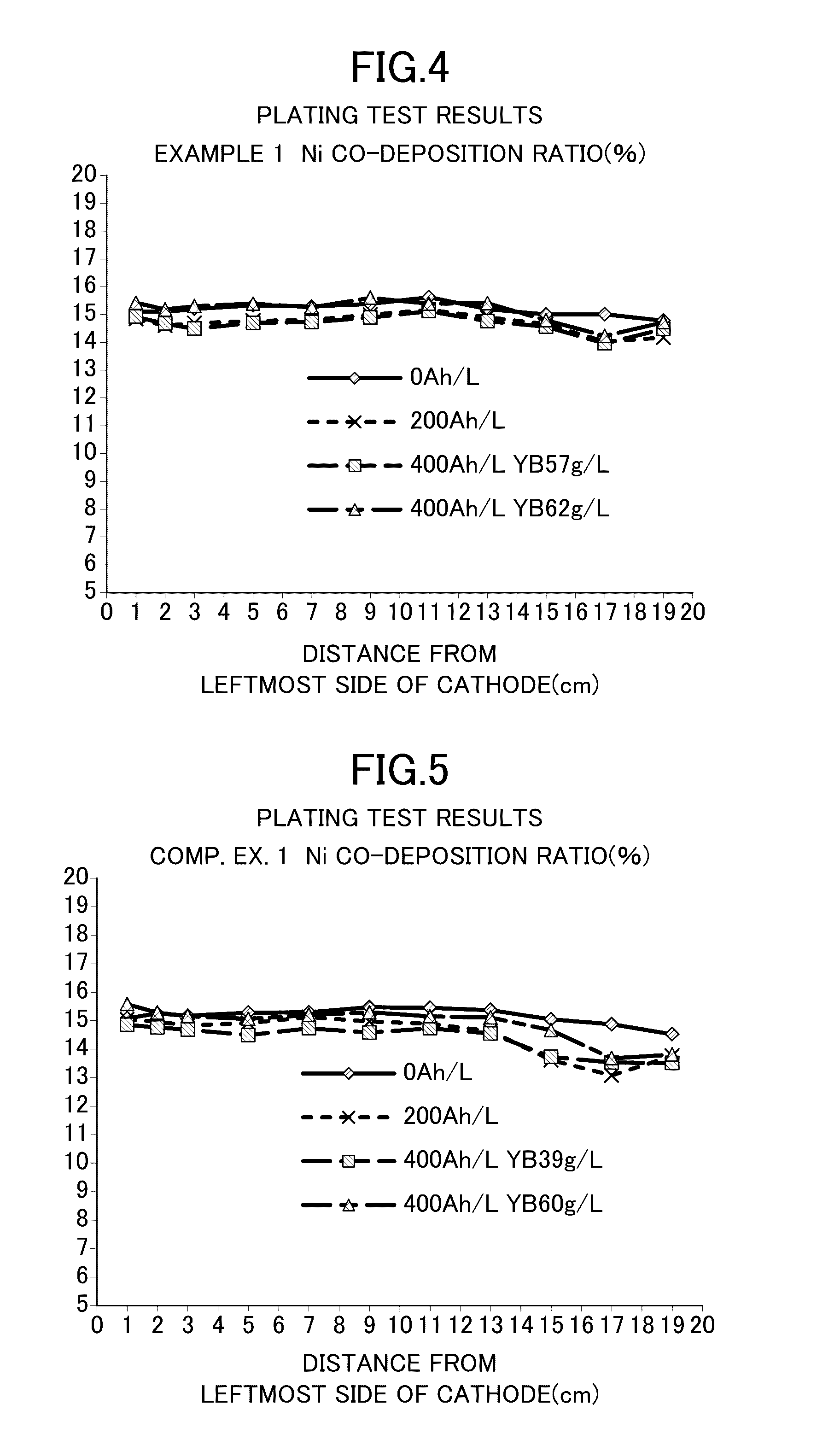
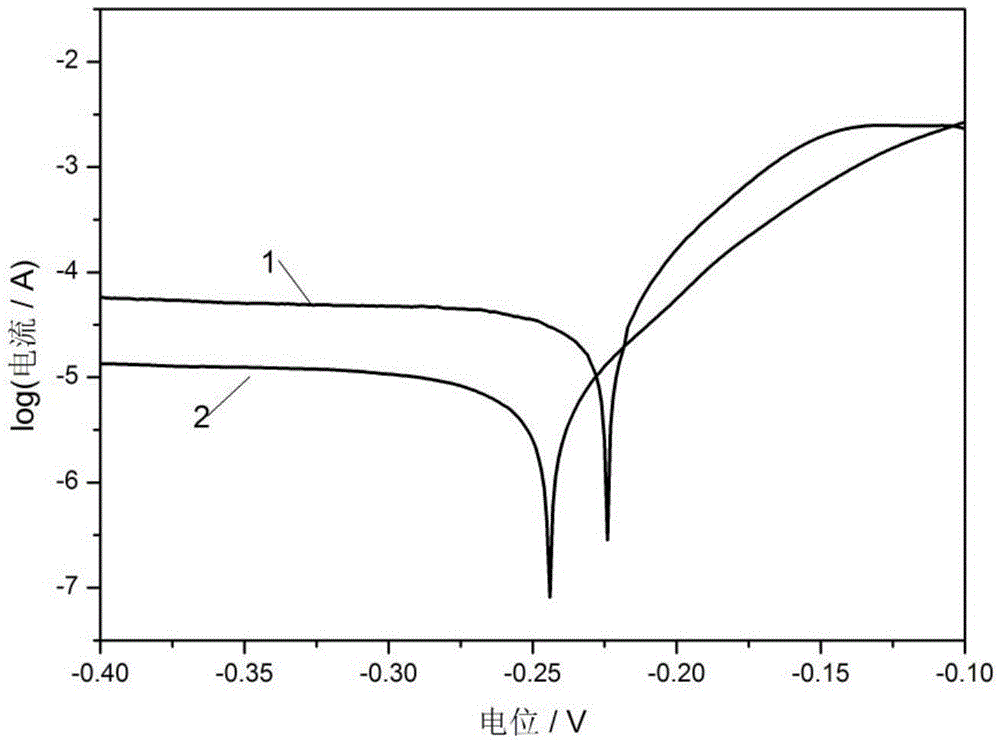
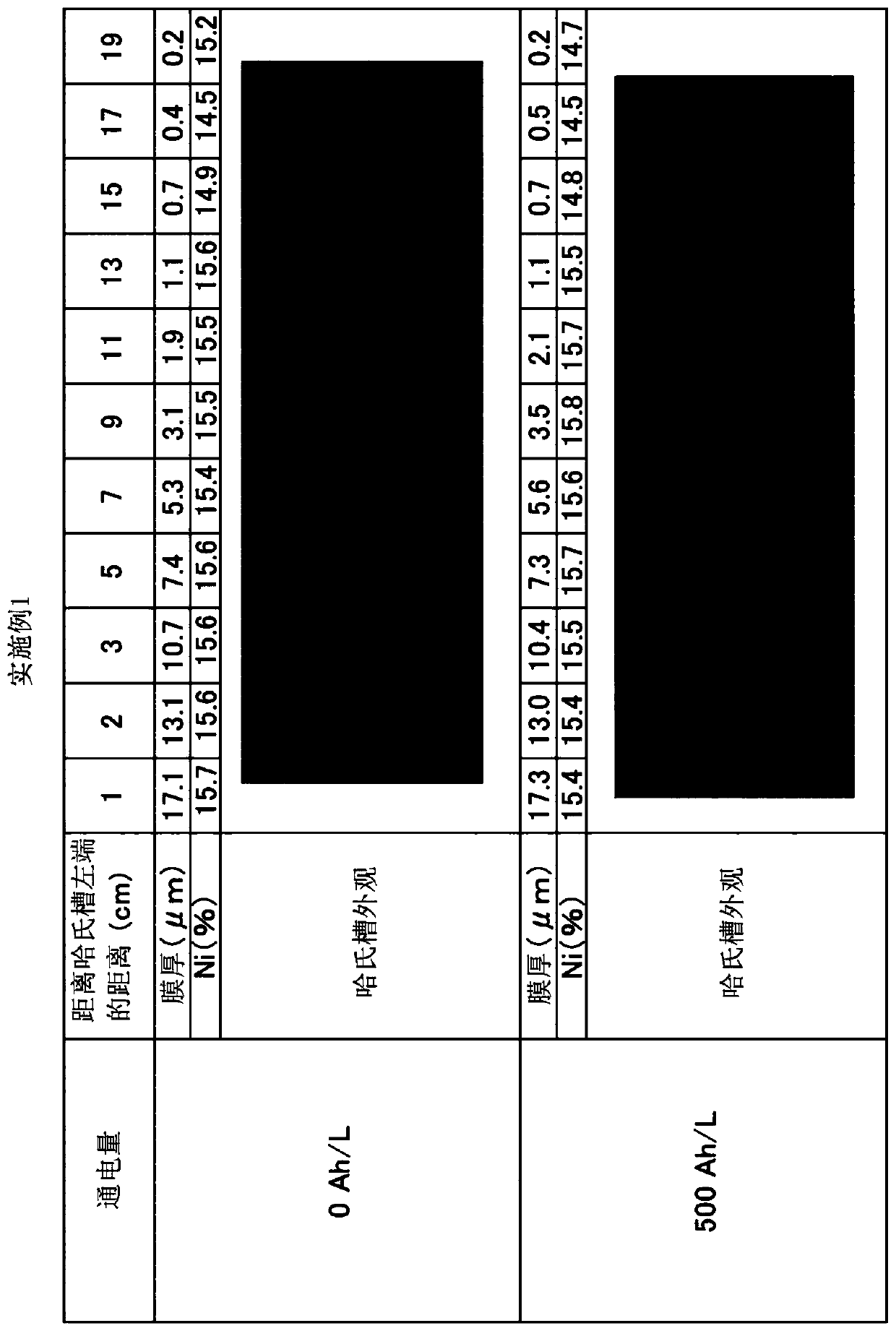
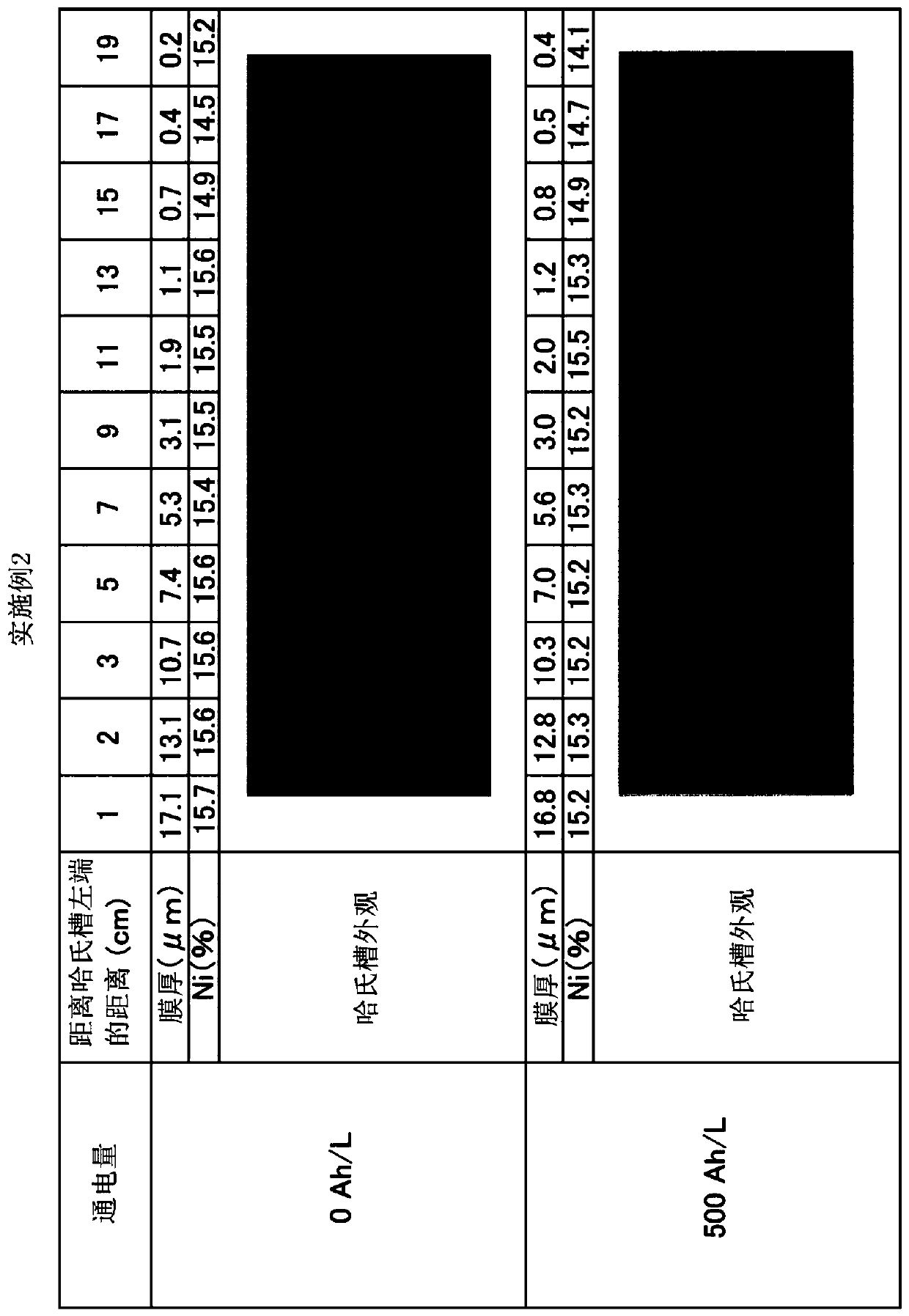
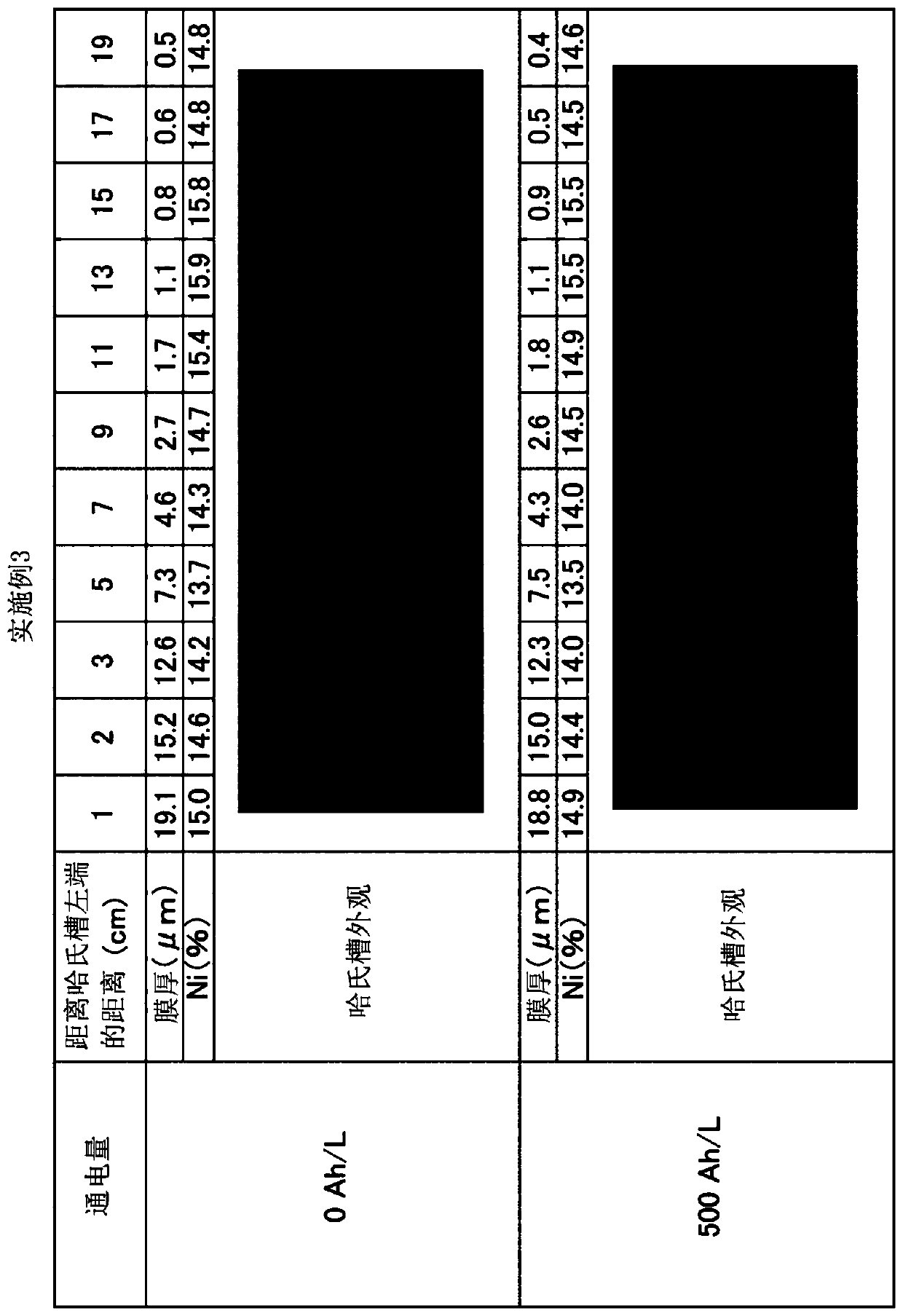
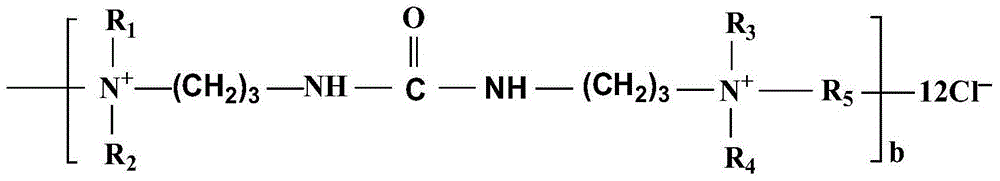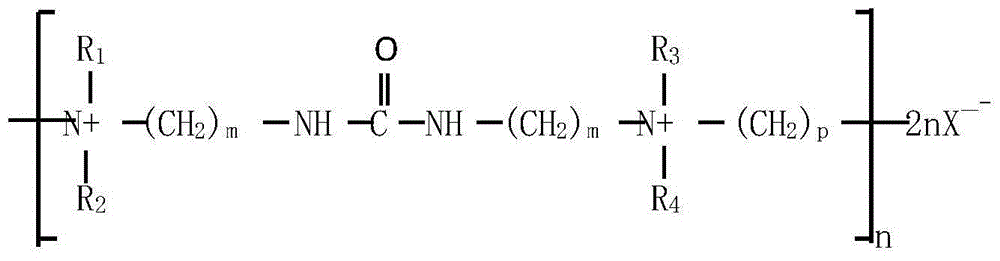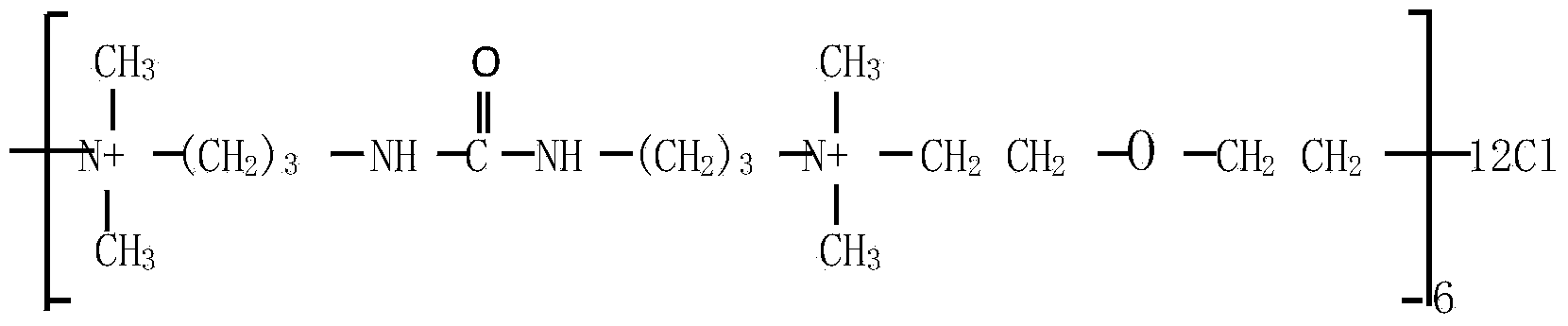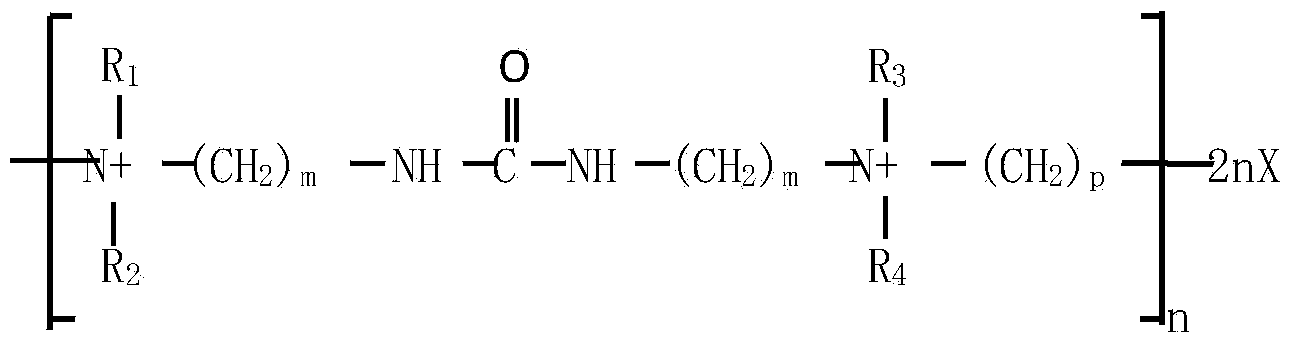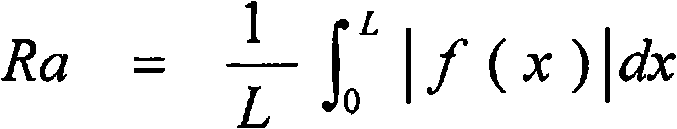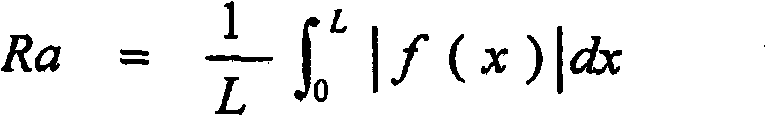Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
64 results about "Zinc alloy electroplating" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Zinc alloy electroplating is an electrogalvanization process for corrosion protection of metal surfaces and increasing their wear resistance.
Zinc and zinc alloy electroplating additives and electroplating methods
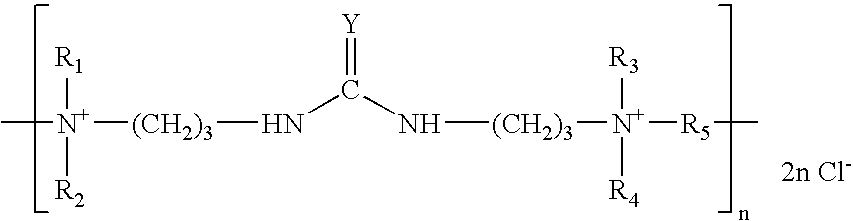
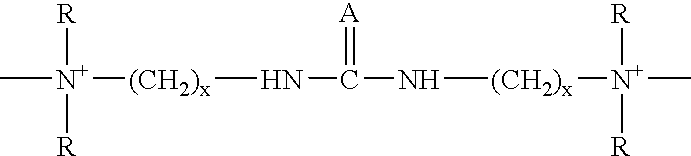
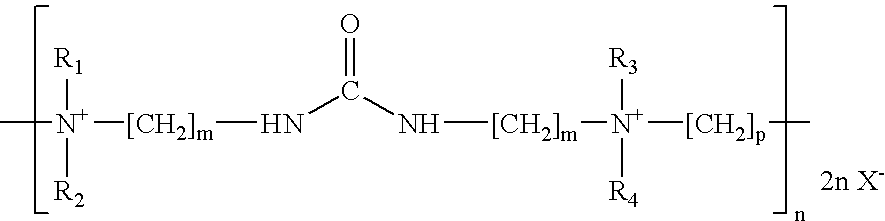
An additive for an alkaline zinc or zinc alloy electroplating bath medium, the additive comprising a random co-polymer comprising the reaction product of: (i) one or more di-tertiary amines including an amide or thioamide functional group, and (ii) optionally, one or more saturated second di-tertiary amines and / or one or more second di-tertiary amines including an unsaturated moiety, with (iii) one or more saturated or unsaturated linking agents capable of reacting with said di-tertiary amines (i) and (ii), provided that, where all the linking agents are saturated, an unsaturated di-tertiary amine must he present. Preferably, the polymer has the general formula n(2x+2y+zEp)j-.
Owner:MACDERMID ACUMEN INC
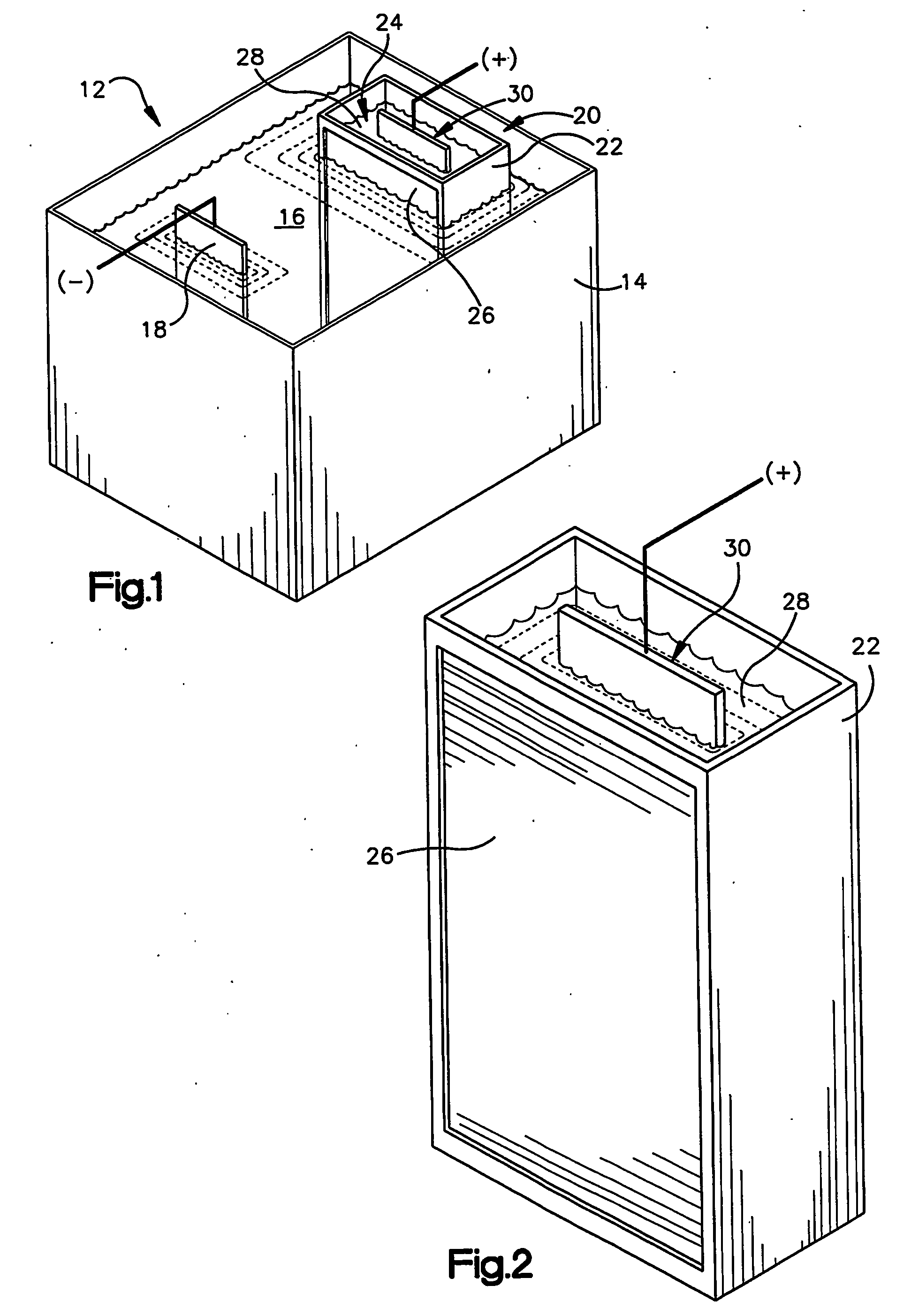
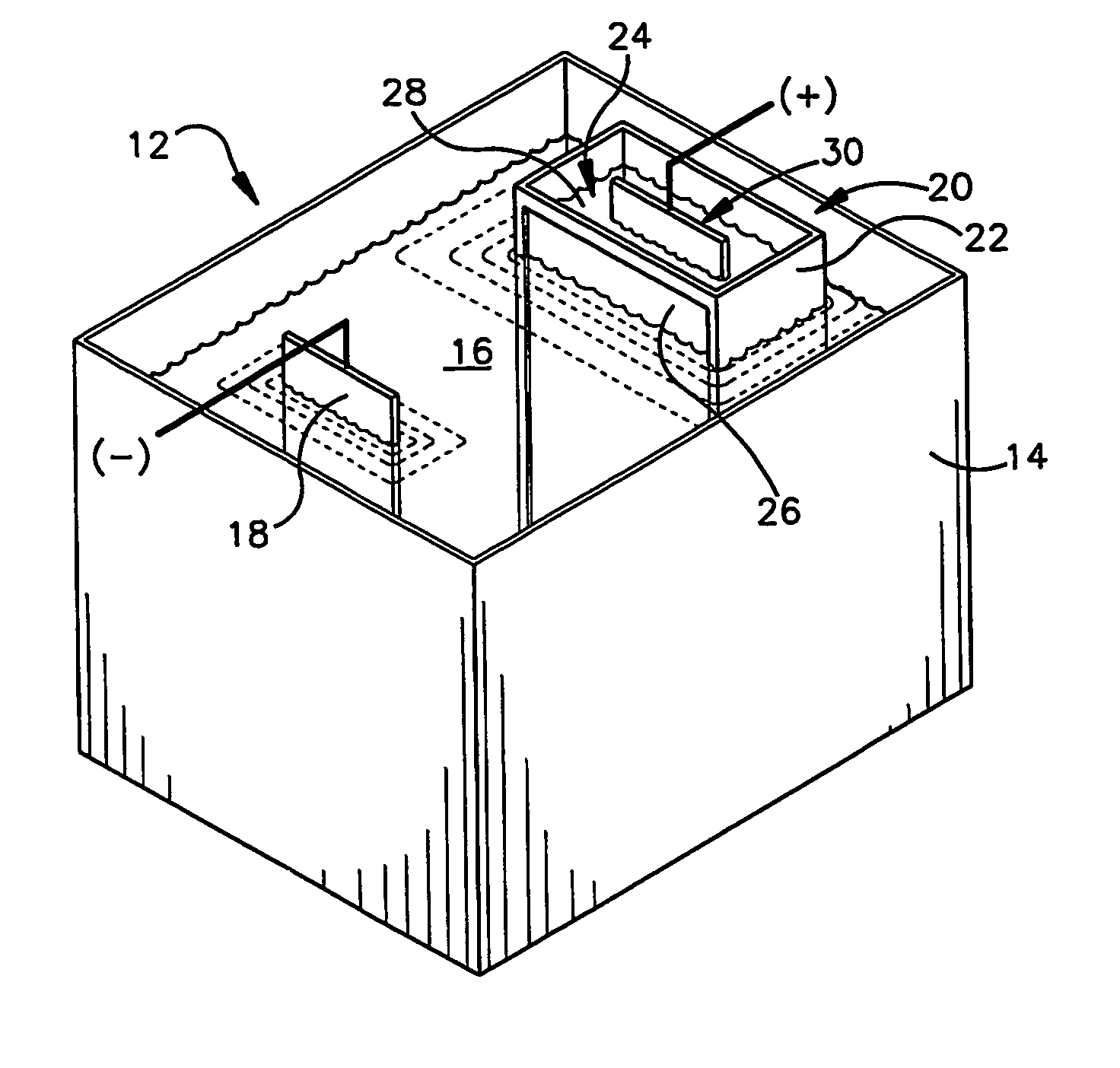
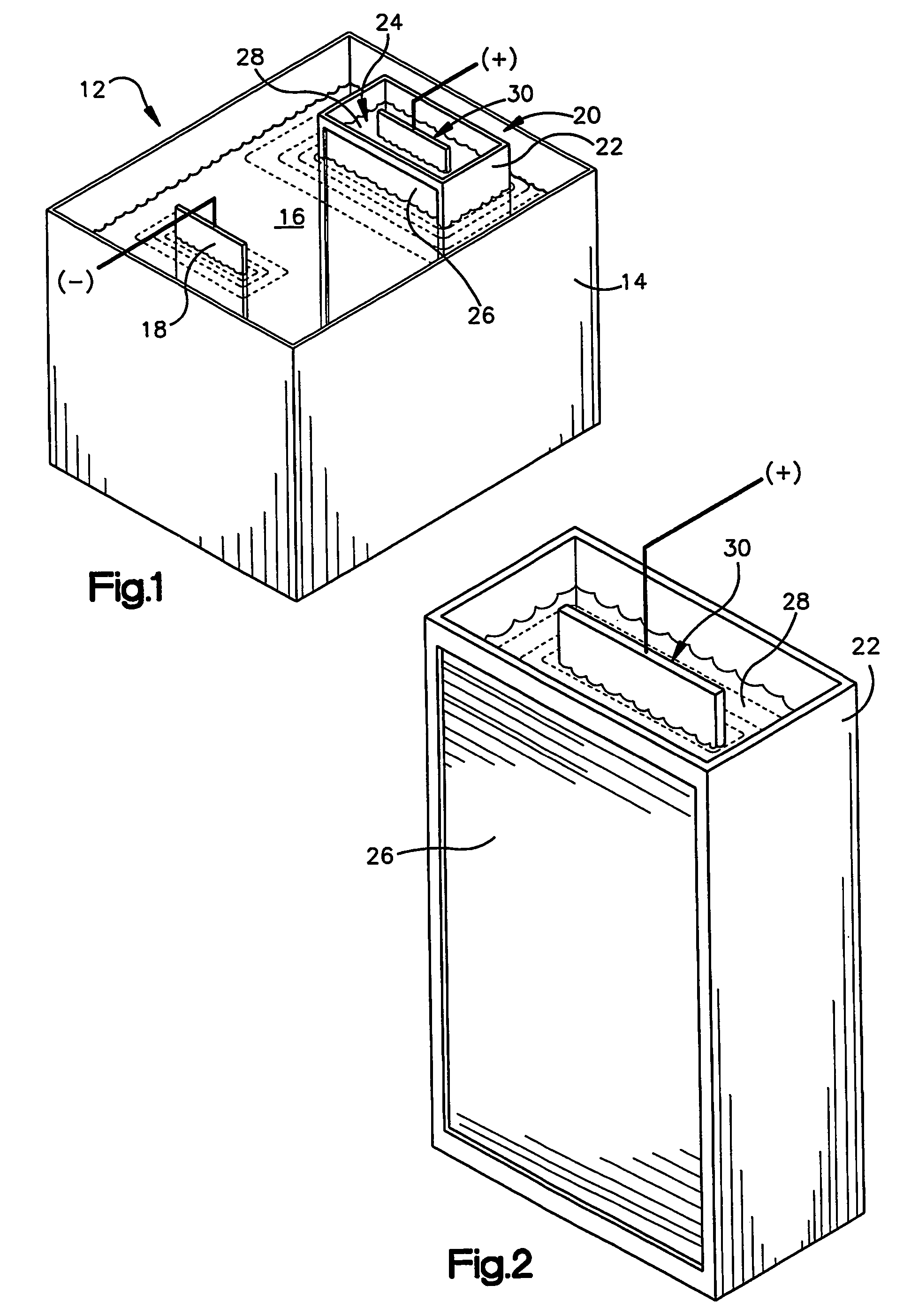
Zinc and zinc-alloy electroplating
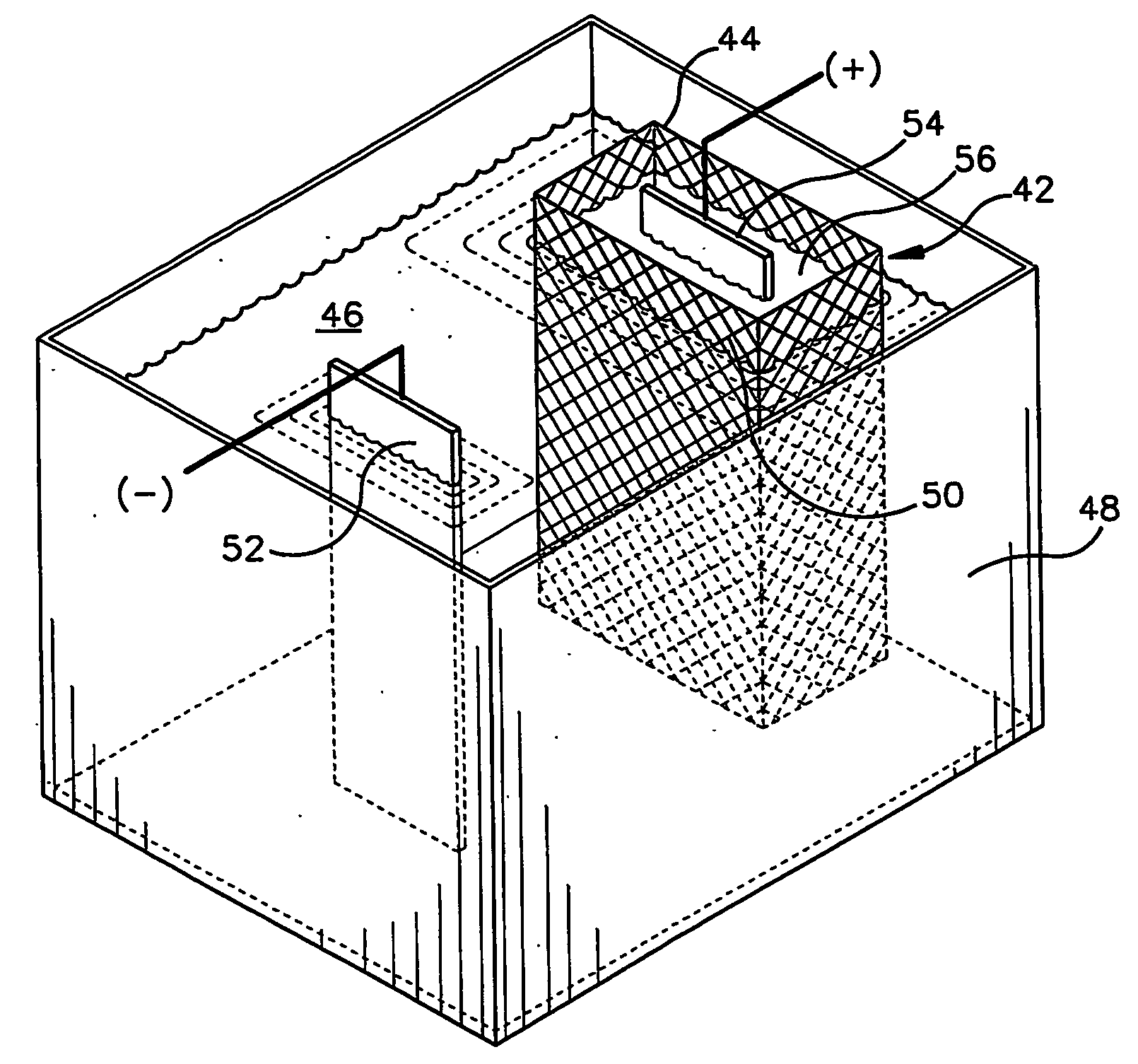
An apparatus (12) for applying a zinc or zinc-alloy electroplate to a workpiece comprises an electroplating bath (16) having a pH more than about 14. The electroplating bath includes zinc ions and an additive. A cathode workpiece (18) is in the bath. An anode assembly (20) contacts the bath. The anode assembly includes an anolyte and an insoluble metal anode in the anolyte. The additive is capable of electrolytically breaking down upon contact with the anode. The anode assembly inhibits the electrolytic breakdown of the additive.
Owner:MACDERMID INC +1
Surface treatment method for zinc alloy electroplating of automobile permanent magnetic material
The invention provides a surface treatment method for zinc alloy electroplating of an automobile permanent magnetic material. The surface treatment method comprises the following steps: (1) chamfering and polishing: carrying out conventional polishing on the permanent magnetic material by adopting mechanical vibration grinding and rolling grinding chamfering methods; (2) degreasing and oil removal: adding sodium phosphate, sodium carbonate or sodium hydroxide, and carrying out conventional degreasing and oil removal on the permanent magnetic material after polishing; (3) pickling and derusting: adding a nitric acid solution, and carrying out conventional pickling and derusting on the permanent magnetic material after degreasing and oil removal; (4) electrogalvanizing to form a galvanized layer: carrying out electrogalvanizing bottoming on the permanent magnetic material after pickling and derusting by adopting a galvanizing liquid to form the galvanized layer; and (5) zinc-nickel alloy electroplating to form an alkaline zinc-nickel alloy electroplated layer: adopting a zinc-nickel alloy electroplating liquid to electroplate the permanent magnetic material formed with the galvanized layer to form an alkaline zinc-nickel alloy electroplated layer. The surface treatment method has the advantages of good plated layer binding force, and high corrosion resistance.
Owner:TIANJIN SANHUAN LUCKY NEW MATERIAL CO LTD +1
Electroplating solution composition for organic polymer-zinc alloy composite plating and plated metal material using such composition
Disclosed is an electroplating solution composition which enables to obtain a plating with excellent corrosion resistance which exhibits excellent adhesion with a coating film without being subjected to a surface treatment. Such an electroplating solution composition for an organic polymer-zinc alloy composite plating is characterized by containing (A) 1-600 g / l of Zn ions, (B) 1-600 g / l of iron group element ions, (C) 0.1-200 g / l of a tungstic acid compound as W ions and (D) 0.5-500 g / l of a water-soluble or water-dispersible organic polymer compound having a number-average molecular weight of 1,000-1,000,000.
Owner:KANSAI PAINT CO LTD
Tin-zinc alloy electroplating method
The invention aims to provide a tin-zinc alloy electroplating method. The method is performed under the following conditions: plating bath temperature: 15-25 DEG C; plating solution stirring speed: 50-200m / min; and cathodic current density: 5-100A / dm2. The tin-zinc alloy electroplating method is obtained by selection of a plating bath temperature, a cathode current density and the like and the use of a specific tin-zinc alloy plating bath, and the tin-zinc alloy plating obtained using the method has strong corrosion resistance and reduced pollution, and is an ideal substitute for cadmium plating.
Owner:东莞市闻誉实业有限公司
Electroplating solution composition for organic polymer-zinc alloy composite plating and plated metal material using such composition
InactiveUS20070170067A1Improve adhesionImprove corrosion resistanceElectrolytic coatingsWater dispersibleIron group
An object of the invention is to provide an electroplating solution composition capable of obtaining a plated film excellent in adhesiveness to a coated film and excellent in corrosion resistance without surface treatment. The present invention relates to an organic polymer composite zinc alloy electroplating solution composition containing: (A) 1 to 600 g / l of Zn ion, (B) 1 to 600 g / l of an iron-group-element ion, and (C) 0.1 to 200 g / l, in terms of W ion, of tungstic acid-based compound, and (D) 0.5 to 500 g / l of water-soluble or water-dispersible organic polymer compound having a number average molecular weight of 1,000 to 1,000,000.
Owner:KANSAI PAINT CO LTD
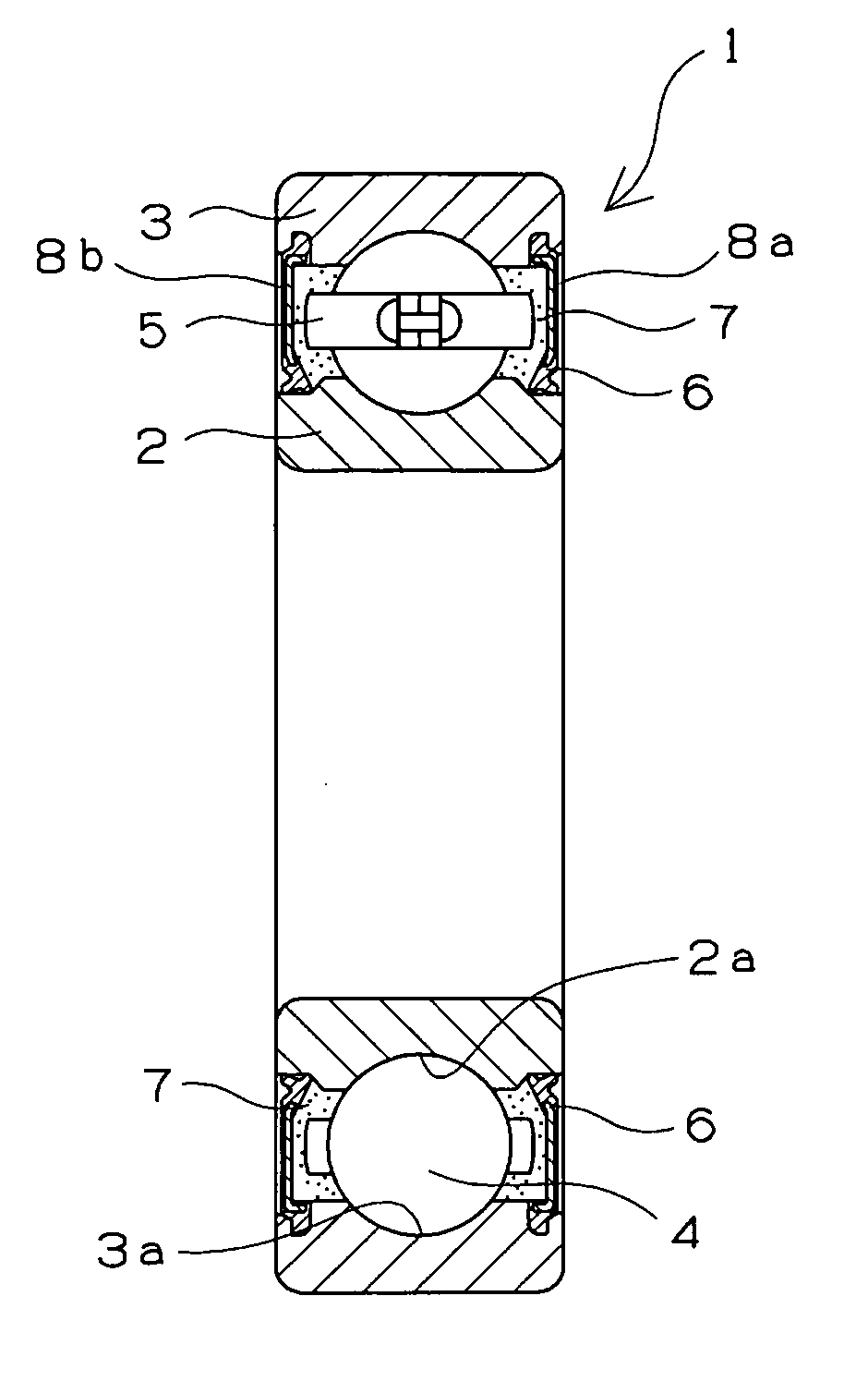
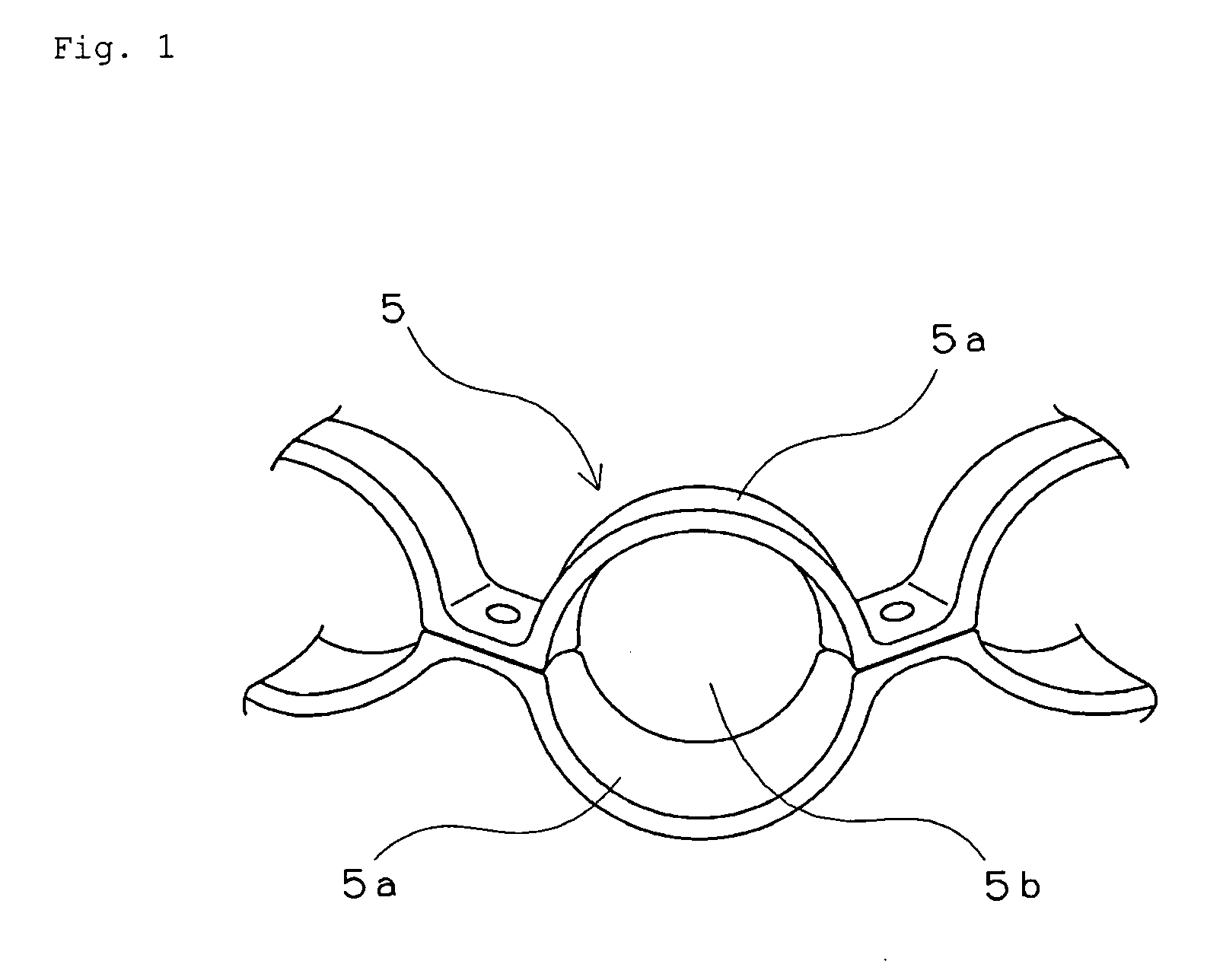
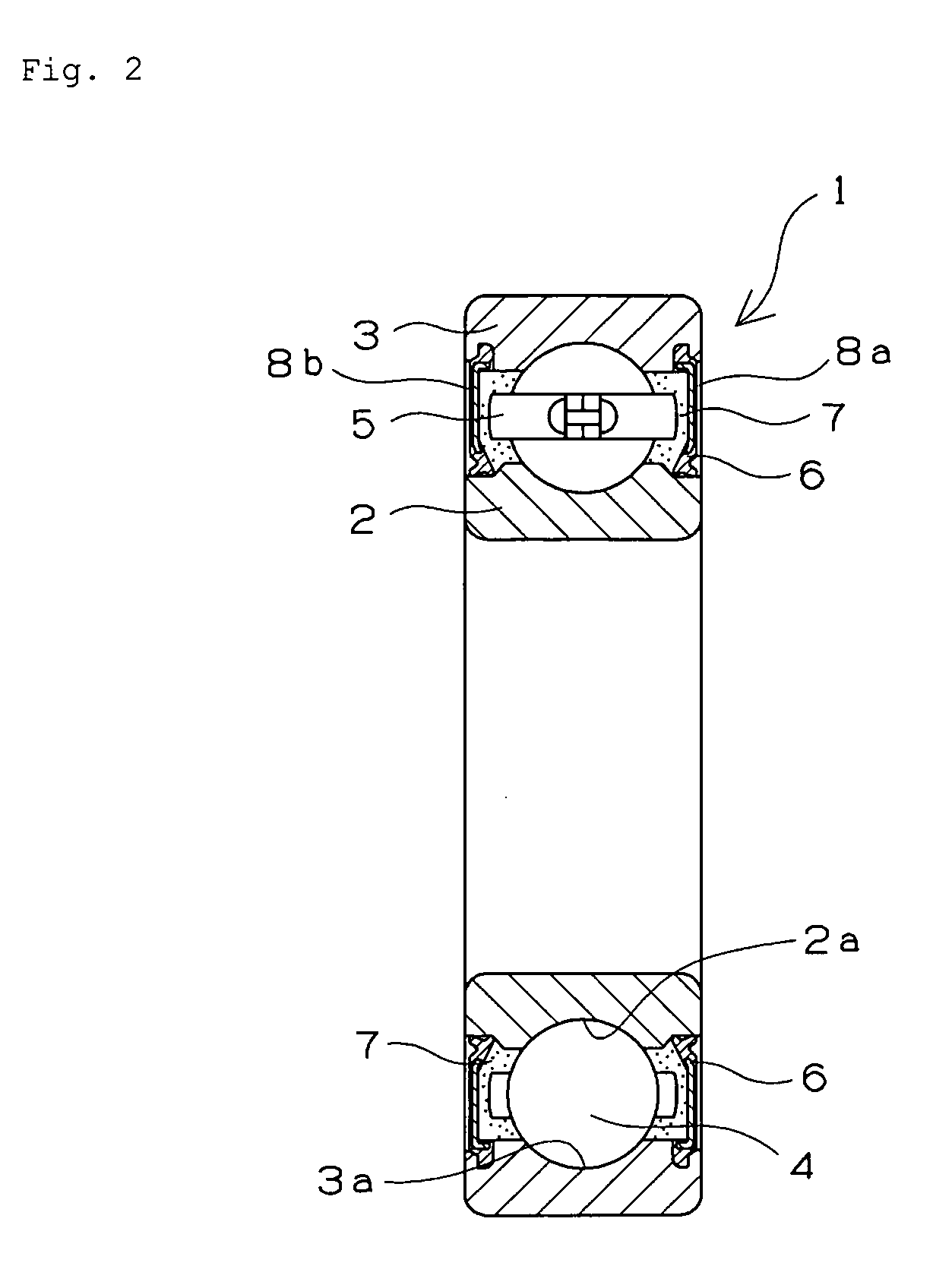
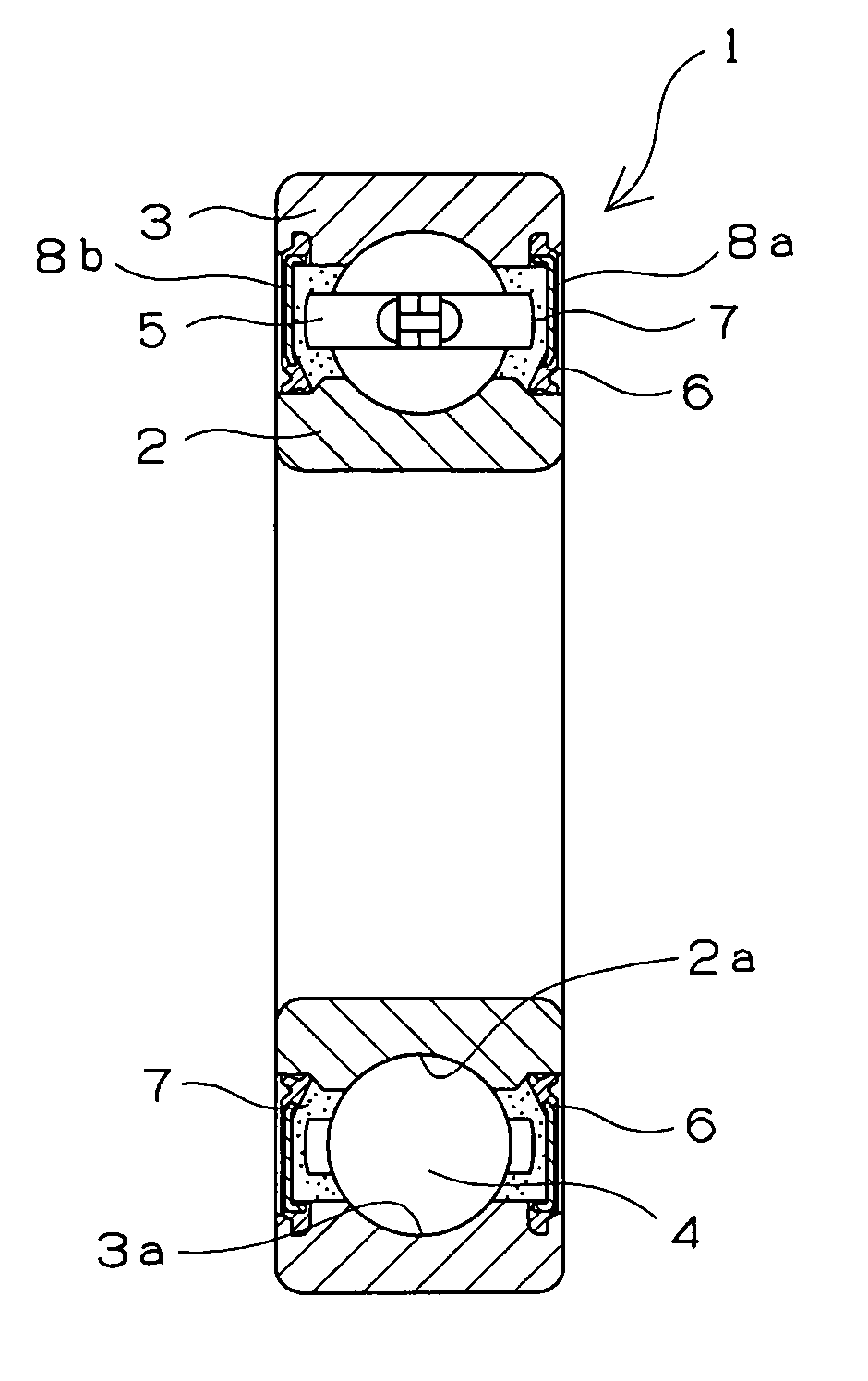
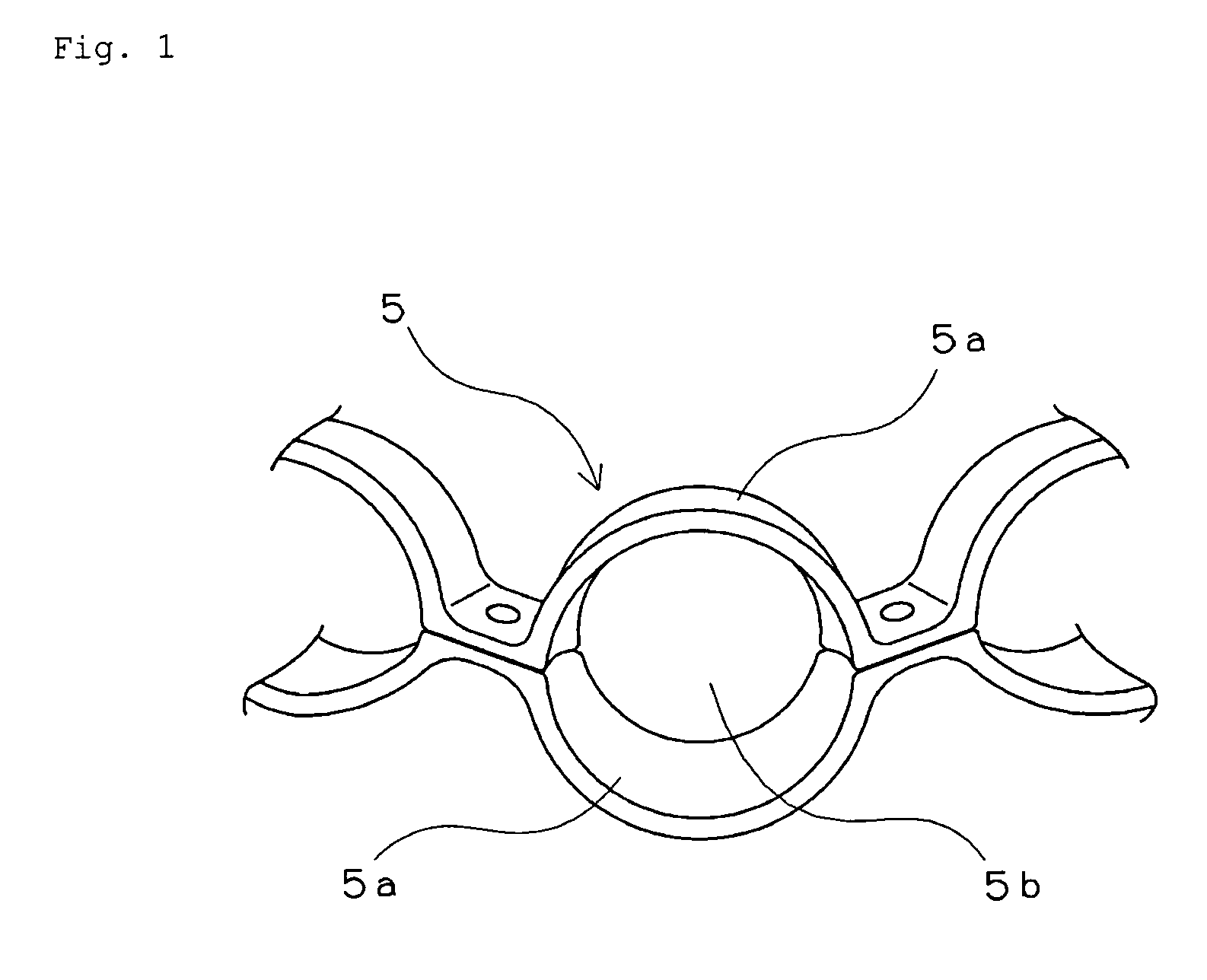
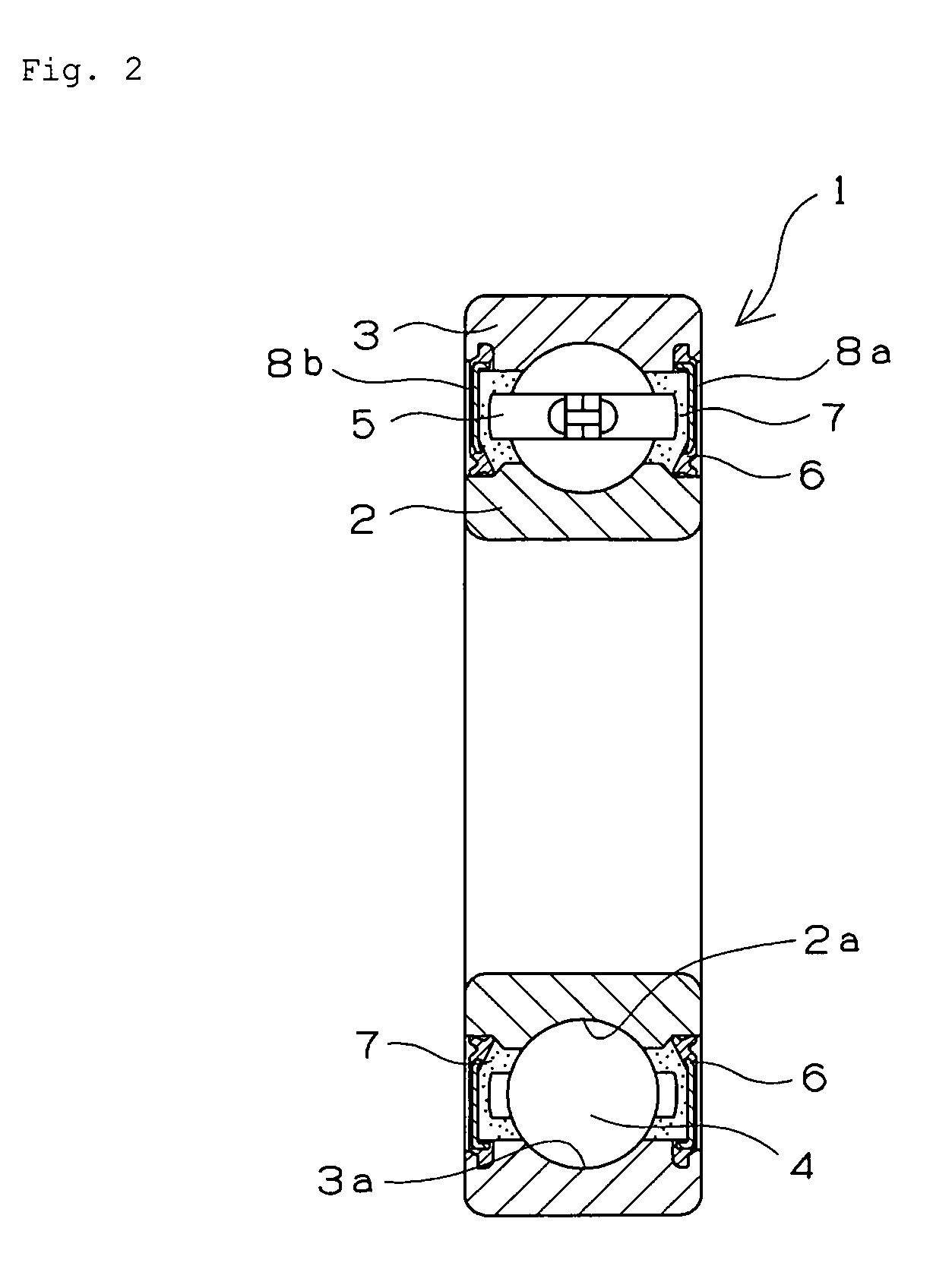
Retainer for rolling bearing and rolling bearing
ActiveUS20100092121A1Hot-dipping/immersion processesBall bearingsHazardous substanceRolling-element bearing
The present invention provides a rolling bearing which does not contain a hazardous substance and thus does not pollute environment. The present invention also provides a retainer, for holding rolling elements of the rolling bearing, which is made of a cold rolled steel plate. The retainer is subjected to at least one kind of surface treatment selected from among electrogalvanizing, electrotinning, electrolytic tin-zinc alloy plating, electrolytic zinc-iron alloy plating, and electrolytic zinc-nickel alloy plating. The retainer is further chromated with trivalent chromium. The rolling bearing uses the retainer for the rolling bearing.
Owner:NTN CORP
Tin-zinc alloy electroplating method
Disclosed is an electroplating method realizing short-time processing which has been difficult for conventional tin-zinc alloy electroplating methods. Specifically disclosed is a method for performing tin-zinc alloy electroplating under the following conditions: the plating liquid temperature is 30-90 DEG C; the stirring rate of the plating liquid is 5-300 m / min; and the cathode current density is 5-200 A / dm<2>. Preferably, the divalent tin ion concentration in the tin-zinc alloy plating bath is 1-100 g / L, while the zinc ion concentration is 0.2-80 g / L.
Owner:DISPOL CHEMICALS CO LTD
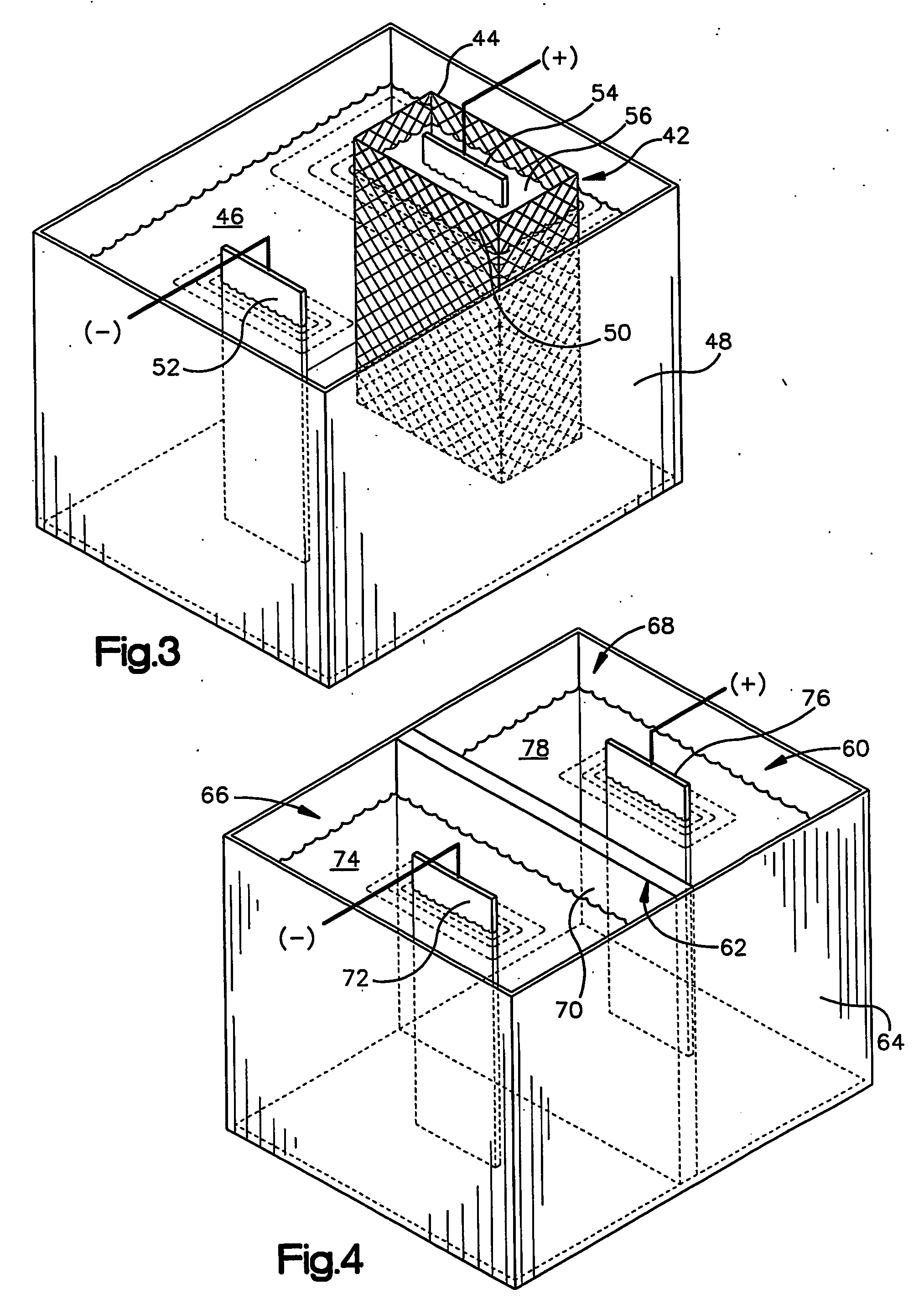
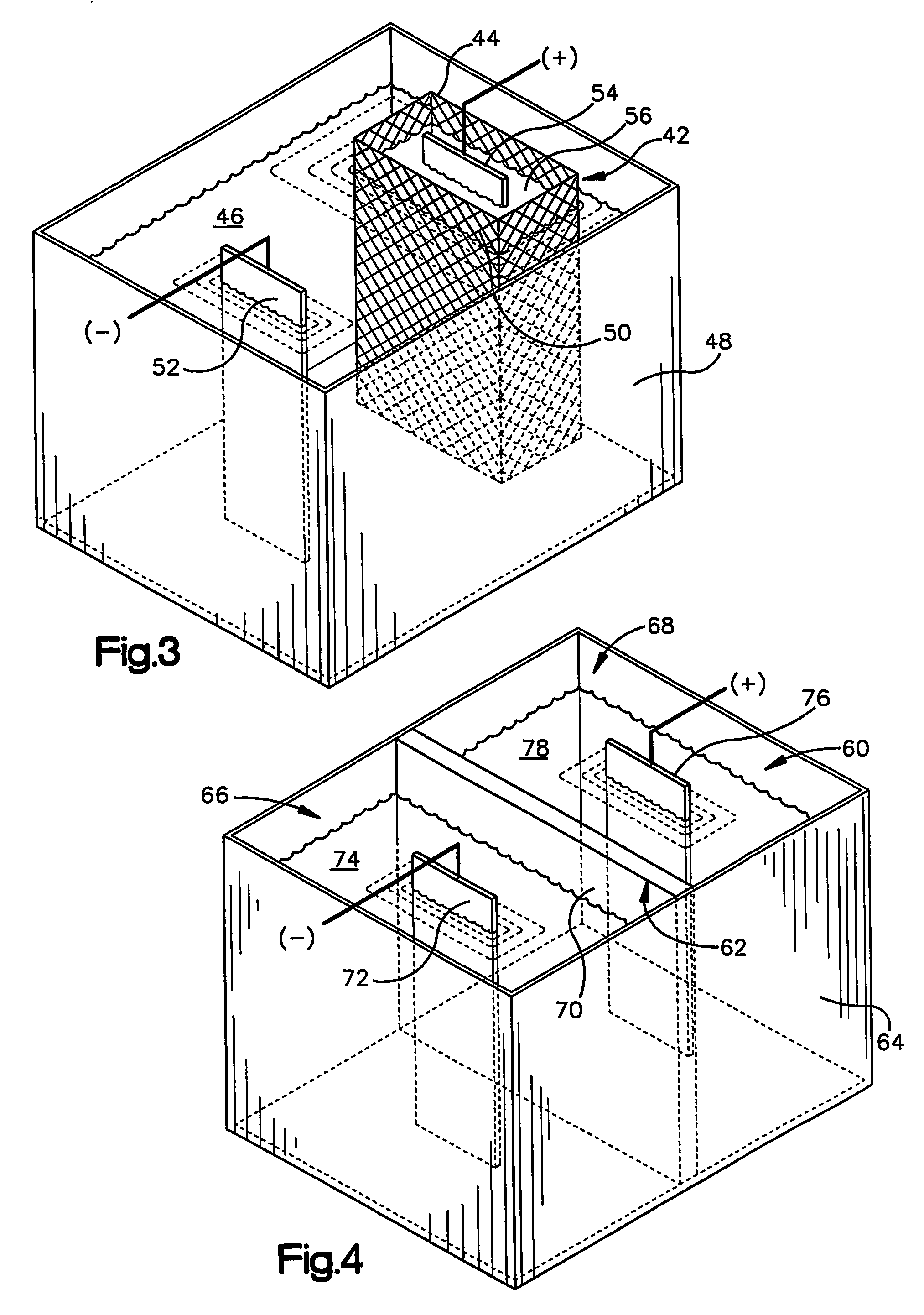
Zinc alloy electroplating baths and processes
The present invention relates to the electrodeposition of zinc nickel alloy on a variety of electrically conducting substrates in a medium which seeks to provide improved deposit distribution and operable current density range. This is achieved through a bath comprising zinc ions, nickel metal ions, and a suitable combination of one or more urea based polymers and non-polymeric complexing agents capable of holding nickel metal ions in alkaline solution and an electrolytic process whereby the bath is used to electrodeposit zinc nickel alloy on electrically conducting substrates.
Owner:MACDERMID INC
Carrier brightener precursor and carrier brightener for alkaline zinc-plating or zinc alloy electroplating solution and electroplating solution
The invention discloses a carrier brightener precursor for an alkaline zinc-plating or zinc alloy electroplating solution. The carrier brightener precursor is obtained by carrying out a one-time chemical reaction of a mixed amine of one amide functional group-containing ditertiary amine and one or more amide functional group-containing monotertiary amines. The invention also discloses a carrier brightener for the alkaline zinc-plating or zinc alloy electroplating solution. The carrier brightener is a random intercondensation polymer; and components participating copolycondensation mainly comprise the following two components: the carrier brightener precursor mixed amine and one or more bridging agents. The intercondensation polymer used as the carrier brightener of the alkaline zinc-plating or zinc alloy electroplating solution has quite good compatibility with a main brightening agent benzyl pyridinium-3-carboxylate which is widely used at present, dependence on sulfonated aromatic aldehyde can be reduced, on one hand, the tendency of blistering of a plating layer is overcome, an operating window is expanded, and at the same time, the dispersion property of the plating solution is relatively improved; and a high-brightness electroplating layer having high dispersibility and high covering power also can be obtained, and the plating layer has excellent spalling (foaming) resistant performance.
Owner:SHAOGUAN MEITUO WEIZHI CHEM
Carrier brightening agent as well as preparation method and application thereof
The invention discloses carrier brightening agent for alkaline galvanization and zinc alloy electroplate liquid. The carrier brightening agent is a random copolycondensate; and the following three components are mainly participated in copolycondensation: (a), one or more di-tertiary amine containing amide functional groups; (b), one or more single tertiary amine containing amide functional groups; and (c), one or more coupling agent-dihalogenation compounds. The carrier brightening agent has good compatibility with key light agent benzyl pyridine onium-3-carboxylate generally used at present, so that the dependence on sulfonated aromatic aldehyde can be reduced; the foaming trend of clad layers is overcome; the operation window is widened; and the dispersing performance of the plating liquid is improved synchronously. Bright electric plating layers with high dispersibility and high covering force can be obtained; and more importantly, the stripping (foaming) resistance of the plating layers is excellent.
Owner:SHAOGUAN MEITUO WEIZHI CHEM
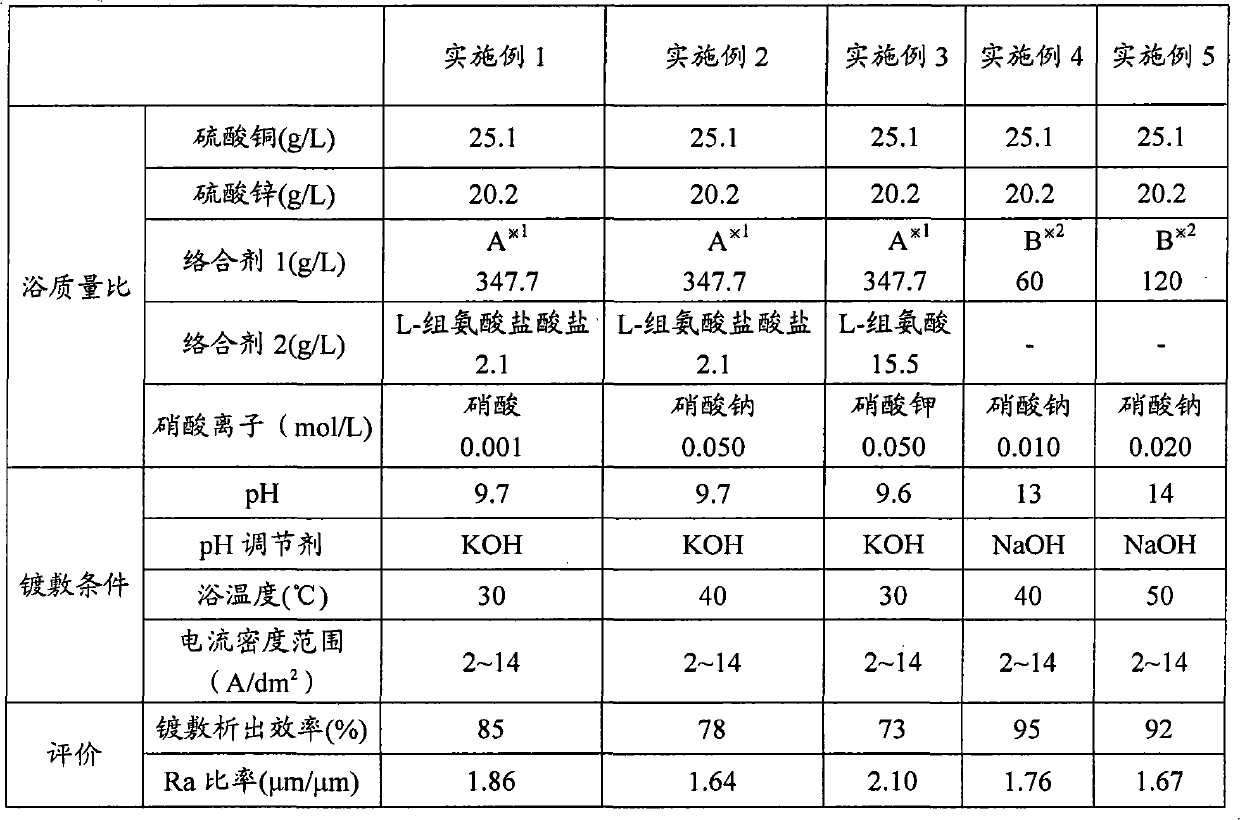
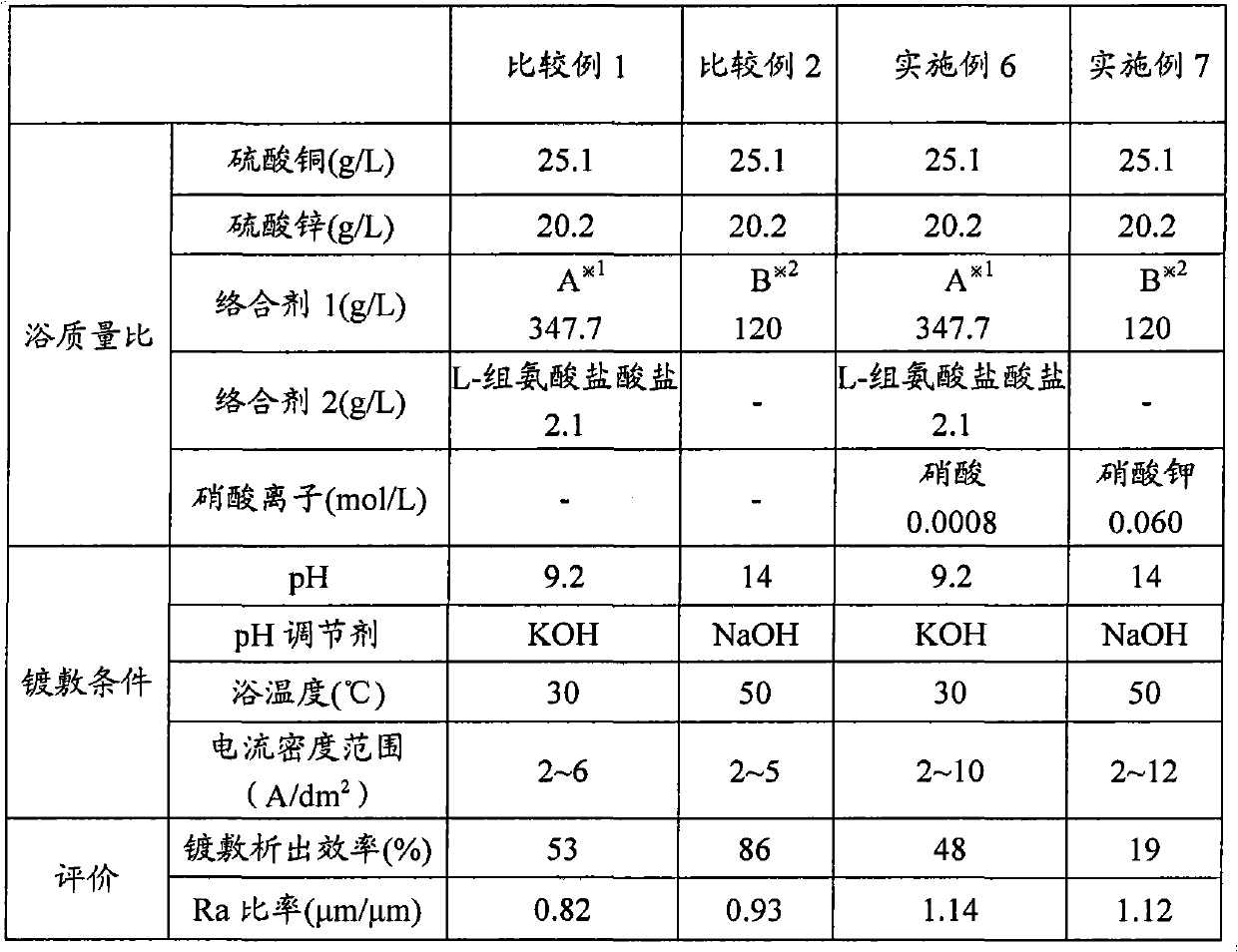
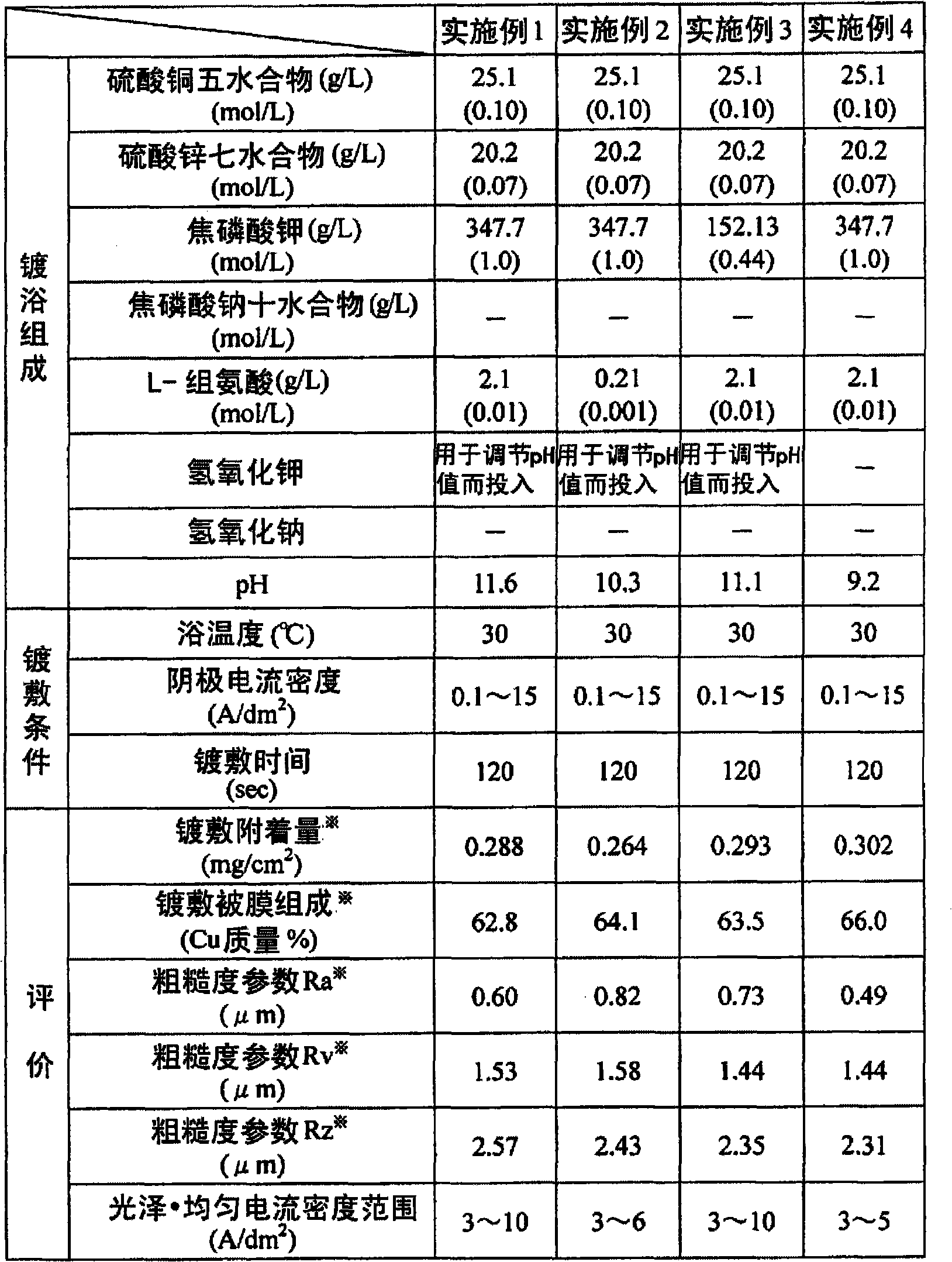
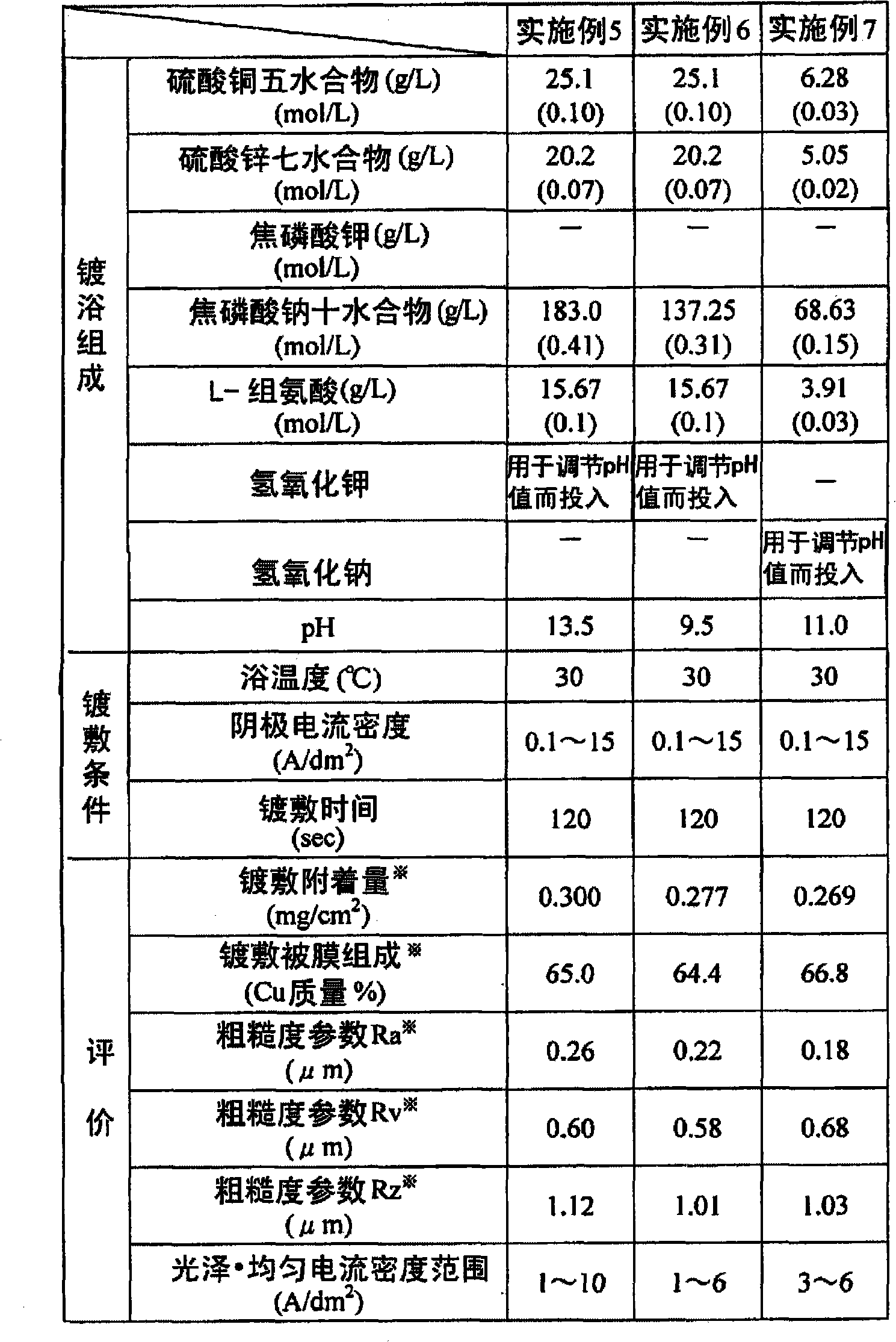
Copper-zinc alloy electroplating bath and plating method using same
Disclosed is a copper-zinc alloy electroplating bath, and a plating method using the same, capable of forming a uniform, brilliant plated layer of the intended composition and having a wide current density range, without the use of a cyanogen compound. The copper-zinc alloy electroplating bath contains a copper salt, a zinc salt, a pyrophosphoric acid alkali metal salt or a tartaric acid alkali metal salt, and nitrate ions. It is preferable that the concentration of the aforementioned nitrate ions be 0.001-0.050 mol / L. Furthermore, it is preferable that the pH of the aforementioned copper-zinc alloy electroplating bath be in the range of 8-14. Furthermore, in addition to the copper salt, zinc salt, pyrophosphoric acid alkali metal salt, and nitrate ions, it is preferable that an amino acid or at least one salt thereof be included, and histidine can be used favorably as the aforementioned amino acid.
Owner:BRIDGESTONE CORP
Nickel plating solution
The invention discloses a nickel plating solution, which includes 90-150g / L nickel sulfate, 45-65g / L sodium chloride, 5-12g / L cobalt chloride, 80-130g / L sodium citrate, a 0.5ml / L neutral nickel brightener, and a 35ml / L neutral nickel prepared solution. The making method of the nickel plating solution consists of: for instance in preparation of 1L of the plating solution, adding 500ml of water, conducting heating to 70DEG C, adding 80-130g of sodium citrate, then adding 90-150g of nickel sulfate and 5-12g of cobalt chloride, stirring the materials evenly, performing dissolving to be clear, adding 45-65g of sodium chloride, and performing dissolving to be clear, then adding 35ml of the prepared solution and 0.5ml of the brightener, adding water to 1000ml, and adjusting the pH value to 6.5-7.0. The nickel plating solution provided by the invention can realize direct nickel plating on zinc, the plating layer is full, pure white, bright, and has little smell, also the defect of required operation on a cyanide copper plating layer during traditional zinc alloy nickel plating can be prevented. Thus, the sewage treatment is simple, the cost is saved and a more environment-friendly effect can be achieved.
Owner:金华市东恩环保科技有限公司
Cyanide-free copper-zinc alloy electroplating solution and preparation method thereof
The invention relates to a cyanide-free copper-zinc alloy electroplating solution and a preparation method thereof. The electroplating solution consists of the following components in percentage by weight: 1-60 percent of complexing agent, 0.3-10 percent of copper salt, 0.2-10 percent of zinc salt and the balance of water, wherein the general formula of the complexing agent is MxHyPnO(3n+1)Rz; M is any one or multiple in alkali metal ions and NH<4+>; R is acyl; the general formula of the copper salt is Cux / 2HyPnO(3n+1)Rz; the general formula of the zinc salt is Znx / 2HyPnO(3n+1)Rz; x, n and z are positive integers; y is 0 or a positive integer; x+y+z is equal to n+2. The cyanide-free copper-zinc alloy electroplating solution is prepared by mixing the complexing agent, the copper salt, the zinc salt and water, the complexing capacity of the complexing agent is high, the complexing constant of copper ions can be 10<26-27>, and the complexing agent is far superior to a conventional complexing agent in the prior art. The stability of the electroplating solution prepared from the complexing agent is greatly improved, the quality of the electroplating solution is high, when the cyanide-free electroplating solution is used for pre-plating, main salt metal ions in the electroplating solution are not subjected to a replacement reaction with a metal base material, a loose replacement layer structure is not formed, and the quality of the electroplating layer is greatly improved.
Owner:浙江洽福科技有限公司
Copper-zinc alloy electroplating bath and plating method using the same
InactiveUS20110052937A1Large current density rangeThin material handlingMetal layered productsCyanidePyrophosphate
Disclosed is a cyanide-free copper-zinc alloy electroplating bath which can form a uniform and glossy plated layer having the desired composition in a large current density range, and a plating method using the same.The copper zinc alloy electroplating bath contains a copper salt, a zinc salt, an alkali metal pyrophosphate or an alkali metal tartrate, and nitrate ions. The concentration of the nitrate ions is preferably 0.001 to 0.050 mol / L. Further, the pH of the copper-zinc alloy electroplating bath is preferably in the range of 8 to 14. Furthermore, in addition to the copper salt, the zinc salt, the alkali metal pyrophosphate and the nitrate ions, at least one selected from amino acids or salts thereof is preferably included, and histidine can be used favorably as the amino acid.
Owner:BRIDGESTONE CORP
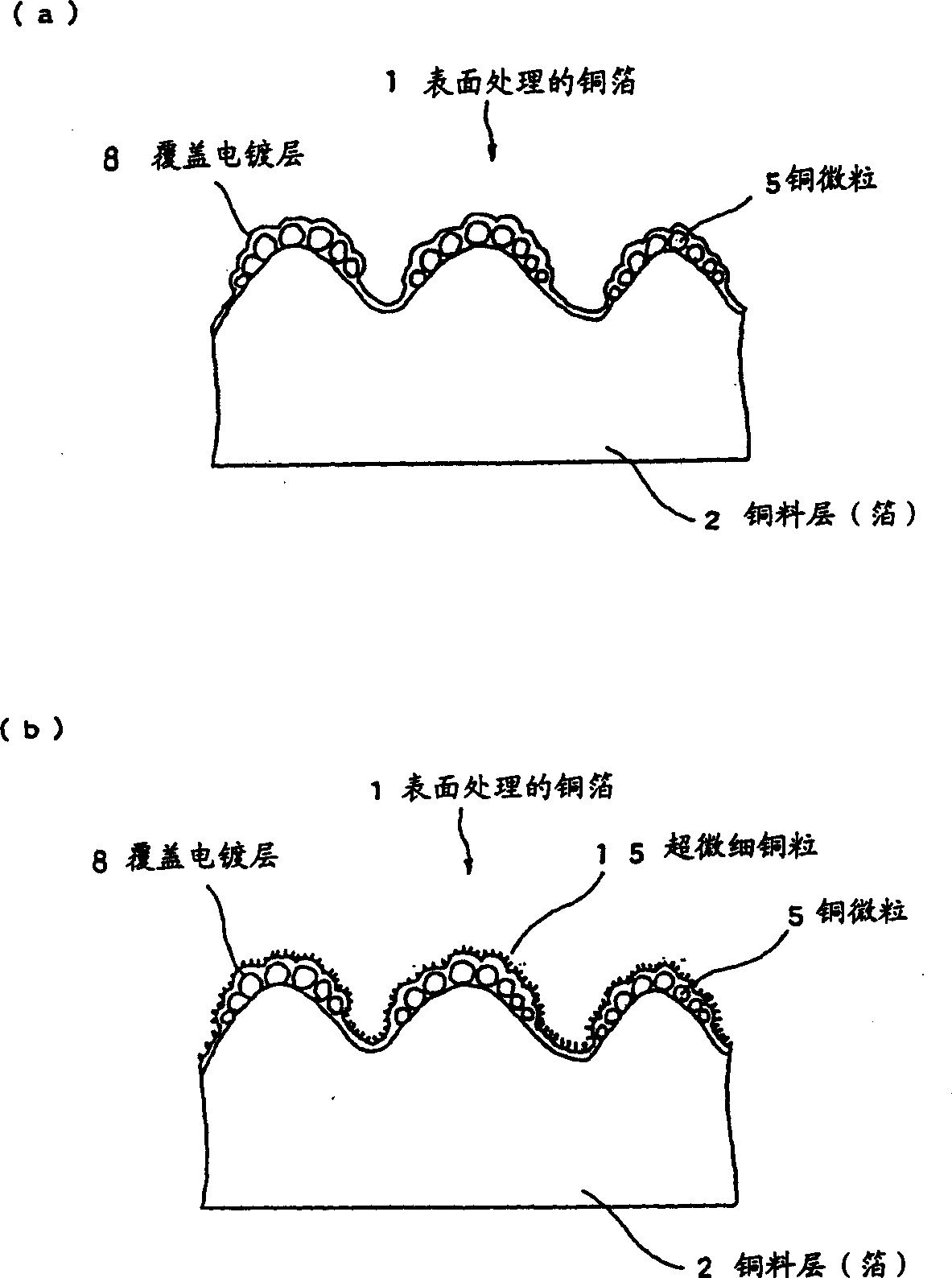
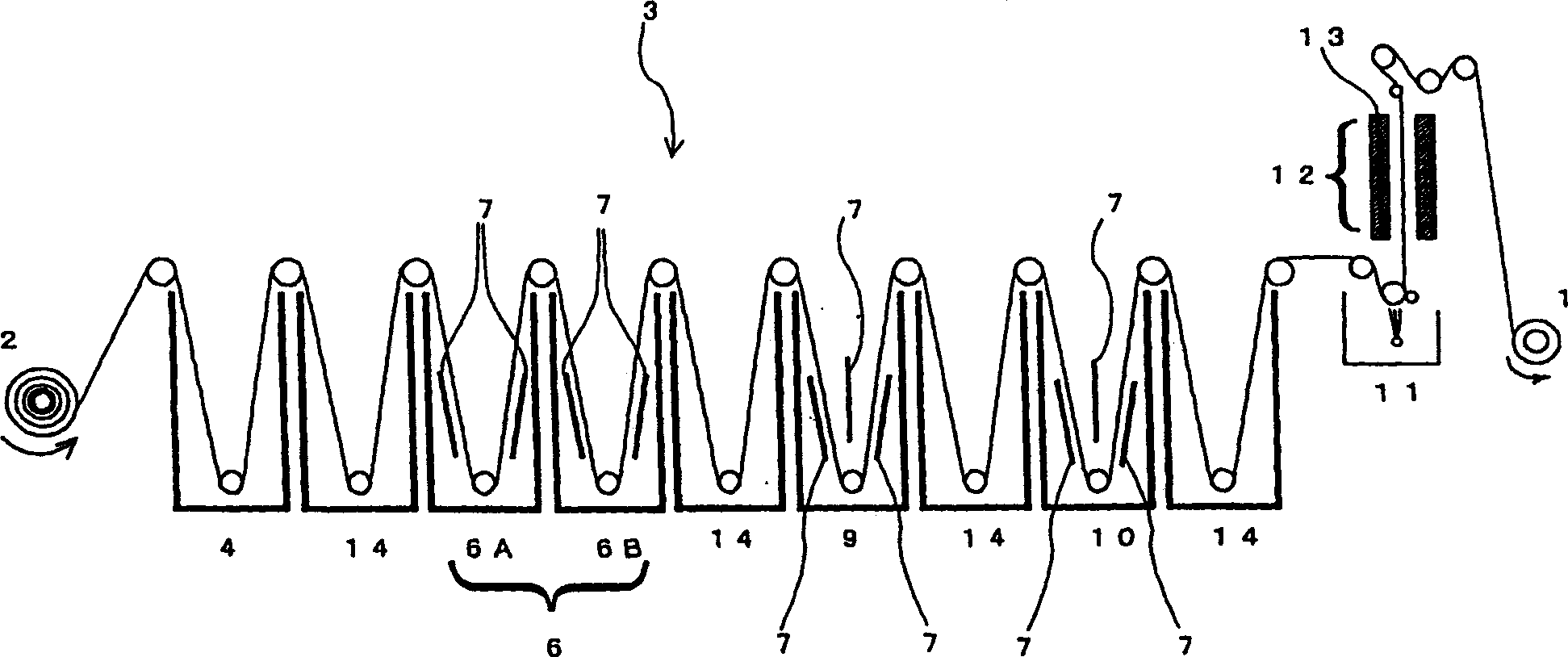
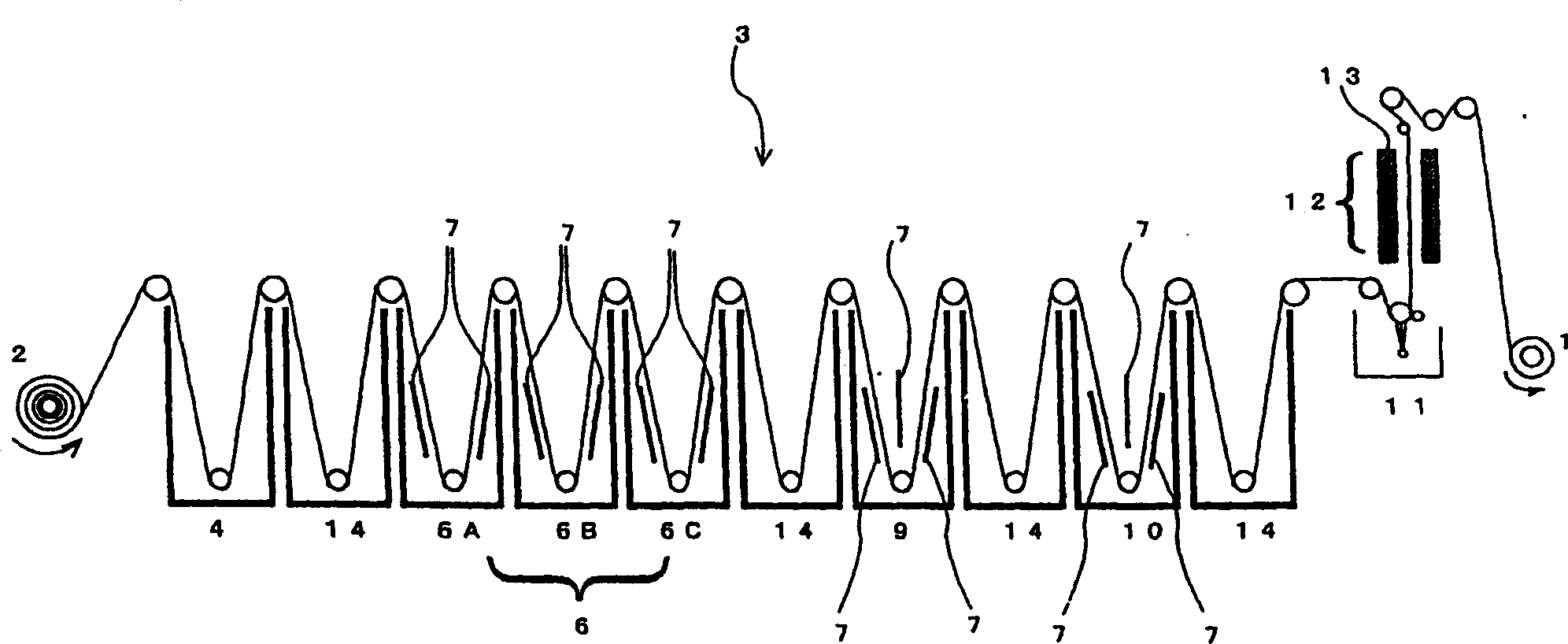
Surface treated copper foil and mehtod for preparing the same and copper-clad laminate using the same
InactiveCN1358410AImprove adhesionImprove stabilityInsulating substrate metal adhesion improvementPrinted circuit aspectsHeat resistanceMoisture resistance
An object of the invention is to provide a surface-treated copper foil capable of consistently attaining a percent loss in peel strength against hydrochloric acid degradation of 10% or less as measured on a copper pattern prepared from the copper foil and having a line width of 0.2 mm, by bringing out the maximum effect of the silane coupling agent employed in zinc-plated or zinc-alloy-plated anti-corrosive copper foil. Another object is to impart excellent moisture resistance, heat resistance, and long-term storage stability to the surface-treated copper foil. In order to attain these objects, the invention provides a surface-treated copper foil for producing printed wiring boards which has been subjected to nodular treatment and anti-corrosion treatment of a surface of a copper foil, wherein the anti-corrosion treatment includes forming a zinc or zinc alloy plating layer on a surface of the copper foil; forming an electrolytic chromate layer on the zinc or zinc alloy plating layer; forming a chromic-ion-containing silane coupling agent-adsorbed layer on the electrolytic chromate layer; and drying the copper foil for 2-6 seconds such that the copper foil reaches 105° C.-200° C.
Owner:MITSUI MINING & SMELTING CO LTD
Copper-zinc alloy electroplating bath and method of plating using same
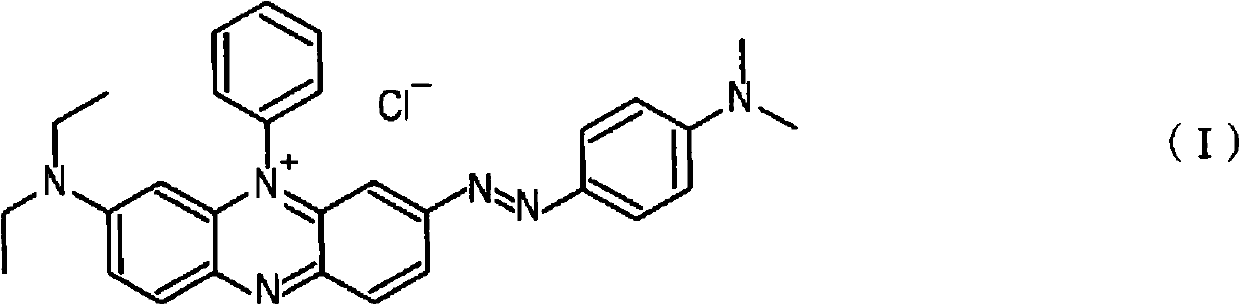
InactiveCN102341530ASurface roughness parameter reductionThin material handlingZinc alloysZinc alloy electroplating
A copper-zinc alloy electroplating bath with which a copper-zinc alloy deposit can be formed with improved throwing power, and a method of plating using the electroplating bath. The copper-zinc alloy electroplating bath contains, as an additive, at least one compound selected from a group consisting of compounds represented by formulae (I) to (III). These additives may be used alone or in combination of two or more thereof. (In formula (II), R1 is a lower alkylene, R2 is H or a lower alkyl, and the weight-average molecular weight is 103 to 105).
Owner:BRIDGESTONE CORP
Retainer for rolling bearing and rolling bearing
The present invention provides a rolling bearing which does not contain a hazardous substance and thus does not pollute environment. The present invention also provides a retainer, for holding rolling elements of the rolling bearing, which is made of a cold rolled steel plate. The retainer is subjected to at least one kind of surface treatment selected from among electrogalvanizing, electrotinning, electrolytic tin-zinc alloy plating, electrolytic zinc-iron alloy plating, and electrolytic zinc-nickel alloy plating. The retainer is further chromated with trivalent chromium. The rolling bearing uses the retainer for the rolling bearing.
Owner:NTN CORP
Agent for reducing coating film overall friction coefficient for trivalent chromate treating solution, trivalent chromate treating solution and method for production thereof, and trivalent chromate co
InactiveCN1950544ALower overall coefficient of frictionImprove corrosion resistanceMetallic material coating processesChromate conversion coatingQuinoline
An agent for reducing a coating film overall friction coefficient for a trivalent chromate treating solution, characterized in that it comprises a quinoline based compound or a derivative thereof. A coating film formed by the contact of a zinc or zinc alloy plating with the trivalent chromate treating solution containing no hexavalent chromium and containing the above agent on the surface of the plating exhibits a corrosion resistance equivalent to that of a coating film formed by a chromate treatment with a treating solution having a conventional hexavalent chromium as a main component, and also exhibits an overall friction coefficient equivalent to or less than that of a coating film formed by a conventional hexavalent chromate.
Owner:DISPOL CHEMICALS CO LTD
Zinc and zinc-alloy electroplating
An apparatus (12) for applying a zinc or zinc-alloy electroplate to a workpiece comprises an electroplating bath (16) having a pH more than about 14. The electroplating bath includes zinc ions and an additive. A cathode workpiece (18) is in the bath. An anode assembly (20) contacts the bath. The anode assembly includes an anolyte and an insoluble metal anode in the anolyte. The additive is capable of electrolytically breaking down upon contact with the anode. The anode assembly inhibits the electrolytic breakdown of the additive.
Owner:MACDERMID INC +1
Zinc alloy plating method
The present invention provides a zinc alloy electroplating method comprising applying a current through an alkaline zinc alloy electroplating bath comprising a cathode and an anode, wherein a cathode region including the cathode and an anode region including the anode are separated from each other by an anion exchange membrane, a catholyte contained in the cathode region is an alkaline zinc alloy plating liquid, and an anolyte contained in the anode region is an aqueous alkaline solution.
Owner:DISPOL CHEMICALS CO LTD
Nano-composite electroplating solution, preparing method of nano-composite electroplating solution and zinc alloy electroplated part
ActiveCN105463534AInhibit growthImprove corrosion resistanceSlide fastenersElectrolytic coatingsPorositySodium potassium tartrate tetrahydrate
The invention provides a nano-composite electroplating solution. The nano-composite electroplating solution is prepared from, by concentration, 23-26 g / L of cuprous cyanide, 10-12 g / L of zinc cyanide, 43-51 g / L of sodium cyanide, 25-35 g / L of sodium carbonate, 10-20 g / L of sodium potassium tartrate tetrahydrate, 4-8 g / L of ammonium chloride, 1-4 g / L of nano SiO2 and the balance water. By adding the nano SiO2, the degree of polarization of the cathode is increased, and growth of Cu-Zn alloy grains is prevented, so that a coating is more refined and compact, the porosity of a deposited layer is decreased, the particle-reinforced metal-based composite coating is formed, and accordingly the corrosion resistance of the coating is improved. It is indicated through test results that the corrosion current of the coating is greatly decreased, and it is proved that the corrosion resistance of the nano-composite coating is obviously improved compared with a Cu-Zn alloy coating. The invention further provides a preparing method of the nano-composite electroplating solution and a zinc alloy electroplated part.
Owner:ZHEJIANG WEIXING IND DEV
Electroplating method of cyanide-free copper-zinc electroplating solution containing ionic liquid
InactiveCN105463535AImprove solubilityReduce energy consumptionSuperimposed coating processSolubilityNo production
The invention discloses an electroplating method of a cyanide-free copper-zinc electroplating solution containing an ionic liquid. The electroplating method comprises the following steps: (1) preparation of the cyanide-free copper-zinc electroplating solution; (2) pretreatment of a stainless steel workpiece; (3) copper pre-plating; (4) copper-zinc alloy electroplating; and (5) after-treatment. The electroplating method provided by the present invention solves the problem of poor solubility between metal ions and the ionic liquid; the electroplating solution contains the ionic liquid additive and thus is non-toxic and pollution-free, and low in energy consumption; meanwhile, due to a relatively low quantity of the added ionic liquid, no production cost is increased; the electroplating method can be used within a relatively wide current density range; and a coating obtained through electroplating under a continuously increased current density is golden yellow, continuous and even, and compact, and further good in binding force with a substrate, and resistant to high temperatures and abrasion.
Owner:苏州市金星工艺镀饰有限公司
Zinc or zinc alloy electroplating method and system
The invention provides a zinc or zinc alloy electroplating method. The zinc or zinc alloy electroplating method comprises the step of electrifying in an alkaline zinc or zinc alloy electroplating bathwith a cathode and an anode, wherein the anode is an anode formed by applying an alkali-resistant ceramic on a conductive substrate in an energizable state, and the alkaline zinc or zinc alloy electroplating bath is an alkaline zinc plating bath containing an organic compound additive, or an alkaline zinc alloy electroplating bath containing an amine chelating agent and an organic compound additive; the oxidative decomposition of an organic compound additive in an alkaline zinc plating bath or an amine-based chelating agent and an organic compound additive in an alkaline zinc alloy plating bath on the surface of the anode due to energization is suppressed as compared to when the same conductive substrate not coated with an alkali-resistant ceramic is used as the anode.
Owner:DIPSOL CO LTD
Copper-zinc alloy electroplate liquid and electroplating method thereof
The invention discloses copper-zinc alloy electroplate liquid and an electroplating method thereof. The copper-zinc alloy electroplate liquid comprises 40-60 g / L of copper sulfate, 6-10 g / L of cupric pyrophosphate, 6-10 g / L of diammine dichloropalladium, 20-40 g / L of zinc sulfate, 20-30 g / L of potassium citrate, 5-10 g / L of amino acid, 4-8 g / L of saccharin and 10-15 g / L of sodium acetate. An electroplating layer obtained in an electroplating manner through the copper-zinc alloy electroplate liquid is free of cracks, and corrosion resistance and abrasion resistance are good.
Owner:WUXI QIAOYANG MACHINERY MFG
Cyanide-free cuprous copper-zinc alloy electroplating solution
The invention relates to a cyanide-free cuprous copper-zinc alloy electroplating solution, particularly a preparation and application method of the cyanide-free cuprous copper-zinc alloy electroplating solution. The invention aims to solve the problem in controllable coating components in the copper-zinc alloy electroplating process and the problem of high electric power consumption due to adoption of divalent copper in the existing cyanide-free technique. The cyanide-free cuprous copper-zinc alloy electroplating solution is prepared from a cuprous compound, zinc chloride, a cuprous non-cyanide complexing agent, an auxiliary complexing agent, a plating solution stabilizer, a brightening agent and sodium acetate. The cyanide-free cuprous copper-zinc alloy electroplating solution is used under the conditions of 30-75 DEG C and 0.1-3.0 A / dm<2> by using a ruthenium-titanium anode or copper-zinc alloy anode. The cuprous ions can be complexed selectively to control the copper / zinc ratio, and the cuprous ions adopted in the plating solution can lower the electric power consumption and is beneficial to saving the energy sources.
Owner:UNIV OF JINAN
Electroplated Coating of Zinc Alloy with Excellent Corrosion Resistance and Plated Metal Material Having Same
InactiveUS20080028976A1Improve corrosion resistanceHeavy environmental loadElectrolytic coatingsAnti-corrosive paintsIron groupZinc alloys
Objects of the present invention is to provide a zinc-based alloy electroplated film having a high corrosion resistance comparable to Zn—Cr alloy plating without containing chromium which gives a heavy environmental load, and a plated metal material using the same. The present invention relates to a zinc-based alloy electroplated film excellent in corrosion resistance containing (A) 30 to 96% by weight of zinc, (B) 2 to 20% by weight of an iron-group metal, and (C) 2 to 50% by weight of tungsten.
Owner:KANSAI PAINT CO LTD
Copper-zinc alloy electroplating bath and plating method using the copper-zinc alloy electroplating bath
Disclosed is a copper-zinc alloy electroplating bath that can form a uniform and glossy alloy layer having a contemplated composition without using any cyanide compound even at a higher current density than the current density in the prior art technique and can realize excellent productivity. The copper-zinc alloy electroplating bath contains a copper salt, a zinc salt, an alkali metal pyrophosphate, and at least one material selected from amino acids or salts thereof, and has a pH value of 8.5 to 14. The pH value is preferably 10.5 to 11.8. The concentration of the amino acid or the salt thereof is preferably 0.08 mol / L to 0.22 mol / L, more preferably 0.1 mol / L to 0.13 mol / L. Histidine or salts thereof are preferred for use as the amino acid or the salt thereof.
Owner:BRIDGESTONE CORP
Rare-earth-yttrium-nickel-zinc-alloy electroplating solution and preparation method thereof
The invention discloses a rare-earth-yttrium-nickel-zinc-alloy electroplating solution and a preparation method thereof. The rare-earth-yttrium-nickel-zinc-alloy electroplating solution comprises 120-200 g / L of nickel sulfate, 15-30 g / L of zinc sulfate, 0.05-1 g / L of yttria, 10-20 g / L of sodium glycolate, 10-25 g / L of citric acid, 2-5 g / L of a stabilizing agent, and 3-8 g / L of a brightener. The are-earth-yttrium-nickel-zinc-alloy electroplating solution has good stability and high cathode current efficiency. The obtained electroplated layer is uniform in dispersion, beautiful in color, and good in compactness and corrosion resistance.
Owner:无锡杨市表面处理科技有限公司
Rare-earth-cerium-copper-zinc-alloy electroplating solution and electroplating method thereof
The invention discloses a rare-earth-cerium-copper-zinc-alloy electroplating solution and an electroplating method thereof. The rare-earth-cerium-copper-zinc-alloy electroplating solution comprises 10-80 g / L of copper sulfate, 4-60 g / L of zinc sulfate, 0.1-1 g / L of cerium dioxide, 4-40 g / L of amino acid, 100-200 g / L of sodium pyrophosphate, and 3-8 g / L of a brightener. The rare-earth-cerium-copper-zinc-alloy electroplating solution has good stability. An obtained electroplated layer has a beautiful color, compactness, and excellent corrosion resistance.
Owner:无锡杨市表面处理科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com