Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
40 results about "Ozonide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
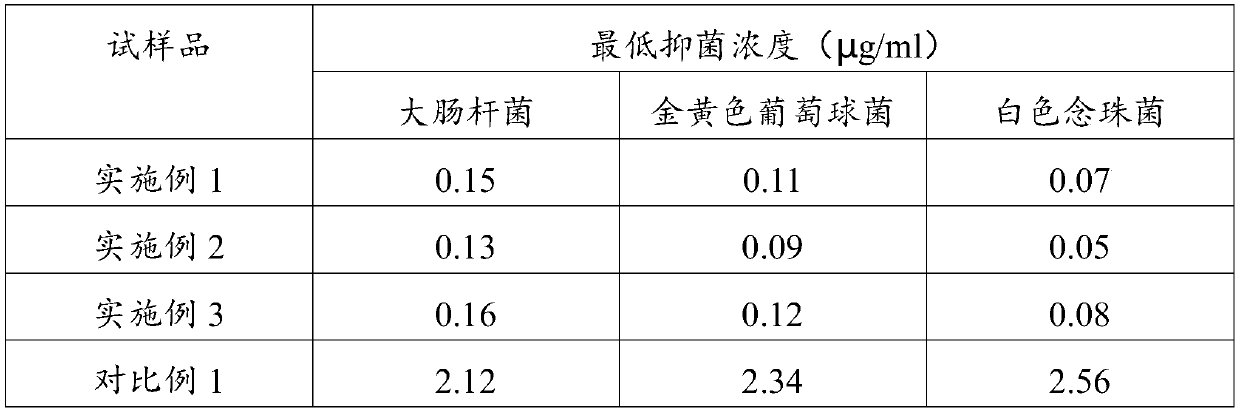
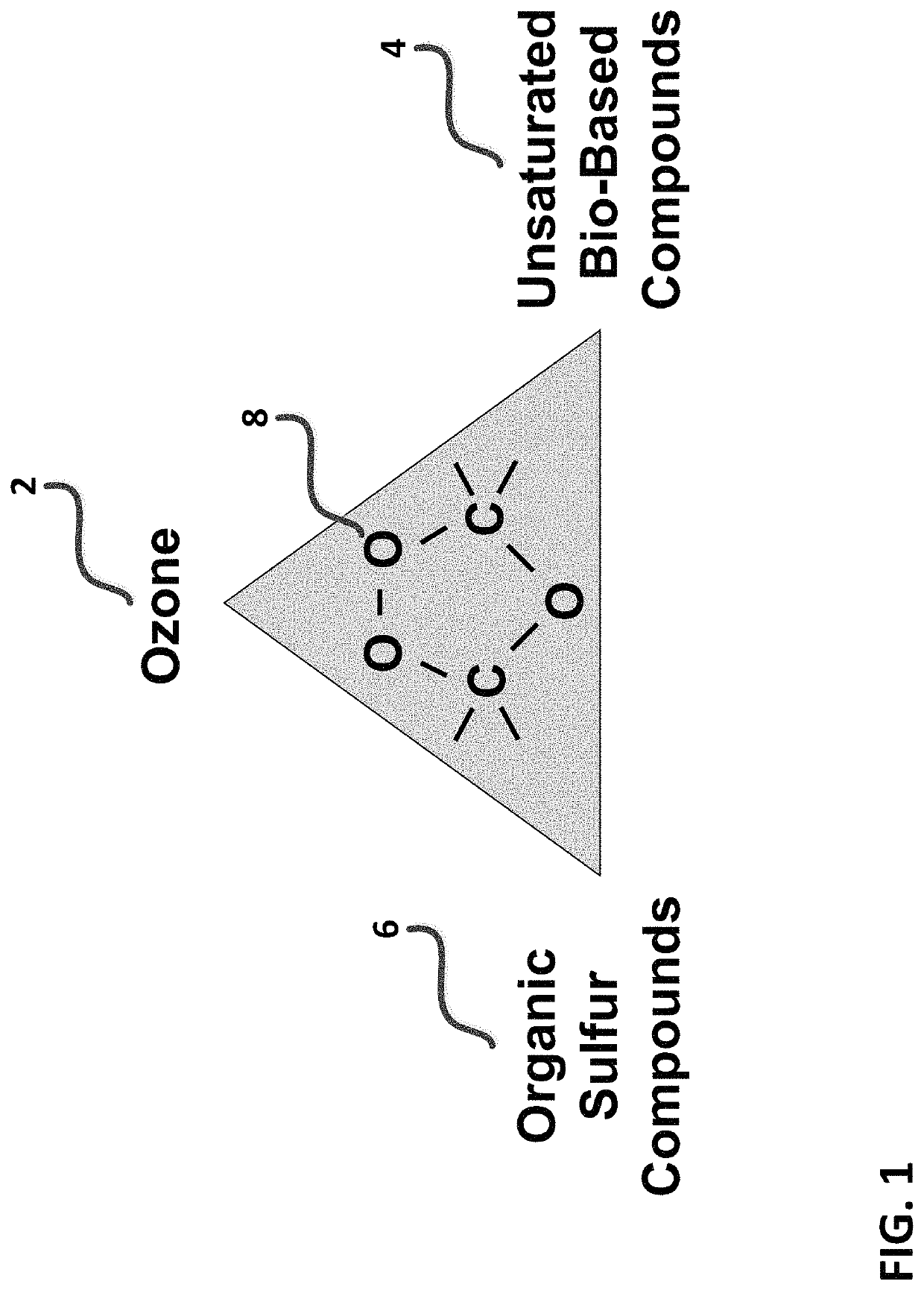
Ozonide is the unstable, reactive polyatomic anion O⁻₃ analog of ozone or any of several classes of organic peroxide compounds similar formed by the reaction of ozone with an unsaturated compound.
Preparation method of natural benzaldehyde
InactiveCN102826978AGood choiceHigh purityPreparation by ozonolysisMetal/metal-oxides/metal-hydroxide catalystsBenzaldehydeDistillation
The invention discloses a preparation method of natural benzaldehyde. The method comprises the following steps of: getting cinnamyl aldehyde or cinnamon oil as a raw material; adding one or more of multi-phase catalysts of 0.5% to 10% of MnO2, TiO2, Al2O3, SnO2, Fe2O3, MgO, CuO, CeO2, ZrO2, Bi2O3, Y2O3 or active carbon; pouring 0.05 to 0.5g of ozone in a bubbling reactor at -5 to 20 DEG C based on 1g of cinnamyl aldehyde per hour; carrying out an ozonization reaction for 0.5 to 10 hours to obtain an ozonide intermediate; dropping the ozonide intermediate into the thiourea aqueous solution to be reduced while agitating at a constant low temperature, so as to obtain an oil-water mixture; separating the oil from the water to obtain a rough benzaldehyde product; and finally operating a molecular distillation device to obtain the benzaldehyde with relatively high purity. The preparation method has the advantages of simple technology, green reaction, being capable of remaining the natural property of the benzaldehyde, high selectivity, and high yield of the benzaldehyde.
Owner:GUANGXI UNIV
Natural benzaldehyde preparation method
The invention discloses a natural benzaldehyde preparation method, which comprises the following steps of: taking cinnamaldehyde as raw material; adding 0.5-10% of activated carbon catalyst modified by 0.1-1.0mol / L of hydrochloric acid; at the temperature of below-10-30DEG C, introducing in 0.05-1.0g of ozone at the rate of 1g of cinnamaldehyde per hour; carrying out ozonization reaction for 0.5-10 hours; under the condition of keeping the low temperature, dipping an ozonide intermediate into thiourea aqueous solution for reduction reaction; carrying out centrifugal separation; and finally carrying out molecular distillation at the temperature of 60DEG C and the pressure of 100Pa by a molecular distillation device to obtain the natural benzaldehyde with higher purity. The natural benzaldehyde preparation method disclosed by the invention has the advantages of simple technology, simplicity in operation, high reaction rate, high ozone use ratio, green and environmental-friendly reaction process, efficient catalyst, and is cheap and non-toxic and easy to separate from a product, the natural degree of the benzaldehyde can be kept, the selectivity of the benzaldehyde is good, and the purity and yield of the benzaldehyde are high.
Owner:GUANGXI UNIV
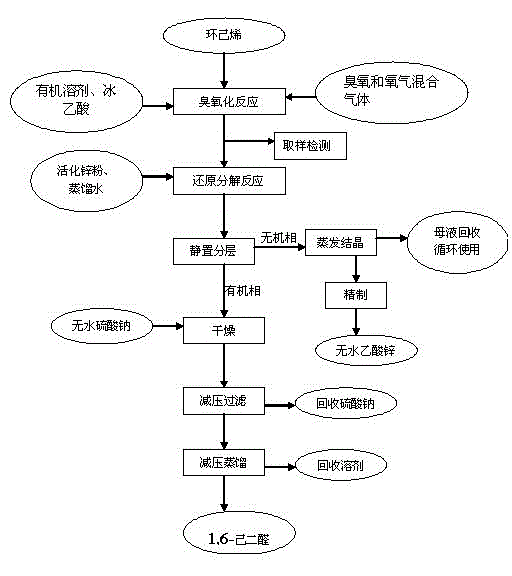
Method for preparing 1,6-adipaldehyde
InactiveCN102746127AHigh activityGood choicePreparation by ozonolysisCarboxylic acid salt preparationCyclohexeneReaction temperature
The invention belongs to the field of synthesis of fine chemicals, and discloses a method for preparing 1,6-adipaldehyde. The method comprises the following steps of: performing an ozonization reaction on cyclohexene serving as a raw material by taking ozone as an oxidant and taking an organic solvent and glacial acetic acid as mixed solvents at the reaction temperature of between 20 DEG C below zero and 10 DEG C to obtain an ozonized reaction liquid, and directly performing a reducing reaction on an ozonide without separating; and reducing and decomposing by adopting zinc powder, and reacting under the protection of nitrogen gas at the room temperature for 0.5-1.5 hours to obtain 1,6-adipaldehyde. Compared with the conventional method, the method has the advantages: ozone is taken as an oxidant, so that high oxidizing capacity and high selectivity are realized, the environmental pollution is low, the requirement of ozonization reaction temperature is low, energy is saved, and consumption is lowered. After reacting, a product is easy and convenient to separate; and zinc acetate is recovered from a water phase by concentrating and crystalizing, so that production cost is lowered, and industrial production is facilitated.
Owner:中国平煤神马控股集团有限公司 +1
Method for producing vegetable oil fuel
InactiveUS7497939B2Improve responseImprove absorbing abilityFatty acid hydrogenationOrganic chemistryVegetable oilTransesterification
A method for producing vegetable oil fuel with low viscosity, including a transesterification step P1 with respect to vegetable oil with triglyceride structure having unsaturated fatty acid, an ozone treatment step P2 with respect to unsaturated fatty acid methyl ester generated in the transesterification step P1, and a reduction step P3 with respect to the ozonide generated in the ozone treatment step P2.
Owner:FOUND FOR ADVANCEMENT OF INT SCI +1
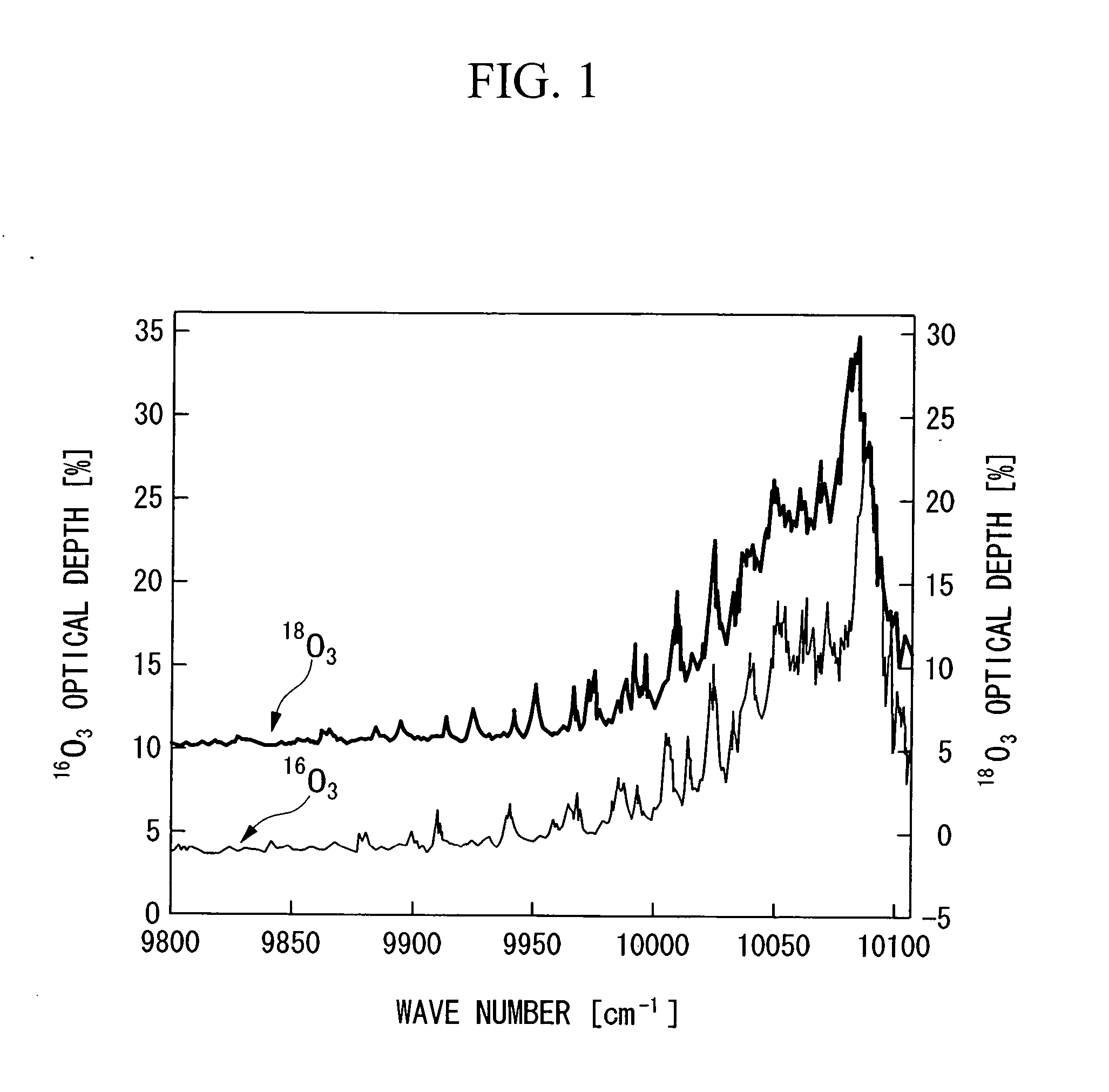
Method for concentrating oxygen isotope
InactiveUS20060249366A1Increased concentration rateReduce ozone concentrationVapor condensationIsotope separationKryptonNoble gas
The object of the present invention is to provide an oxygen isotope concentration method capable of concentrating the stable oxygen isotopes of 17O and 18O which can be carried out using a simple device configuration. The present invention relates to a method for concentrating an oxygen isotope in separated oxygen by irradiating ozone with light, selectively dissociating an isotopomer of ozone containing an oxygen isotope in its molecule into oxygen, followed by dissociating the ozone and separating the formed oxygen from the non-dissociated ozone. The basic configuration of the device is provided with an ozone formation unit 11 that forms ozone from raw material oxygen, an ozone separation unit 12 that separates ozone formed with said ozone generation unit and raw material oxygen, an ozone photodissociation unit 13 that radiates light of a specific wavelength onto the ozone separated by said ozone separation unit 12 and selectively dissociates ozone containing an oxygen isotope in its molecule into oxygen, and an oxygen separation unit that separates the oxygen formed by dissociating ozone in said ozone photodissociation unit 13 and non-dissociated ozone to concentrate the oxygen isotope in the oxygen. The present invention also relates to a method for concentrating an oxygen isotope comprising an ozone photodissociation step, in which light is radiated onto a rare gas-ozone mixed gas containing ozone and at least one type of rare gas selected from krypton, xenon and radon to selectively dissociate ozone containing a specific oxygen isotope in its molecule into oxygen, and an oxygen isotope concentration step, in which the oxygen separated from ozone in said ozone photodissociation step is separated from non-dissociated ozone and rare gas to concentrate the oxygen isotope present in the separated oxygen. An oxygen isotope may also be concentrated in a dissociation reaction product by irradiating a gas containing a peroxide selected from hydroperoxides, (di)alkyl peroxides, peroxyacids including peracids, (di)acyl peroxides, peroxy esters, peroxycarbonates, peroxydicarbonates, diperoxycarbonates, peroxalates, cyclic peroxides, ozonides and endoperoxides, nitrite esters and nitrate esters, and selectively dissociating a peroxide containing a specific oxygen isotope in its molecule.
Owner:NIPPON SANSO CORP
Ozonide oil blasting bead and use method and dressing plaster
InactiveCN105597138AGuaranteed concentrationPromote healingNon-adhesive dressingsAdhesive dressingsWound healingMedical waste
The invention relates to the technical field of medical treatment, in particular to an ozonide oil blasting bead and a use method and a dressing plaster. The ozonide oil blasting bead comprises a capsule and ozonide oil, the capsule is provided with an inner cavity, and the ozonide oil is arranged in the inner cavity of the capsule; the ozonide oil is arranged in the capsule so that carrying is convenient, the odor of the ozonide oil is blocked, the ozone concentration is guaranteed by a closed environment, and the action time of the ozonide oil is prolonged; when the ozonide oil blasting bead is used by being matched with a liquid dressing or a conventional dressing, the capsule is crumbed, the ozonide oil arranged in the inner cavity of the capsule flows out, the affected part is coated with the ozonide oil, the wound surface can be coated with the liquid dressing according to the size of a wound, a biological membrane is formed, the conventional dressing covers the surface of the wound, ozone can be released slowly, the time for acting on the wound directly is prolonged, antibacterial and disinfectant effects are improved, healing of the wound is facilitated, replacing frequencies of the liquid dressing is decreased, medical waste is not produced or reduced, environmental friendliness is achieved, the resources are saved, and use is convenient.
Owner:TIANJIN TAICHUANG BIOTECH CO LTD
Natural benzaldehyde preparation method
The invention discloses a natural benzaldehyde preparation method, which comprises the following steps of: taking cinnamaldehyde as raw material; adding 0.5-10% of activated carbon catalyst modified by 0.1-1.0mol / L of hydrochloric acid; at the temperature of below-10-30DEG C, introducing in 0.05-1.0g of ozone at the rate of 1g of cinnamaldehyde per hour; carrying out ozonization reaction for 0.5-10 hours; under the condition of keeping the low temperature, dipping an ozonide intermediate into thiourea aqueous solution for reduction reaction; carrying out centrifugal separation; and finally carrying out molecular distillation at the temperature of 60DEG C and the pressure of 100Pa by a molecular distillation device to obtain the natural benzaldehyde with higher purity. The natural benzaldehyde preparation method disclosed by the invention has the advantages of simple technology, simplicity in operation, high reaction rate, high ozone use ratio, green and environmental-friendly reaction process, efficient catalyst, and is cheap and non-toxic and easy to separate from a product, the natural degree of the benzaldehyde can be kept, the selectivity of the benzaldehyde is good, and the purity and yield of the benzaldehyde are high.
Owner:GUANGXI UNIV
Preparation method of natural benzaldehyde
InactiveCN102826978BGood choiceHigh purityPreparation by ozonolysisMetal/metal-oxides/metal-hydroxide catalystsBenzaldehydeDistillation
The invention discloses a preparation method of natural benzaldehyde. The method comprises the following steps of: getting cinnamyl aldehyde or cinnamon oil as a raw material; adding one or more of multi-phase catalysts of 0.5% to 10% of MnO2, TiO2, Al2O3, SnO2, Fe2O3, MgO, CuO, CeO2, ZrO2, Bi2O3, Y2O3 or active carbon; pouring 0.05 to 0.5g of ozone in a bubbling reactor at -5 to 20 DEG C based on 1g of cinnamyl aldehyde per hour; carrying out an ozonization reaction for 0.5 to 10 hours to obtain an ozonide intermediate; dropping the ozonide intermediate into the thiourea aqueous solution to be reduced while agitating at a constant low temperature, so as to obtain an oil-water mixture; separating the oil from the water to obtain a rough benzaldehyde product; and finally operating a molecular distillation device to obtain the benzaldehyde with relatively high purity. The preparation method has the advantages of simple technology, green reaction, being capable of remaining the natural property of the benzaldehyde, high selectivity, and high yield of the benzaldehyde.
Owner:GUANGXI UNIV
Medical ozone oil and preparation method thereof
InactiveCN110179856AKeep aliveEnhance metabolic functionOrganic active ingredientsInorganic active ingredientsSophocarpidineWound healing
The invention provides medical ozone oil and a preparation method thereof. The effective components of the medical ozone oil are as follows: 0.5 to 3 parts of ozone, 70 to 90 parts of soybean oil, 0.5to 2.5 parts of mint oil, 10 to 20 parts of olive oil and 0.1 to 0.8 part of sophocarpidine. The preparation method comprises the steps of adding a certain amount of soybean oil into a stirring tank,stirring and then introducing dried ozone. The content of ozonide in the ozone oil is detected, and whether the ozonide content is qualified or not is judged. The olive oil, the mint oil and the sophocarpidine are sequentially added into the qualified ozone oil according to certain weight percentage and then are stirred. The medical ozone oil is subjected to sterilization treatment and subpackaging. The medical ozone oil provided by the invention can increase the bacteriostasis rate of the ozone oil, can accelerate blood circulation of blood capillary, can reduce piercing pain generated aftera skin mucous membrane is coated with the ozone oil, and can promote wound healing.
Owner:深圳市橘井舒泉技术有限公司
Method for ozonization preparation of 2-nonenyl aldehyde
InactiveCN101508634ANo pollution in the processImprove reducibilityOrganic compound preparationPreparation by hydrogenolysisReductive decomposition2-Nonenal
The invention relates to a method for preparing 2-nonenal by ozonization with castor methyl oleate as the raw material, ethanol and n-hexane as the solvent, comprising performing ozonization by ventilating O3 / O2 gas mixture after uniformly mixing the raw material and the solvent at the low temperature, slowly dropping aqueous solution of NaHSO3 in ozonides, raising temperature for reductive decomposition, regulating the pH to 9-11 by sodium hydroxide solution, setting the mixture aside for layering, removing the water layer, rinsing the organic phase to neutrality by de-ionized water, adding dilute sulphuric acid for reflux, and recovering solution through reduced pressure distillation, and obtaining the target product, 2-nonenal, through the molecular distillation of the reaction mixture. In the method, no high pressure is needed during the reaction; the solvent of n-hexane can be recovered for repeated use; the mixed solvent system solves the serious corrosion to the devices by using acetic acid as solvent; the method has clean process, environmental protection, safe execution, simple operation and convenient mass production. The separation and purification of the products are simple.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
Application of sodium peroxide mixing solution containing sodium ozonide in preparing paper pulp
InactiveCN101078185AIncrease productivityReduce the requirements for heat resistance, pressure resistance and corrosion resistancePulping with inorganic basesChemical/chemomechanical pulpResource utilizationBleach
A sodium peroxide with ozonide applies into the producing of paper pulp. The pretreatment straw mixed with the sodium peroxide with ozonide, and it cooks at the pressure of 1-200kpa to get the wheat or white paper pulp. Comparing to the current technology, it has the advantage as fellow, 1. The volume of the alkali reduces 4-5times than the traditional technology, and the waste water can directly use. 2. The JHO3 has the bleach function at the same time, and there is no need chlorine and the chloric derivatives as the bleach agent. 3. The cooking pressure reduces 4-5 times than the traditional technology, and it can reduce the consumption of the energy and the investment of the equipment. 4. The cooking time reduces 1-3 times can improve the efficiency of wording and the ratio of equipment usage. 5. The output of fibre can improve 3-5 percent, and it can reduce the cost and improve the ratio of source.6. The waste water can be as the organic complex fertilizer.
Owner:STATE GRID CORP OF CHINA +1
System, method and apparatus for controlling microbiological contamination in commercial freeze dryers using UV energy and photohydroionization cell technology
InactiveUS20100183782A1Prevent potential recontaminationEliminate or reduce biological hazardsHydrogen peroxideDeodrantsSuperoxideCell system
The system, method and apparatus involves the control of microbiological contamination of commercial freeze dryers using advanced oxidation gases produced by Photohydroionization (PHI) Cell technology. The PHI Cell system produces hydro peroxides, super oxide ions, ozonide ions, hydroxides and other oxidative gases. These compounds act as anti-microbial agents and systematically inactivate bacteria, viruses, yeast and mold in the air and on surfaces inside commercial freeze dryers.
Owner:STELLA & CHEWYS
Method for preparing vanillin through sodium isoeugenol process
InactiveCN104086390AEasy to useEasy to preparePreparation by ozonolysisChemical industrySulfite salt
A method for preparing vanillin through a sodium isoeugenol process relates to the technical field of the chemical industry. The method comprises the following steps: mixing sodium isoeugenol and water, adding the obtained mixture into an oxidation reaction kettle, carrying out an oxidation reaction, connecting with a vent pipe to input ozone, increasing the ozone rate to drive the stirring and overturning of a liquid in the oxidation reaction kettle in order to carry out a liquid phase oxidation reaction, layering the liquid obtained after the liquid phase oxidation reaction, adding a sodium sulfite solution to decompose ozonides, neutralizing the ozonides, washing with water to obtain crude vanillin, adding to a refining kettle, carrying out refining crystallization, drying to obtain finished vanillin, packaging and warehousing. The method has the advantages of convenient and simple preparation, environmental protection, no pollution, less equipment investment, high purity and convenient operation, and the prepared vanillin has the advantages of good use effect, safety and reliability.
Owner:安徽佑骏商品混凝土有限公司
Process and apparatus for organic compound fertilizer co-produced with straw papermaking
ActiveCN103184701AReduce consumptionIncrease organic matterClimate change adaptationSewage/sludge fertilisersPapermakingWastewater
The invention relates to a process and an apparatus for organic compound fertilizer co-produced with straw papermaking. The process comprises the steps of mixing a medicine liquid and straw truncated materials uniformly and thoroughly, promoting a straw wetting reaction by using hot water for heat preservation, making the mixture into a mixed pulp through mechanical pulping, separating paper pulp and waste water by a steaming and spraying method on a steaming and spraying device, thus obtaining neutral paper pulp and concentrated waste water capable of directly being used as raw materials for the organic compound fertilizer at the same time, wherein the medicine liquid is a mixed solution JH03 of sodium peroxide containing sodium ozonide or an alkaline solution JH02 of sodium peroxide. Liquid-solid separation is carried out by using the steaming and spraying method, so that clean paper pulp and concentrated waste water capable of directly being used as raw materials for the organic compound fertilizer can be obtained without washing water, all organic impurities after the paper pulp is extracted from the straws can be utilized, full utilization of a large amount of agricultural straws can be realized and a circular economy system of paper-fertilizer circulation, water circulation, zero waste water discharge and zero CO2 discharge. The process and apparatus are also suitable for pulping by other papermaking raw materials.
Owner:黄正华
Method for preparing anisaldehyde through micro-channel continuous ozone oxidation
ActiveCN111302915ATake advantage ofReduce generationChemical/physical/physico-chemical microreactorsPreparation by hydrogenolysisSodium hydrogen sulphiteSodium bisulfate
The invention belongs to the technical field of chemical application, and particularly relates to a method for preparing anisaldehyde through micro-channel continuous ozone oxidation. According to themethod, anethole is used as a raw material, ozone is used as an oxidizing agent, sodium hydrogen sulfite is used as a reducing agent, micro-channel equipment is adopted for mixing, a continuous reaction is carried out, anise oil is oxidized by ozone in the presence of a solvent or in the absence of a solvent, oxydol is reduced by sodium hydrogen sulfite, and the target product anisaldehyde is obtained. Compared with the present synthesis technology in the prior art, the method has the advantages of realization of continuous reaction, easiness in automatic control, simplicity, convenience andsafety in operation, mild reaction conditions, few byproducts, low cost and less pollution and is suitable for industrial production.
Owner:FUDAN UNIV
Method for synthesizing glyoxalic acid by oxidation of glyoxal with maleuric ozonide
InactiveCN1709849AHigh selectivityGood choiceCarboxylic preparation by ozone oxidationHigh concentrationGlyoxylic acid
This invention has disclosed a kind of method to use maleic acid ozonide to oxidize glyoxal in order to gain glyoxylic acid. This method succeeds in combining maleic acid ozonide oxidizing method with other glyoxal catalyzed oxidizing method, devised a new way of to use maleic acid ozonide to oxidize glyoxal in order to gain glyoxylic acid, it has high concentration and purity with the simple step. This method craft is simple, the whole course reflects mild condition, the supplies are saved, and pollution-free to the environment, help industrialization to cosmically produce.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
Method for producing nonandioic acid, pelargonic acid by ozonization-oxidative decomposition of oleic acid
ActiveCN101244998BReduce entrainmentGuaranteed entrainmentCarboxylic preparation by ozone oxidationKetopinic acidDecomposition
Owner:SICHUAN SIPO CHEMICAL CO LTD
Hydrated catalytic coating
InactiveUS20070245928A1Increase surface areaIncrease kinetic rateMaterial nanotechnologyCoatingsNano sizeUltraviolet lights
The hydrated catalytic coating is a coating that is applied to a substrate or target structure in the path of a broad spectrum ultraviolet light in the 100 nm to 300 nm range to decompose ozone. The coating is particularly useful in conjunction with germicidal UV lamps emitting UV radiation at 185 nm and 254 nm. The coating is a combination of a hydrophilic agent and the following metals: titanium dioxide (TiO2); silver (Ag); copper (Cu); nickel (Ni); and rhodium (Rh). At least three of the five metals are present as particles in the 50-100 nm range. The nanosize particles increase the surface area of the catalyst, improving the kinetic rate of reaction and the total reduction in ozone concentration. The particular mixture of catalytic metals and absorbed water also results in reaction products that have antimicrobial effect, including hydroxyl radicals and ions, superoxides, hydro peroxides and ozonide ions.
Owner:BENNERT JEFF E +3
Method for ozonization preparation of 2-nonenyl aldehyde
InactiveCN101508634BNo pollution in the processImprove reducibilityOrganic compound preparationPreparation by hydrogenolysisReductive decomposition2-Nonenal
The invention relates to a method for preparing 2-nonenal by ozonization with castor methyl oleate as the raw material, ethanol and n-hexane as the solvent, comprising performing ozonization by ventilating O3 / O2 gas mixture after uniformly mixing the raw material and the solvent at the low temperature, slowly dropping aqueous solution of NaHSO3 in ozonides, raising temperature for reductive decomposition, regulating the pH to 9-11 by sodium hydroxide solution, setting the mixture aside for layering, removing the water layer, rinsing the organic phase to neutrality by de-ionized water, adding dilute sulphuric acid for reflux, and recovering solution through reduced pressure distillation, and obtaining the target product, 2-nonenal, through the molecular distillation of the reaction mixture. In the method, no high pressure is needed during the reaction; the solvent of n-hexane can be recovered for repeated use; the mixed solvent system solves the serious corrosion to the devices by using acetic acid as solvent; the method has clean process, environmental protection, safe execution, simple operation and convenient mass production. The separation and purification of the products are simple.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
Ozone antibacterial and antivirus ointment and preparation method thereof
PendingCN109758421AAdd antibacterial and antivirus functionNo side effectsAntibacterial agentsInorganic active ingredientsSide effectLanolin
The invention discloses an ozone antibacterial and antivirus ointment and a preparation method thereof. The ointment is prepared from the following raw materials including a ozone oil agent, vaseline,stearic acid, azone, glyceryl monostearate, polyethylpyrrolidone, lanolin and liquid paraffin. The prepared ointment has high antibacterial and antivirus functions through ozone treatment, has no toxic or side effects on the human body, and is free of residues and drug resistance, high in stability and capable of being preserved for a long time, and the active ingredients cannot be reduced; the preparation method of the ointment is simple, stable ozonide is formed by ozone and the unsaturated fatty acid, and the antibacterial and antivirus functions of the ointment are improved; the preparedointment can be preserved for 2 years or longer at normal temperature and normal pressure, the active ingredients are stable, and the ointment can be preserved for 3 years or longer in a 4-DEG C environment.
Owner:重庆渠济生物科技有限公司
Method for Producing Oxygen-Containing Compound
InactiveUS20120053354A1Guaranteed heat exchange effectImprove reaction efficiencyProcess control/regulationOrganic oxidationSimple Organic CompoundsOzonolysis
[Problem] There is provided a method for producing an oxygen-containing compound safely and with improved reaction efficiency, in which an undesired peroxide is unlikely to be produced, and efficient heat exchange of the ozonization can be achieved.[Mean for solving the Problem] The method comprises an ozonization reaction step of continuously supplying, together with an organic compound, ozone having an oxygen content of less than 10% in a dissolved state in high-pressure carbon dioxide to an ozonization reaction section having a thin tubular shape, and reacting the ozone and the organic compound under conditions that suppress generation of oxygen due to thermal decomposition of the ozone, thereby continuously producing an ozonide; and a decomposition reaction step of continuously supplying the ozonide produced in the ozonization reaction step to a decomposition reaction section having a thin tubular shape, thereby continuously producing an oxygen-containing compound, the decomposition reaction step being provided in a manner continuous with the ozonization reaction step.
Owner:UTSUNOMIYA UNIV
Method for forming and applying an oxygenated machining fluid
ActiveUS10639691B1Efficient and effective deliveryImproves boundary layer oxygenated fluid-cutting surface interactionMetal working apparatusMaintainance and safety accessoriesChemical reactionAlcohol
The present invention describes a chemically-assisted machining process that converts conventional lubricant chemistries to produce reactive oxygenated species that accelerate the formation of friction-reducing boundary layer lubrication during cutting operations—termed “Ozonolytic Machining”. The new type of cooling-lubricant chemistry is based on chemical reactions between unsaturated bio-based oils and alcohols, and other types of machining lubricants, containing carbon-carbon double or triple bonds, with ozone gas to form variously reacted or polymerized ozonides—termed “super-oxygenated fluids, oils or alcohols, aldehydes or ketones,”“sulfurized ozonides” and “super-oxygenated gels.”
Owner:HITACHI HIGH-TECH CORP
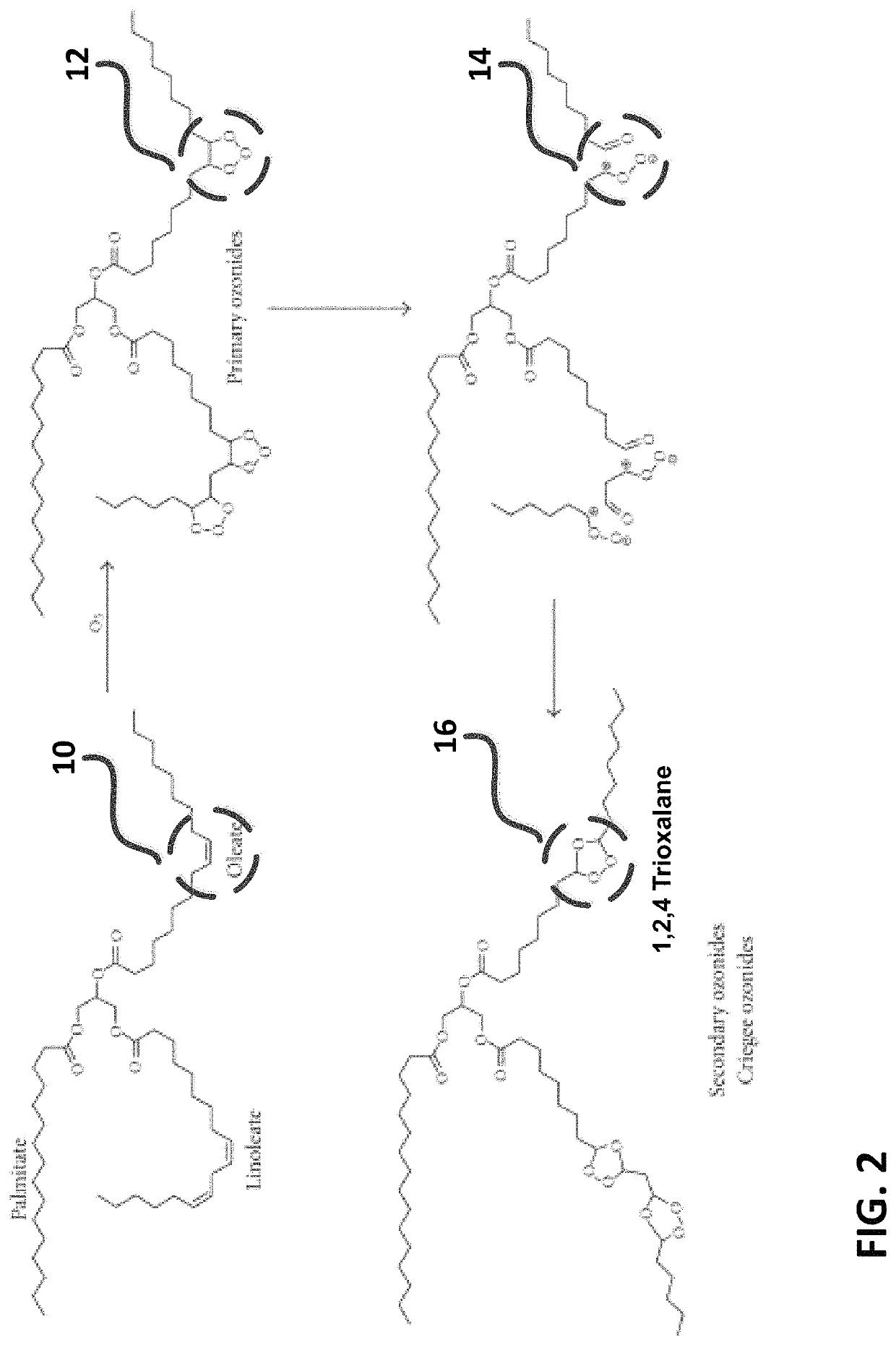
Method for preparing azelaic acid, tridecanedioic acid and other long-chain dicarboxylic acids from high erucic acid rapeseed oil
ActiveCN112110815AIncrease profitQuick responsePreparation from carboxylic acid saltsOrganic compound preparationTridecanedioic acidSaponification
The invention discloses a method for preparing azelaic acid, tridecanedioic acid and other long-chain dicarboxylic acids from high-erucic-acid rapeseed oil. The method comprises the following steps: mixing high-erucic-acid rapeseed oil, a solvent and a foaming agent, putting the mixture into a reaction kettle, introducing ozone while stirring, and carrying out an ozonization reaction; carrying outan oxidation reaction on the erucic acid ozonization product obtained by the reaction under a microwave condition to obtain an oxidation product mixture containing dibasic acid glyceride and nonanoicacid, and removing the solvent and the nonanoic acid by a distillation method to obtain dibasic acid glyceride; and saponifying, crystallizing, separating and acidifying the dibasic acid glyceride torespectively obtain azelaic acid and tridecanedioic acid. According to the invention, a foaming agent is introduced, so that ozone and oxygen are in full contact with reaction materials in the ozonization reaction stage and the oxidation reaction stage respectively, the gas utilization rate is increased, and the reaction speed is increased; and high erucic acid rapeseed oil is used as a raw material, so that the production process is shortened.
Owner:SICHUAN SIPO CHEMICAL CO LTD
Energy-saving and environment-friendly bacteria stick preparing method
InactiveCN108551975AReasonable formulaGuarantee the growth of the journeyCultivating equipmentsMushroom cultivationPotassiumHumidity ratio
The invention provides an energy-saving and environment-friendly bacteria stick preparing method, and relates to the technical field of edible fungi production. The energy-saving and environment-friendly bacteria stick preparing method comprises the following steps of S1, primordium mixing, wherein raw material dried powder is mixed first, and then water is added for adjusting the water content; S2, ozone sterilization, wherein sterilization is conducted with ozone water; S3,adjustment of the humidity ratio of primordia; S4, mother strain mixing, wherein the second generation of strains and potassium ozonide are added into the primordia; S5, stick preparation; S6, spawn running; S7, generation of the finished product. According to the bacteria stick preparing method, the formula of the adopted primordium raw materials is reasonable, the nitrogen source is abundant, nutrients required for the growth of edible fungi are ensured, the growth of the edible fungi is promoted, carbon dioxideis prevented from being accumulated in the preparation process, sufficient oxygen is provided for the edible fungi, the preparation process is energy-saving and environmentally friendly, thorough sterilization is achieved, strain mixing is uniform, the spawn running time is short, and the edible fungi produced by using the bacteria sticks are high in yield.
Owner:北京亚盛增光物理农业科技开发有限公司
Ozone hemorrhoids cream and preparation method thereof
InactiveCN110680846AEasy to prepareEasy to operateHydroxy compound active ingredientsInorganic active ingredientsBiotechnologyVegetable oil
The invention discloses ozone hemorrhoids cream and a preparation method thereof. The ozone hemorrhoids cream comprises components as follows: 35-50 parts by weight of edible vegetable oil, 10-20 parts by weight of liquid paraffin, 18-26 parts by weight of linseed oil, 2-8 parts by weight of beeswax, 2-7 parts by weight of Vaseline, 2-5 parts by weight of notoginseng, 0.8-1.2 parts by weight of calculus bovis artifactus, 0.5-1.0 part by weight of borneol and 0.9-2 parts by weight of ozonide. The ozone hemorrhoids cream has no irritation or adverse reaction to skin, the therapeutic effects of product are not affected, the product quality is stable and reliable, and the necrotic tissue removal and granulation promoting effects are good.
Owner:湖北仟枝生物科技有限公司 +1
Ozonides for treating or preventing virus infections
Described are methods of treating or preventing a virus in a subject comprising administering ozonides to the subject.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
Method for producing oxygen-containing compound
InactiveUS8716501B2Guaranteed heat exchange effectProducing an oxygen-containing compound safelyProcess control/regulationOrganic oxidationDecompositionHigh pressure
[Problem] There is provided a method for producing an oxygen-containing compound safely and with improved reaction efficiency, in which an undesired peroxide is unlikely to be produced, and efficient heat exchange of the ozonization can be achieved.[Mean for solving the Problem] The method comprises an ozonization reaction step of continuously supplying, together with an organic compound, ozone having an oxygen content of less than 10% in a dissolved state in high-pressure carbon dioxide to an ozonization reaction section having a thin tubular shape, and reacting the ozone and the organic compound under conditions that suppress generation of oxygen due to thermal decomposition of the ozone, thereby continuously producing an ozonide; and a decomposition reaction step of continuously supplying the ozonide produced in the ozonization reaction step to a decomposition reaction section having a thin tubular shape, thereby continuously producing an oxygen-containing compound, the decomposition reaction step being provided in a manner continuous with the ozonization reaction step.
Owner:UTSUNOMIYA UNIV
Method for extracting deacetylating chitin by fast hydrolyzing shrimp and crab carapace
The fast hydrolysis process of extracting deacetyl chitin from shrimp and crab shell includes the following steps: crushing fresh shrimp and crab shell and adding water to form solid-liquid mixture, eliminating protein with sodium peroxide mixture solution containing sodium ozonide, eliminating calcium carbonate with hydrochloric acid, eliminating acetyl radical with sodium hydroxide, eliminatinginsoluble impurity with dilute acetic acid solution, and spray drying to obtain scaly deacetyl chitin acetate product. The present invention has the advantages of simple technological process and short production period. During the production process, great amount of protein and calcium carbonate are converted into composite amino acid and calcium ion entering to the filtrate and may be used in producing feed in low cost.
Owner:黄正华
A process and equipment for producing organic compound fertilizer with straw papermaking chain
ActiveCN103184701BImprove the effect of impurity removalRealize continuous productionClimate change adaptationSewage/sludge fertilisersWater dischargePapermaking
Owner:黄正华
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com