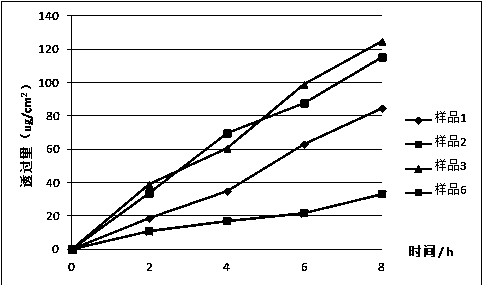
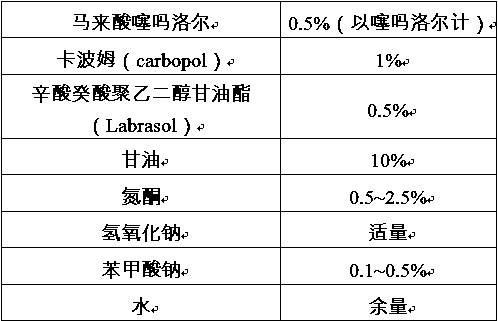
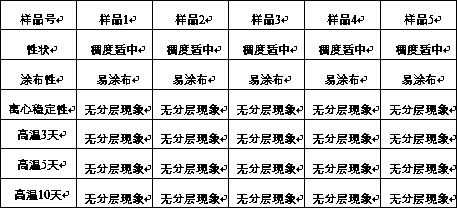
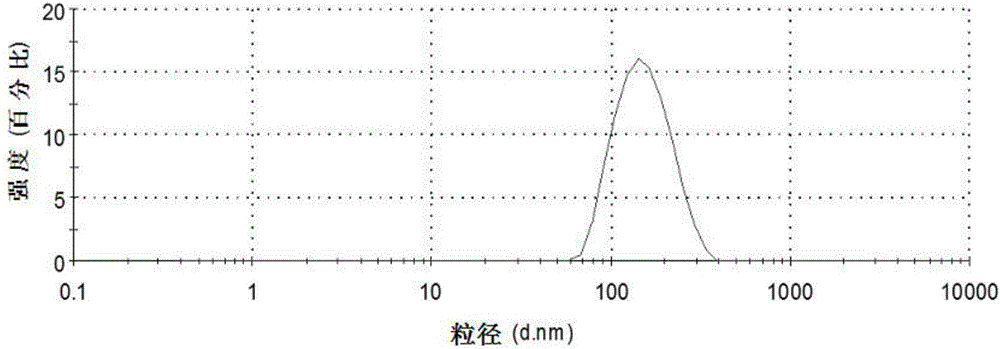
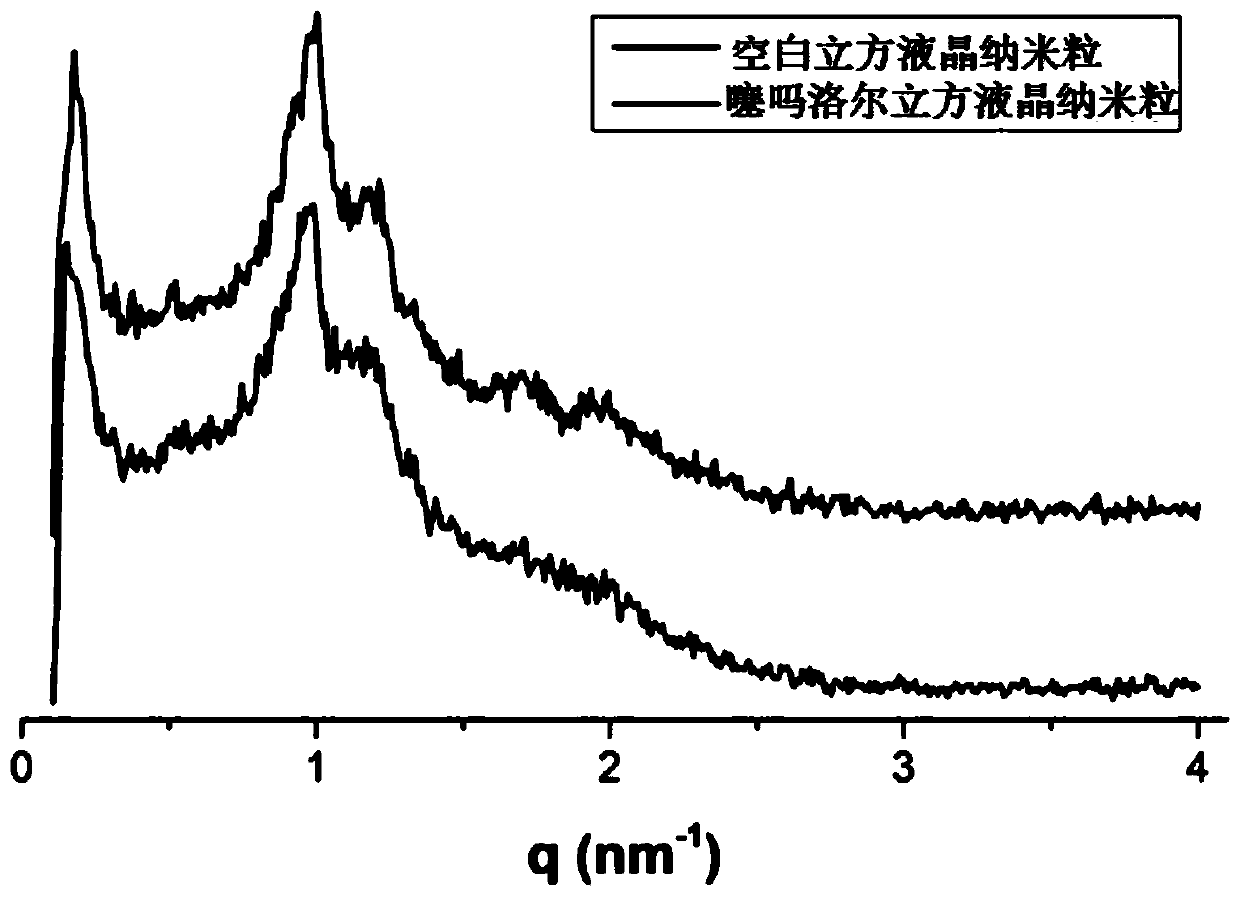
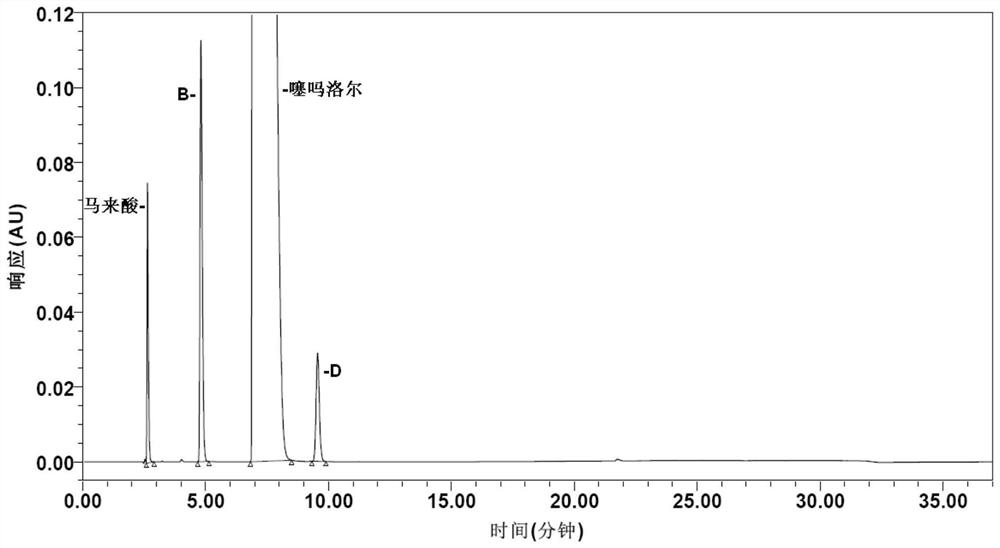
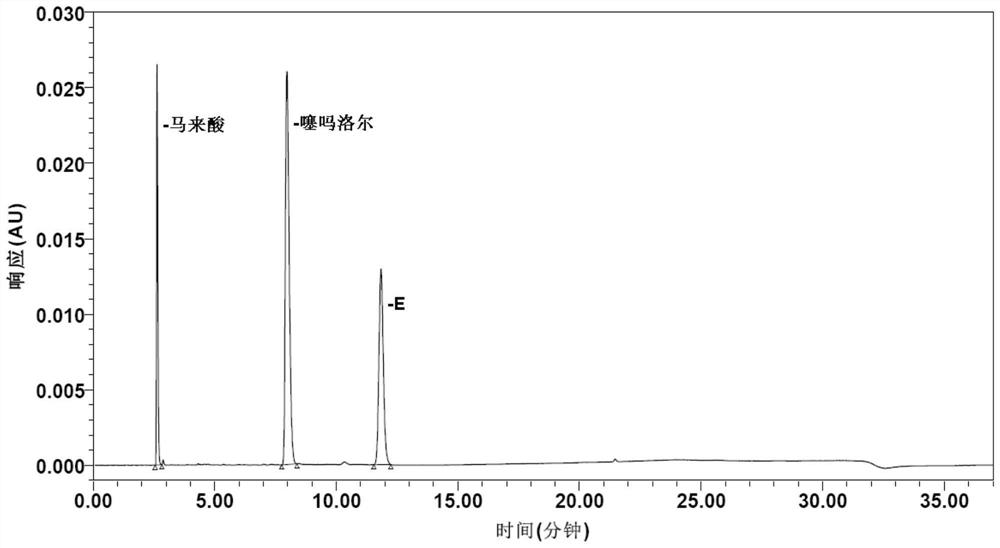
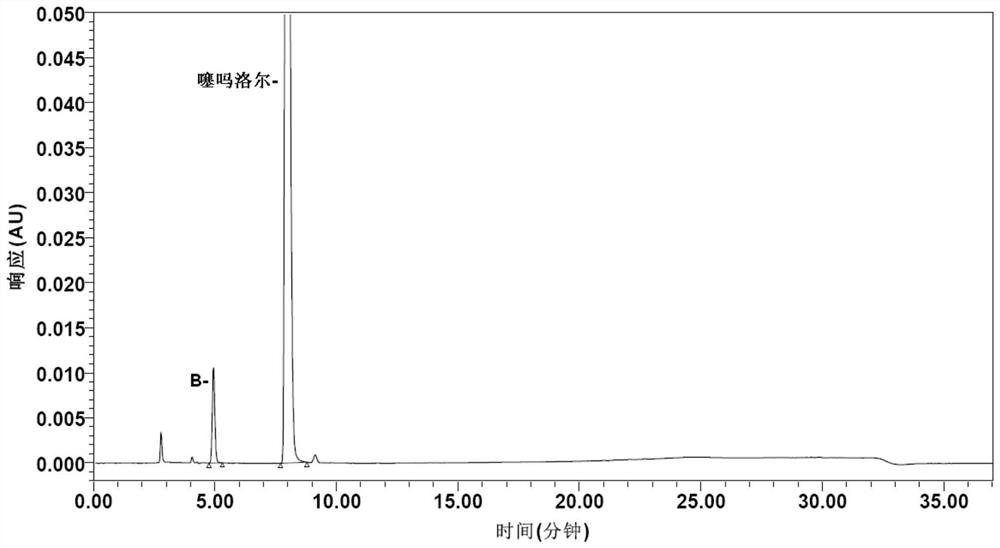
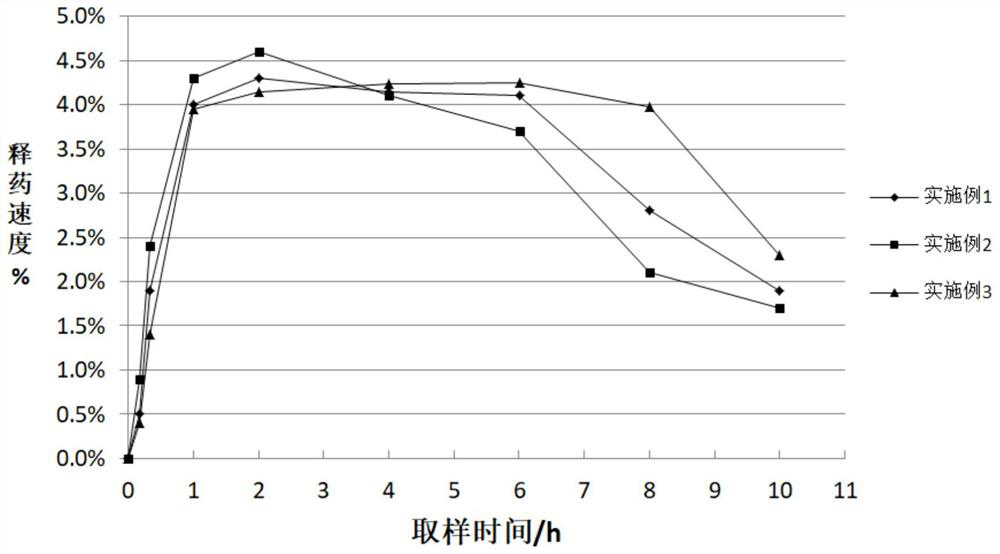
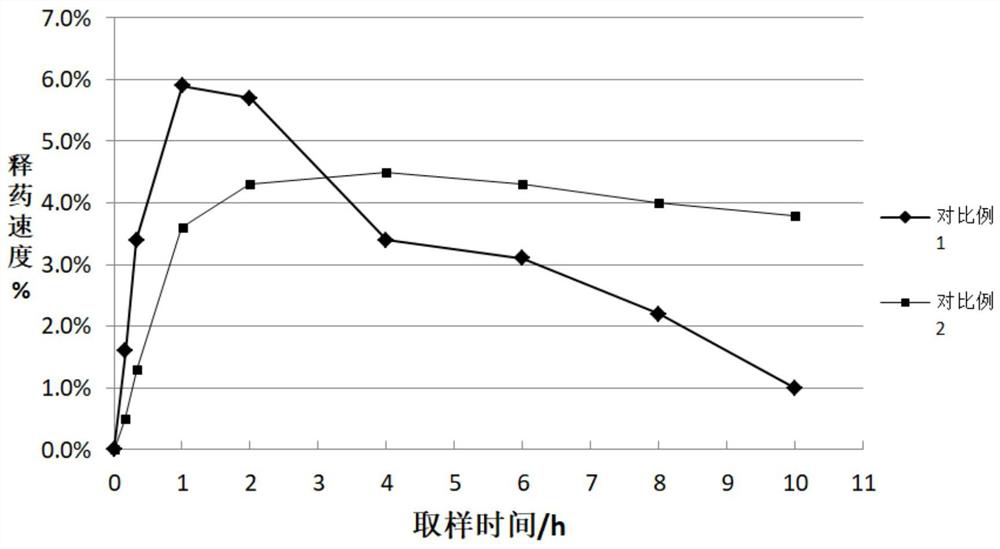
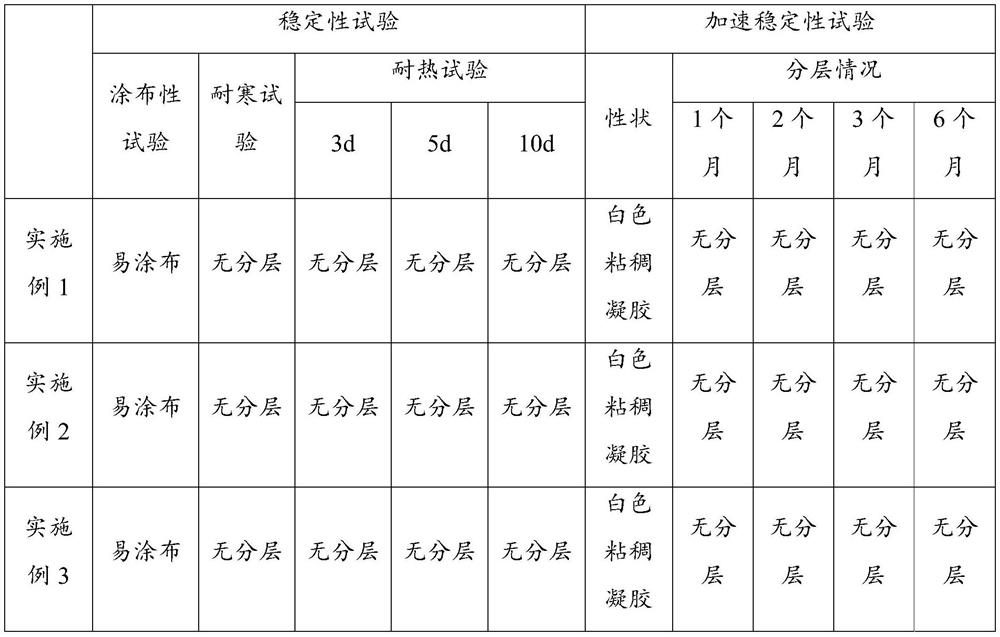
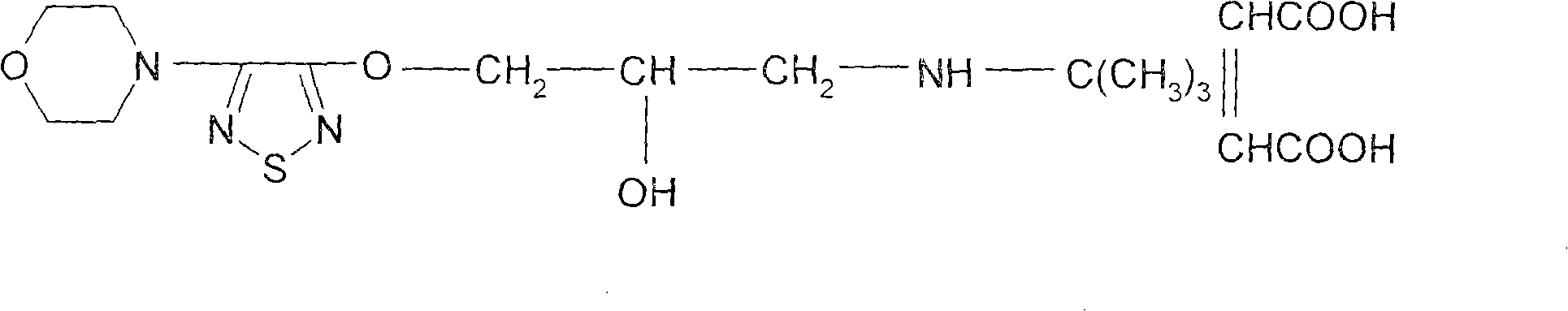
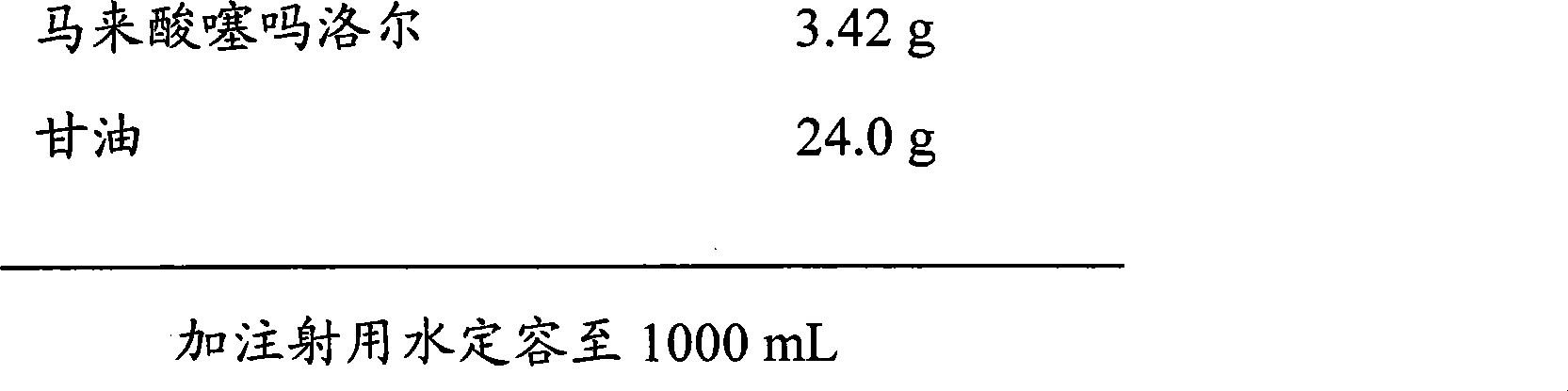Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
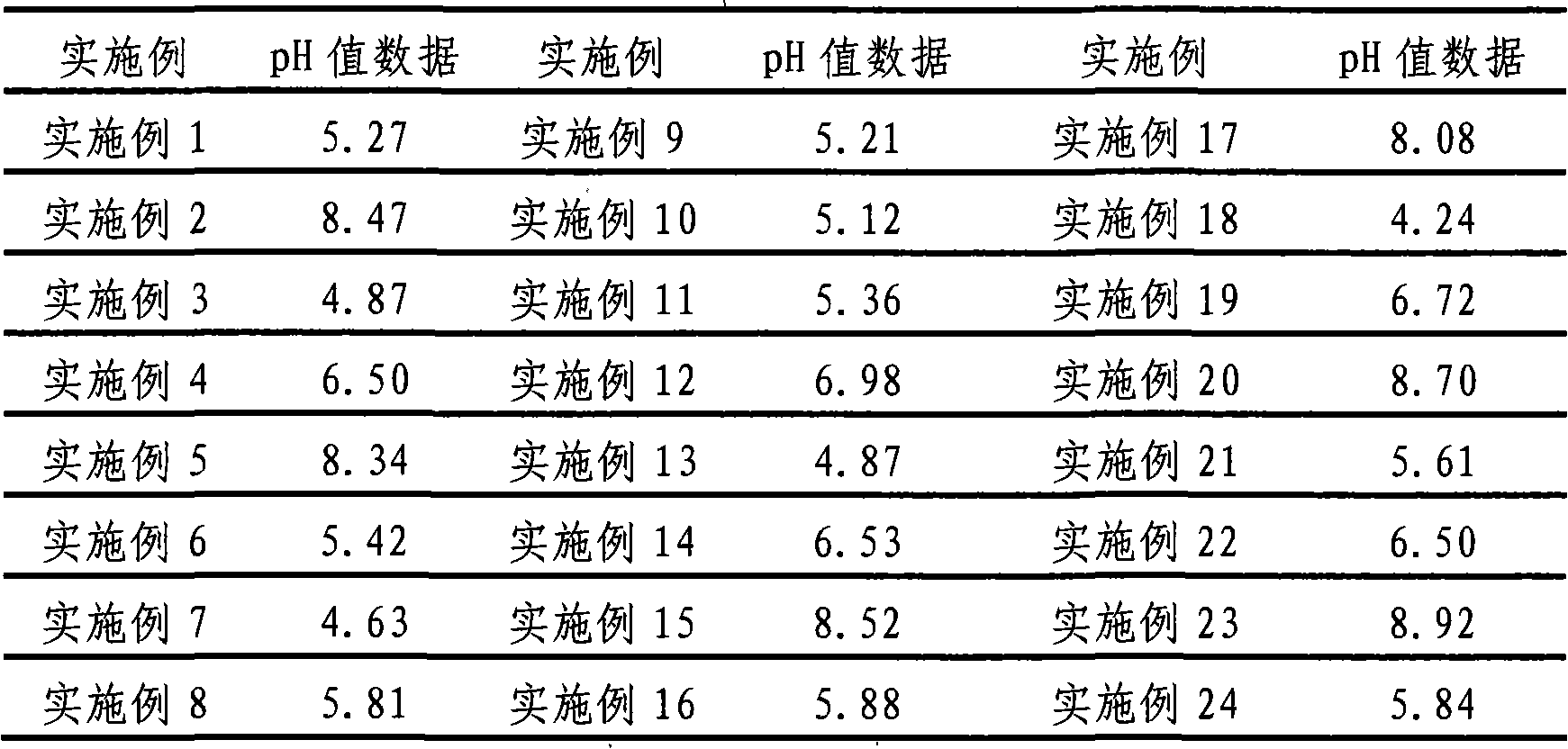
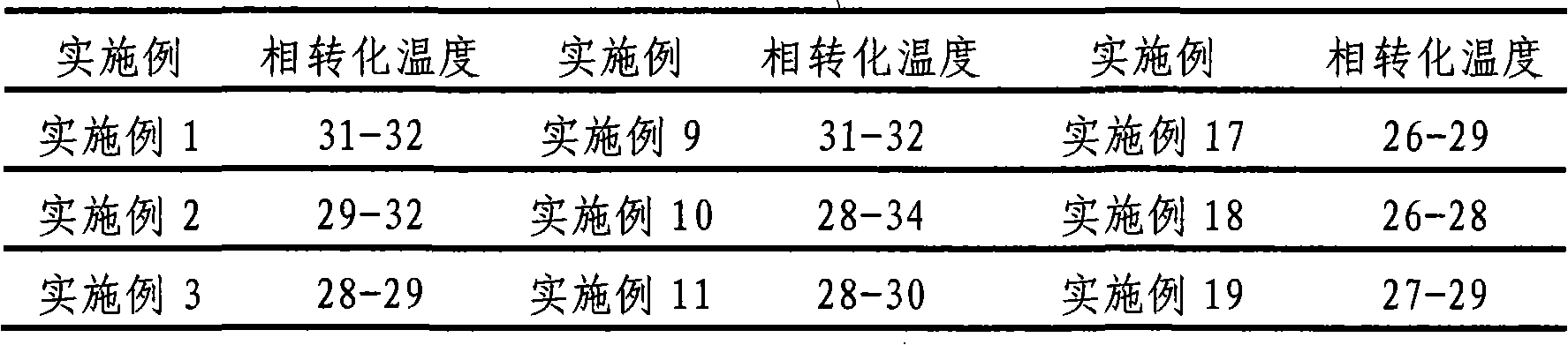
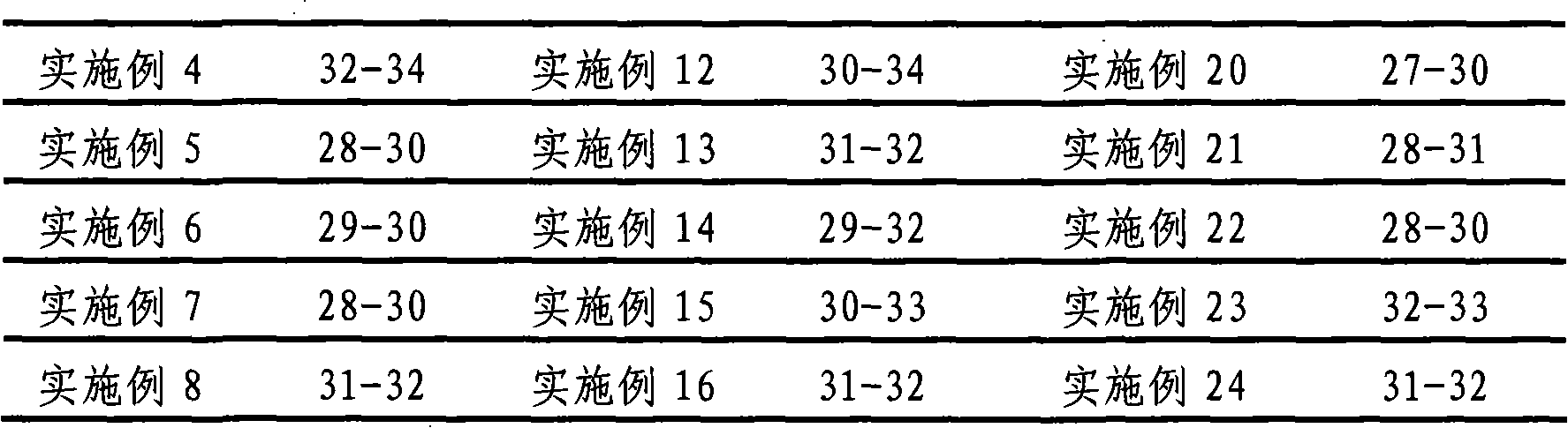
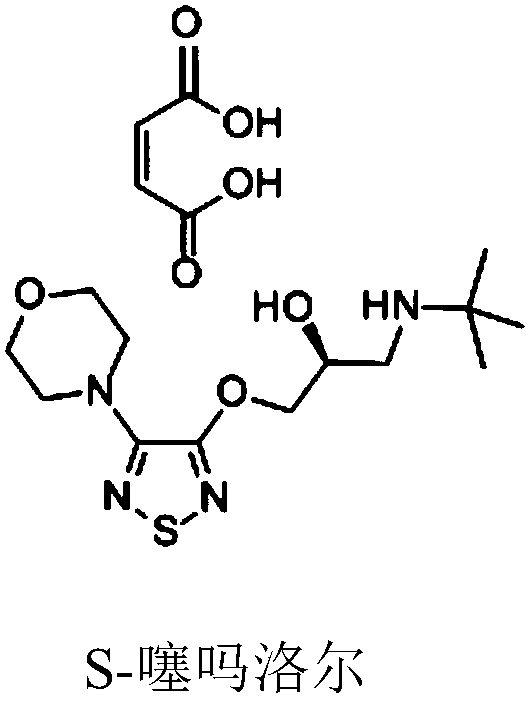
34 results about "Maleate timolol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
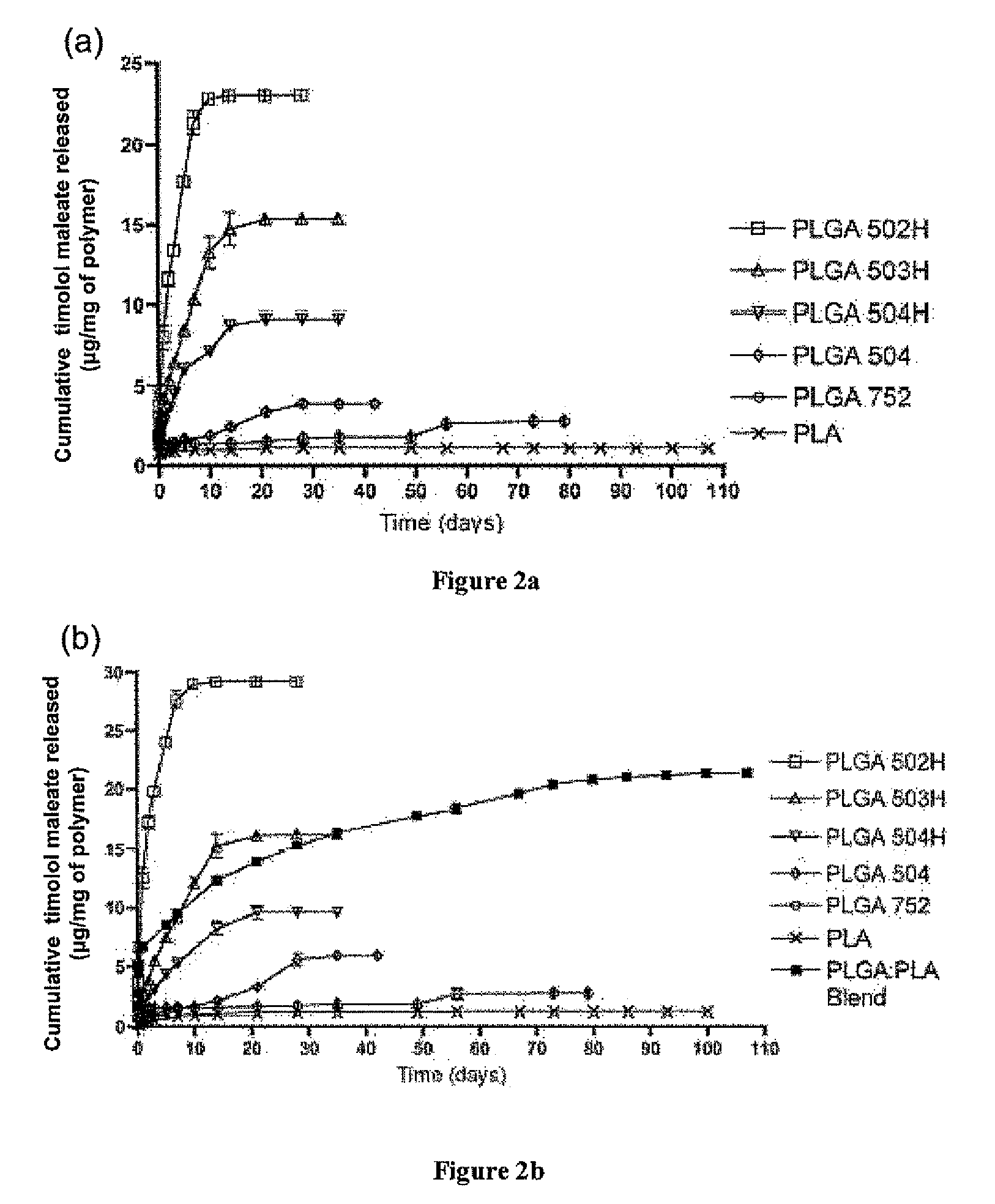
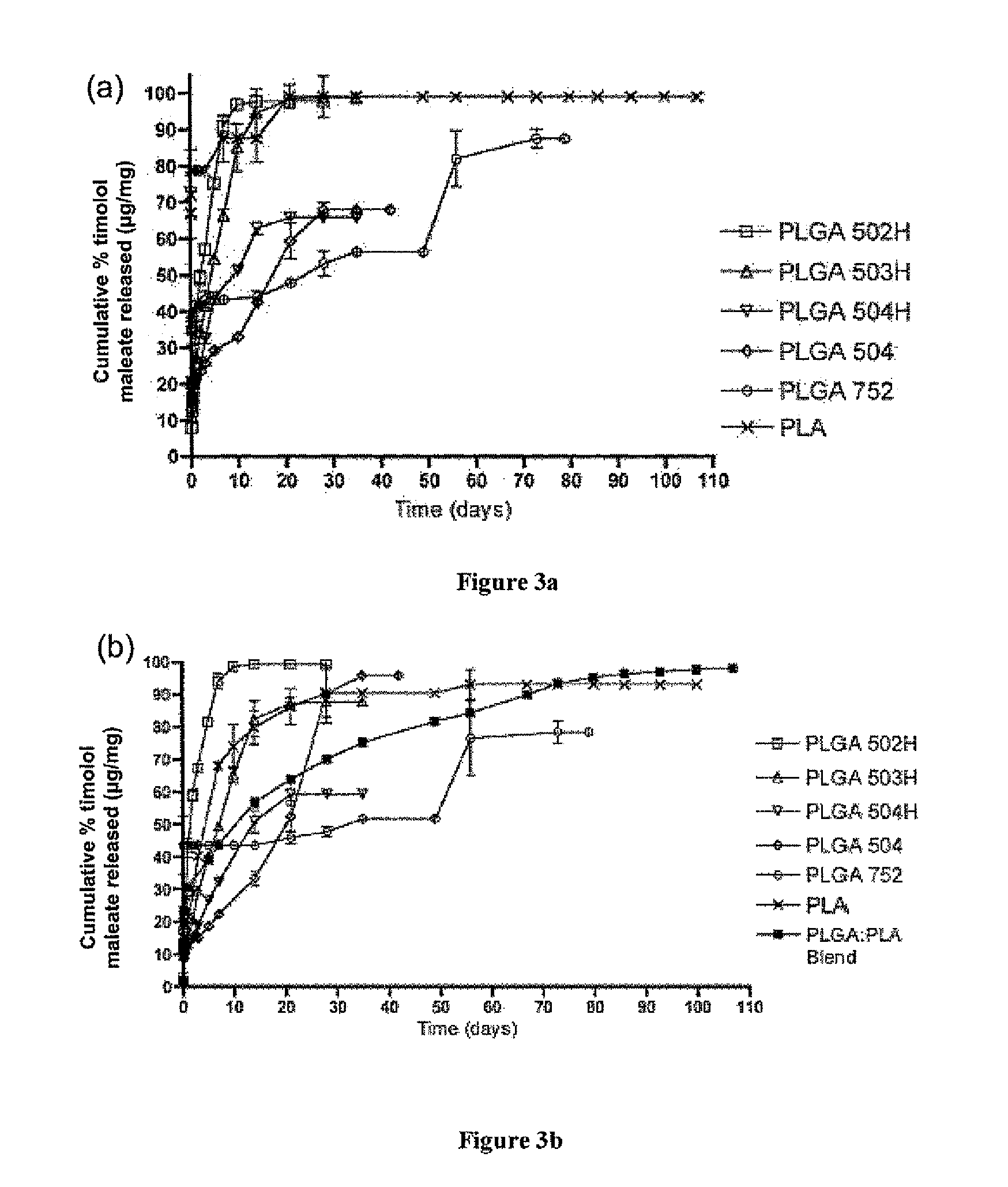
Sustained intraocular delivery of drugs from biodegradable polymeric microparticles
ActiveUS20100261646A1Lower eye pressureSustained releaseHormone peptidesBiocideMicrosphereActive agent
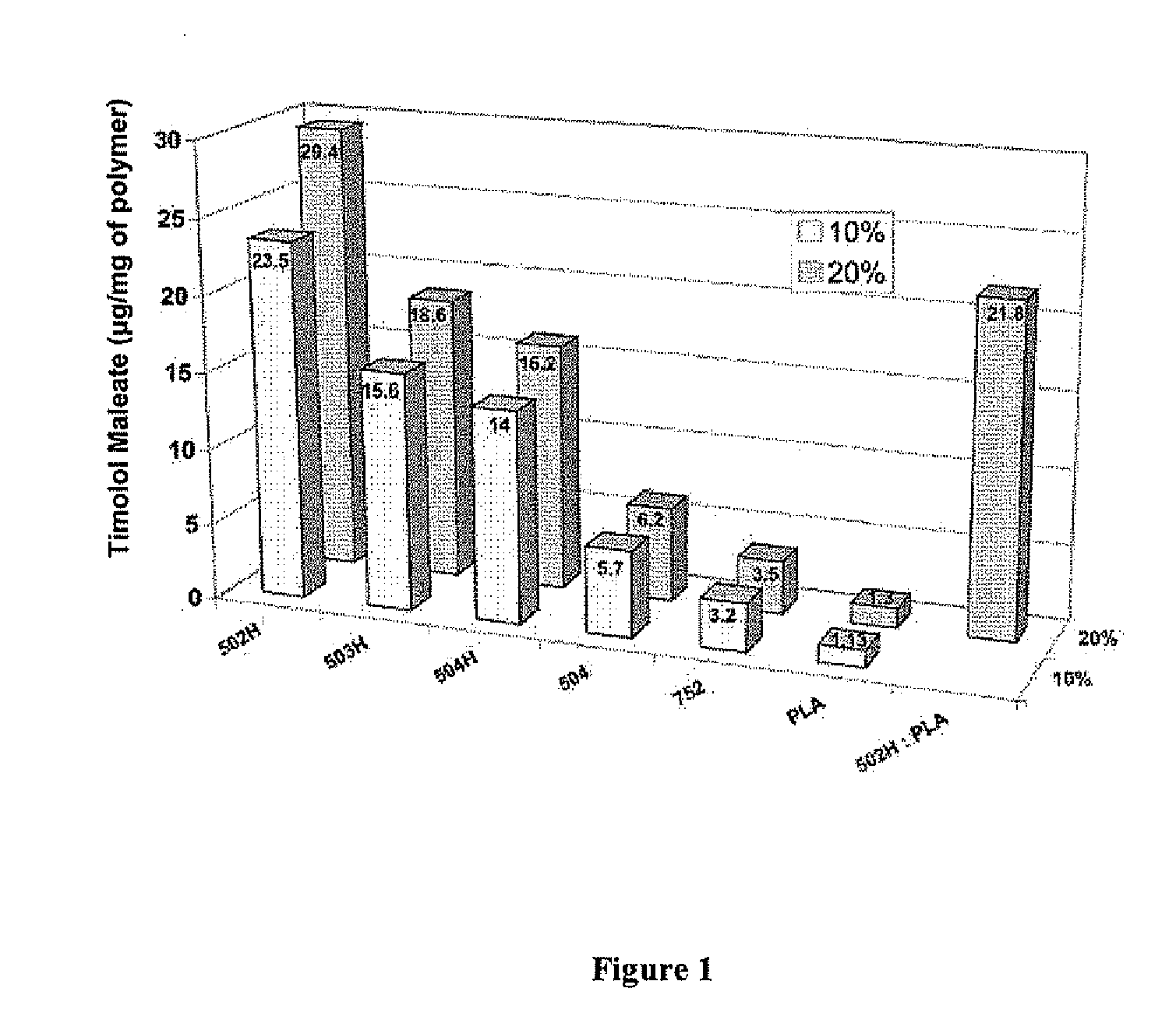
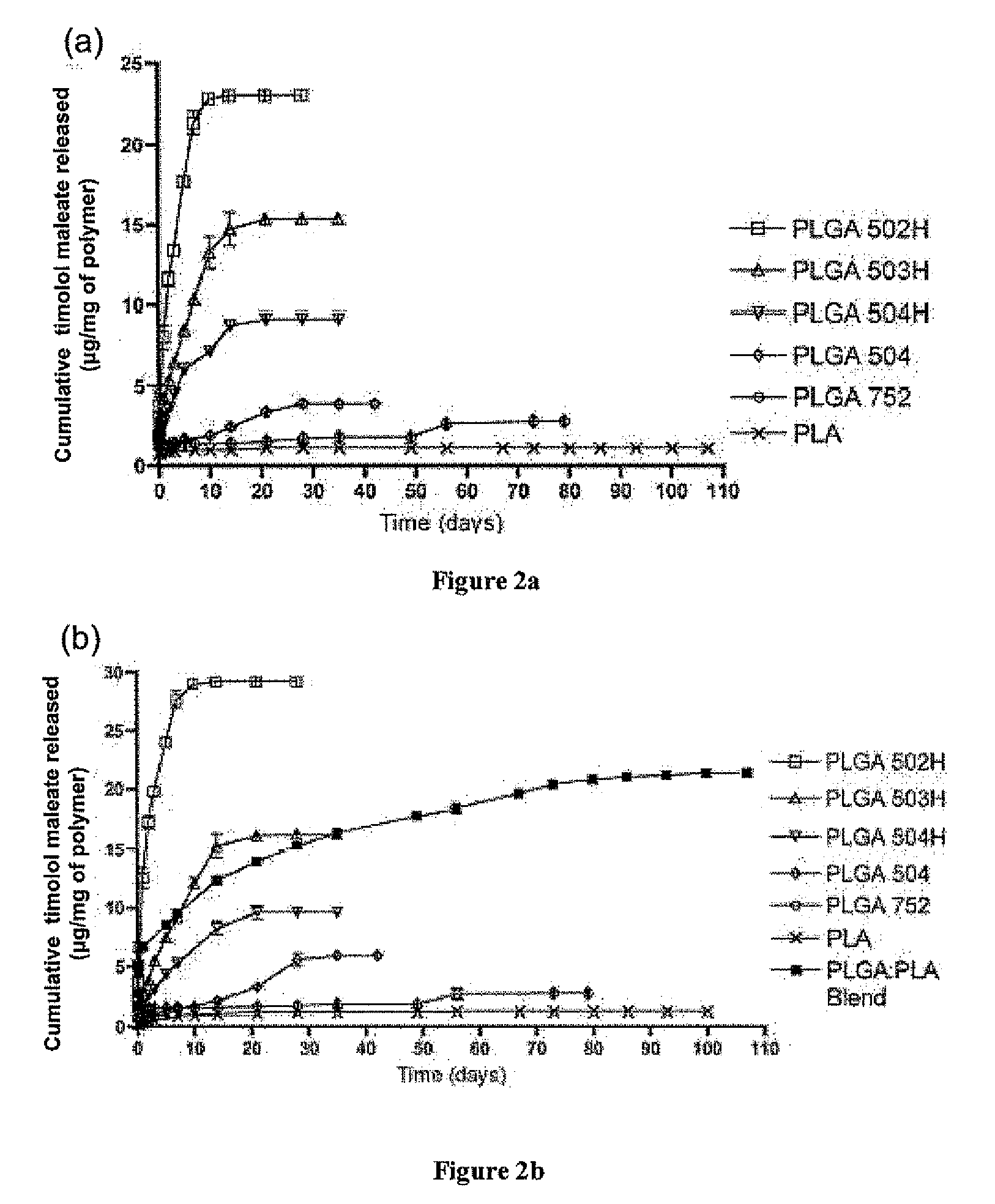
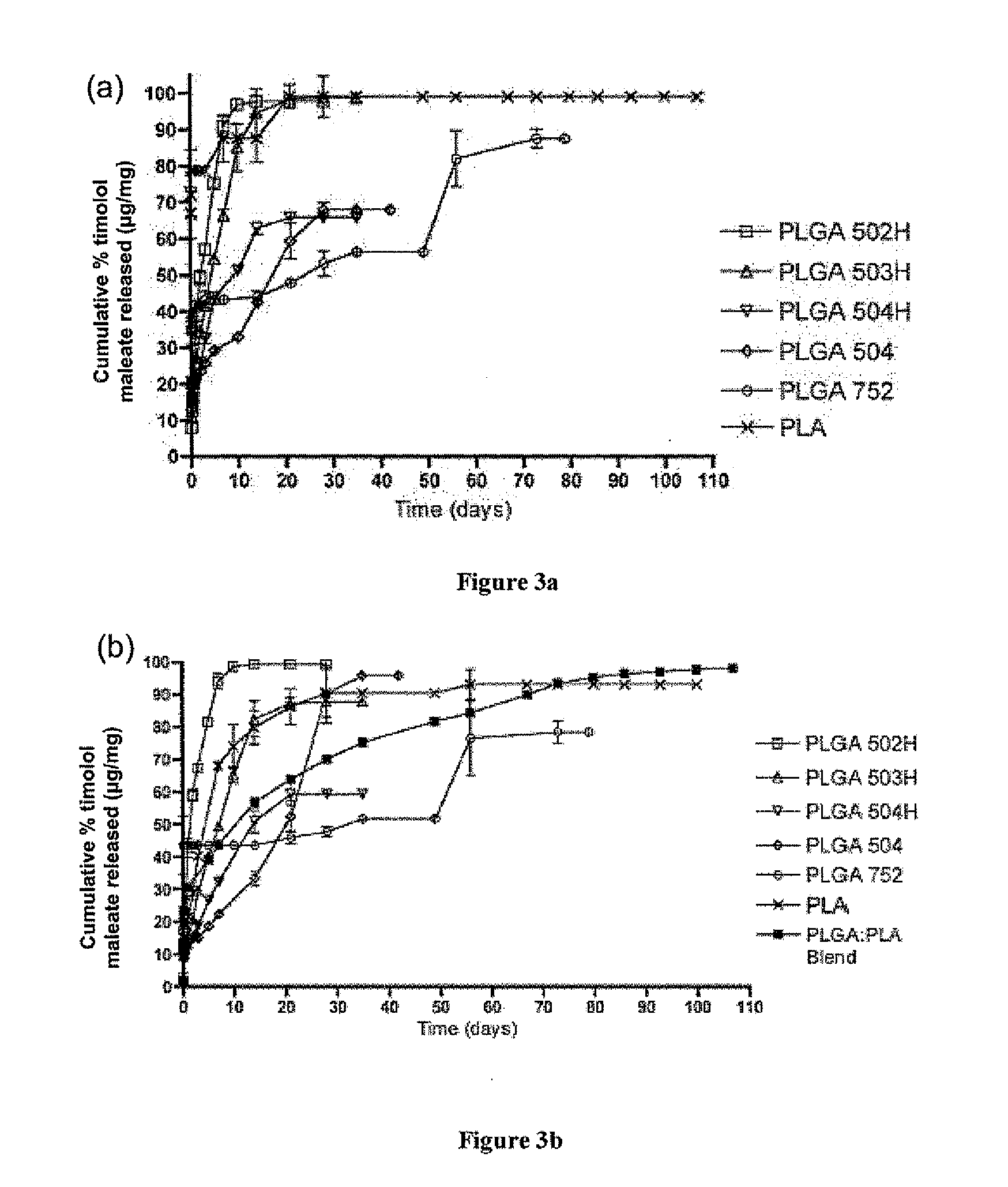
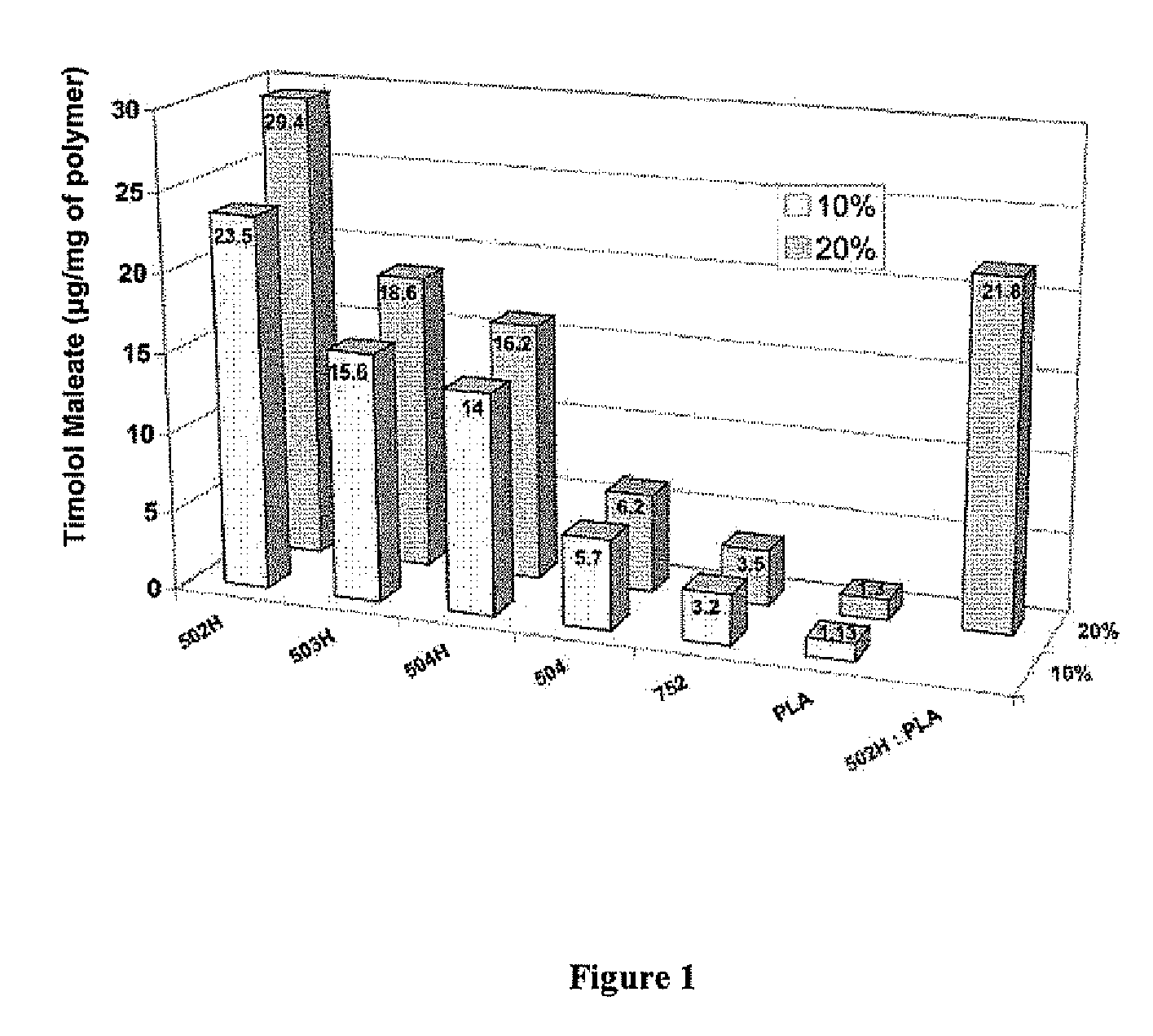
Biodegradable polymeric microparticle compositions containing one or more active agents, especially those useful for treating or preventing or one or more diseases or disorders of the eye, and methods of making and using thereof, are described. The microsphere compositions release an effective amount of the one or more active agents for a period greater than 14 days in vivo, preferably greater than 60 days in vivo, more preferably up to 73 days in vivo, more preferably greater than 90 days in vivo, even more preferably over 100 days in vivo, and most preferably greater than 107 days in vivo. In a preferred embodiment, the microparticle compositions contain one or more active agents useful for managing elevated intraocular pressure (TOP) in the eye. In one embodiment, the microspheres are formed from polylactide-co-glycolide (“PLGA”); in another embodiment, the microspheres are formed from a blend PLGA and poly lactic acid (“PLA”). Relatively hydrophilic, and preferably carboxylated, polymeric materials such as PLGA are used for a drug such as timolol maleate, which is relatively water soluble, to increase drug loading. Higher molecular weight polymers, as well as the ratio of LA (which has a longer degradation time, up to one to two years) to GA (which has a short degradation time, as short as a few days to a week), are used to provide release over a longer period of time. The combination of drug loading and release rate, as well as the minimization of initial burst release, result in prolonged release of a higher amount of drug.
Owner:YALE UNIV +1
Sustained intraocular delivery of drugs from biodegradable polymeric microparticles
ActiveUS8492334B2Maximizes loading of drugMaximize release of an effective amount of the drugBiocideHormone peptidesDiseaseActive agent
Owner:YALE UNIV +1
Preparation method of ointment for treating infantile hemangioma
InactiveCN106729614AAvoid bleedingPromote circulationOrganic active ingredientsAerosol deliveryVitamin CVitamin B12
The invention relates to a preparation method of an ointment for treating infantile hemangioma. The preparation method comprises the following steps: adding timolol hydrogenmaleate, propranolole hydrochloride, tacrolimus, pingyangmycin, vitamin C, vitamin E and vitamin B12 to water according to certain mass ratio, heating to 30-85 DEG C of the temperature, adding a thickener, imiquimod and a wetting agent according to certain mass ratio after stirring and dissolving, and uniformly stirring, and preparing the ointment for treating the infantile hemangioma after cooling. The ointment for treating the infantile hemangioma is an external used medicine, particularly suitable for the infants inconvenient for taking medicines and injection.
Owner:刘腾
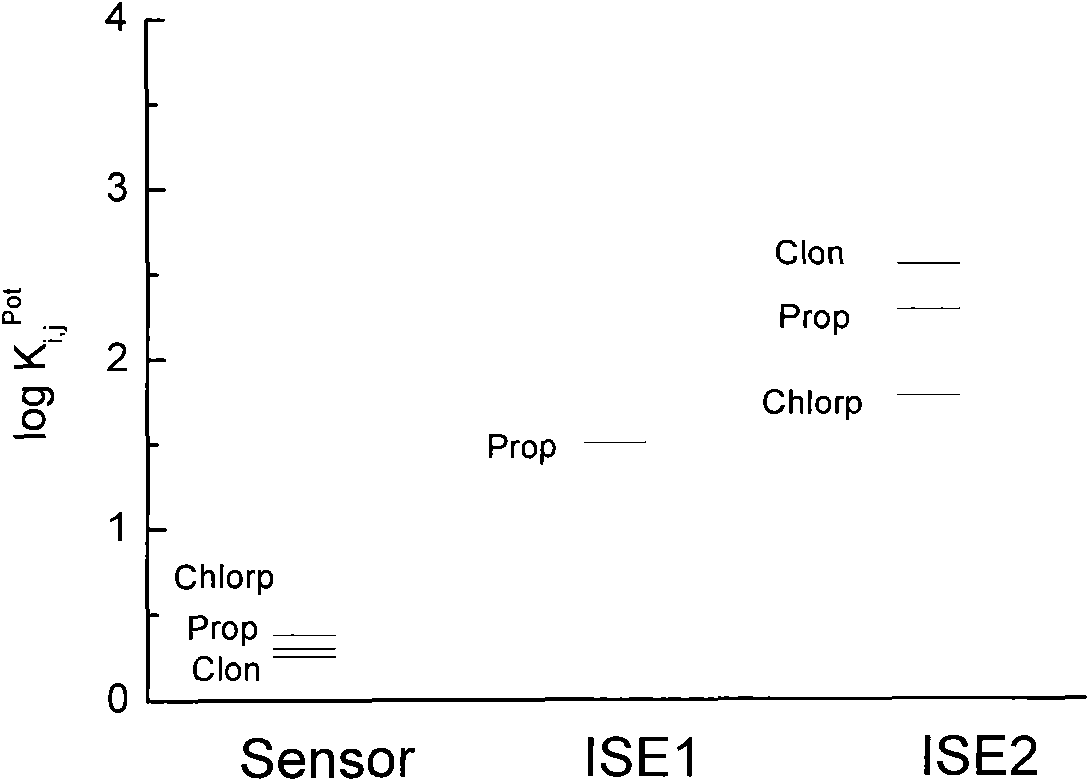
Timolol maleate potentiometric chemical sensor and preparation method thereof
ActiveCN101556258AEliminate internal reference solutionHigh selectivityMaterial electrochemical variablesDip-coatingMolecularly imprinted polymer
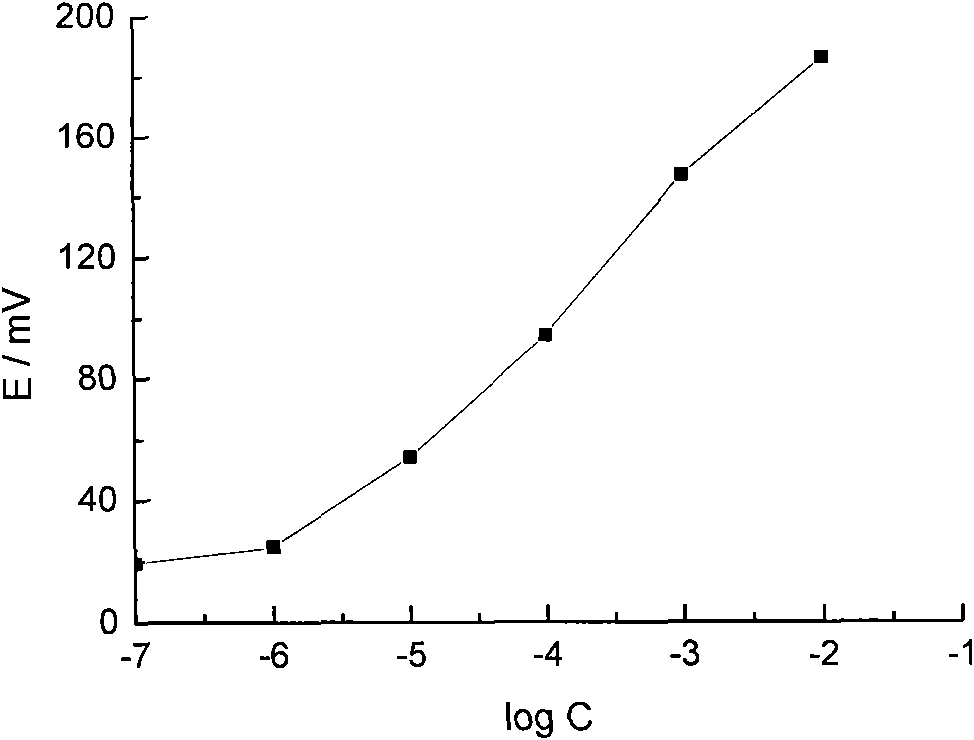
The invention relates to and belongs to a potentiometric chemical sensor, in particular to a timolol maleate potentiometric chemical sensor and a preparation method thereof. The preparation method comprises the following steps: (1) carrying out the chemical modification by the periodic scanning electro-polymerization method after pre-processing the surface of the matrix electrode to prepare the chemically modified electrode; (2) preparing a PVC-film sensitive solution by the conventional method with the solvent being tetrahydrofuran, and coating the modified electrode with the PVC sensitive film by the dip-coating method to prepare a chemical sensor (I); (3) preparing the molecularly imprinted polymer (Polymer A) of timolol maleate, and preparing the PVC film (Film II) containing Polymer A; and (4) compounding the Film II onto the PVC sensitive film of the chemical sensor I. The invention can effectively overcome the disadvantages of the existence of internal reference solution electrodes in the prior art, furthermore, the chemical sensor of the invention is significantly superior to the existing ion-selective electrodes in term of the selectivity of the drug (such as propranolol hydrochloride and the like) ions with the molecule with the structures, such as alcohol amine, imido and the like.
Owner:溧阳常大技术转移中心有限公司
Timolol maleate (TM) eye gel and preparation method thereof
ActiveCN102178644AImprove complianceLess irritatingOrganic active ingredientsSenses disorderChemistryTimolol maleate
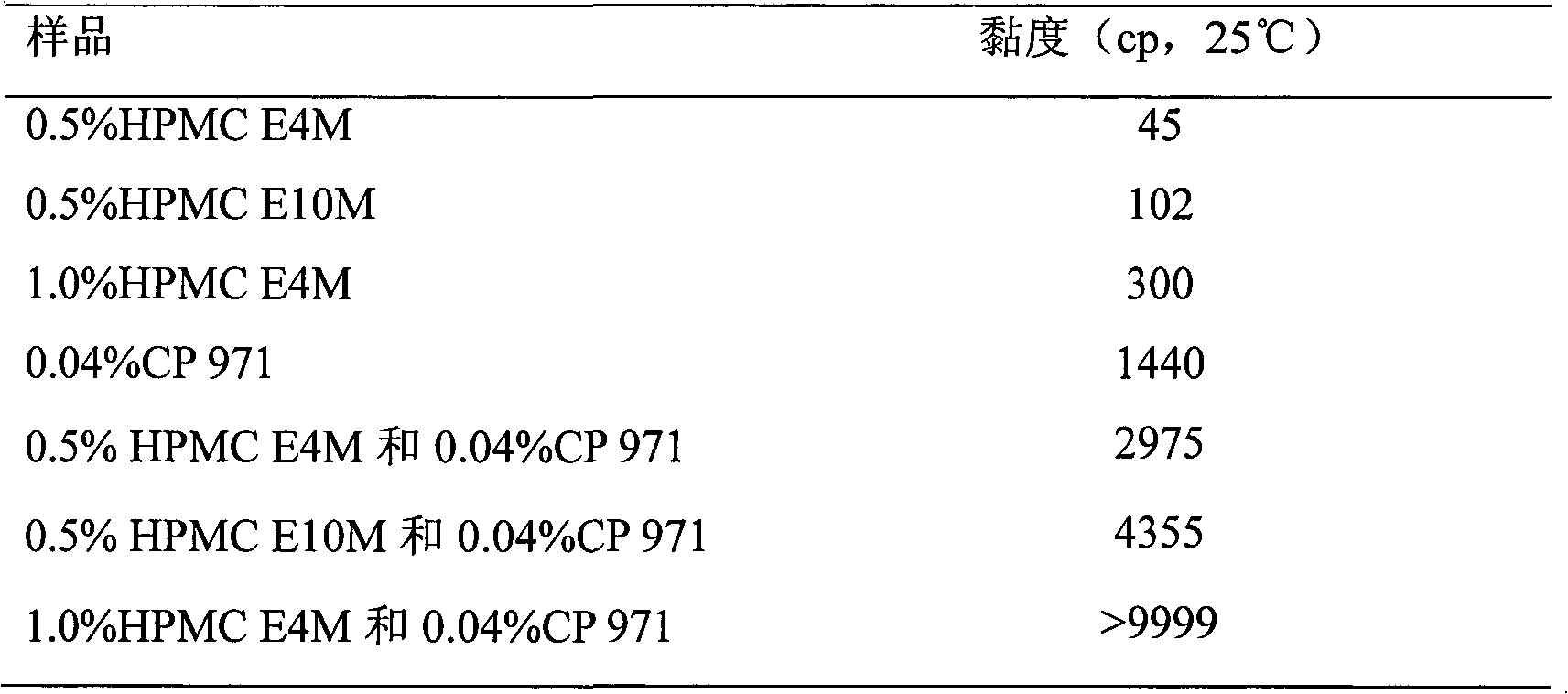
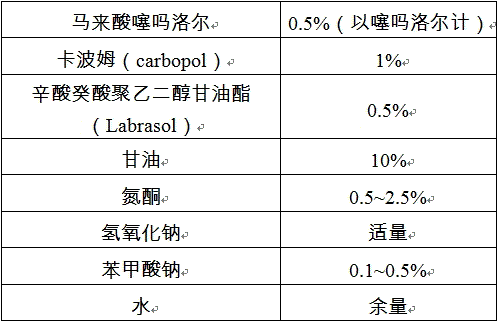
The invention provides timolol maleate (TM) eye gel and a preparation method thereof. One hundred grams of the eye gel comprises the following components by weight: 0.10-0.70g of TM, 0.10-2.0g of gel matrix, 0.2-5.0g of osmotic pressure regulator and the balance of water, wherein the gel matrix is a mixture of cellulose derivatives and carbopol and the weight ratio of cellulose derivatives to carbopol is 1:0.02-1, the cellulose derivatives comprise more than one of methylcellulose, hydroxymethyl cellulose, hydroxypropyl methylcellulose (HPMC) and carboxymethyl cellulose (CMC), and the carbopol comprises one of polyacrylic acid (PAA), polyacrylate or crosslinking polyacrylic resin. Patients with glaucoma and ocular hypertension use the controlled-release TM eye preparation once a day; and the prescription of the eye preparation is free of preservatives, thus lowering stimulation to eyes and improving medication safety if the gel is used for a long term.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Timolol maleate eye drops without bacteriostatic agent and preparation method thereof
InactiveCN101474146AAvoid side effectsAvoid potential dangerOrganic active ingredientsSenses disorderEye dropMaleate timolol
The invention discloses a timolol maleate eye drop which does not contain bacteriostat and a preparation method thereof. The timolol maleate eye drop contains timolol maleate, pH regulator, isotonic agent, stabilizing agent, thickening agent, freshener, etc. The preparation method adopts antiseptic technique filling technology or heat pressing sterilizing technique, and the eye drop uses one-off individual package with single dose, so that the aseptic performance of the product is ensured, and the product is safer, more reliable, simpler, more convenient and sanitary.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Pharmaceutically Stable Compound Consisting of Timolol, Dorzolamide and Brimonidine
The present invention is related to ophthalmic formulations for the treatment of ocular ailments. More specifically, it is related to the pharmaceutical industry in the production of ophthalmic medication for the treatment of ocular hypertension. The advantage of the present invention over other state of the art treatments is that it achieves a composition of Dorzolamide Hydrochloride, Timolol Maleate and Brimonidine Tartrate with excellent properties of stability; it does not give rise to chemical reactions which produce modifications in the active molecules; with no antagonistic effects among the components. The present invention consists of a stable pharmaceutical composition for the treatment of ocular hypertension characterized by consisting of the following excipients: Polyoxyl 40 Stearate, Sodium Borate crystals, Sodium Chloride, Mannitol and Benzalkonium chloride.
Owner:BAYARDO ARTURO JIMENEZ
A kind of timolol maleate gel and preparation method thereof
ActiveCN106727278BEasy to useGood treatment effectOrganic active ingredientsAerosol deliveryMedicineTimolol
Owner:BEIJING MERSON PHARMA CO LTD
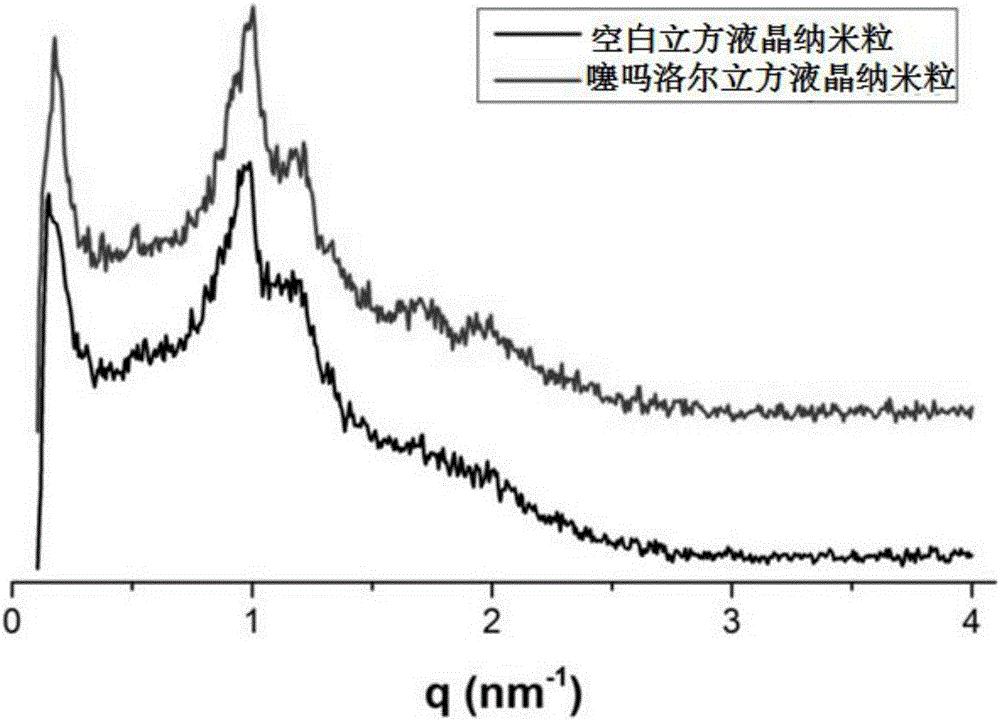
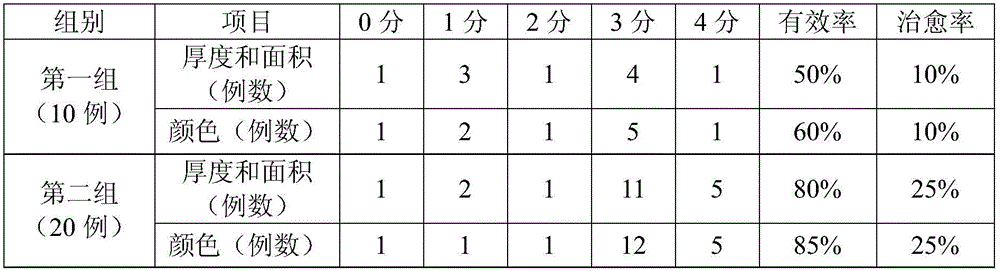
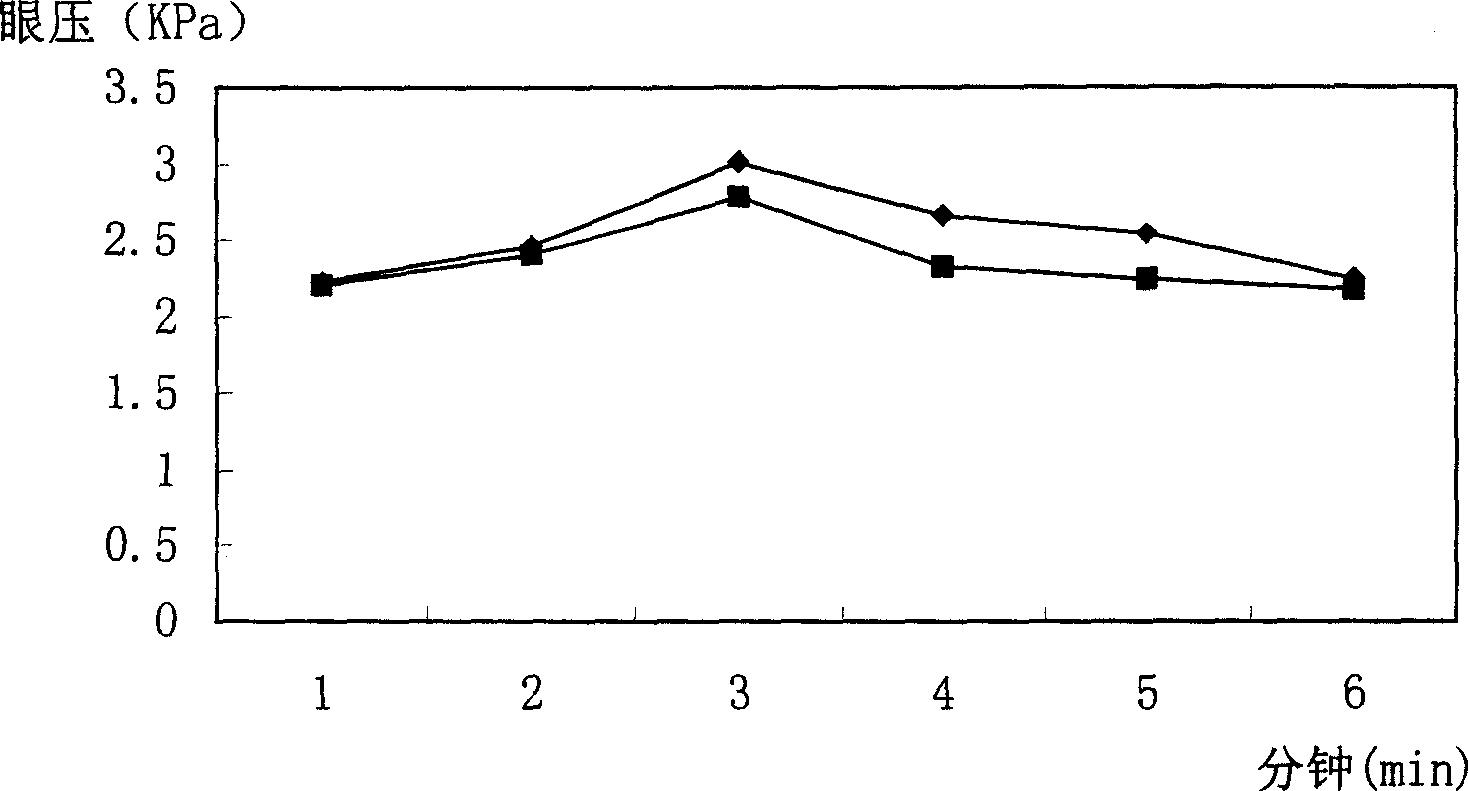
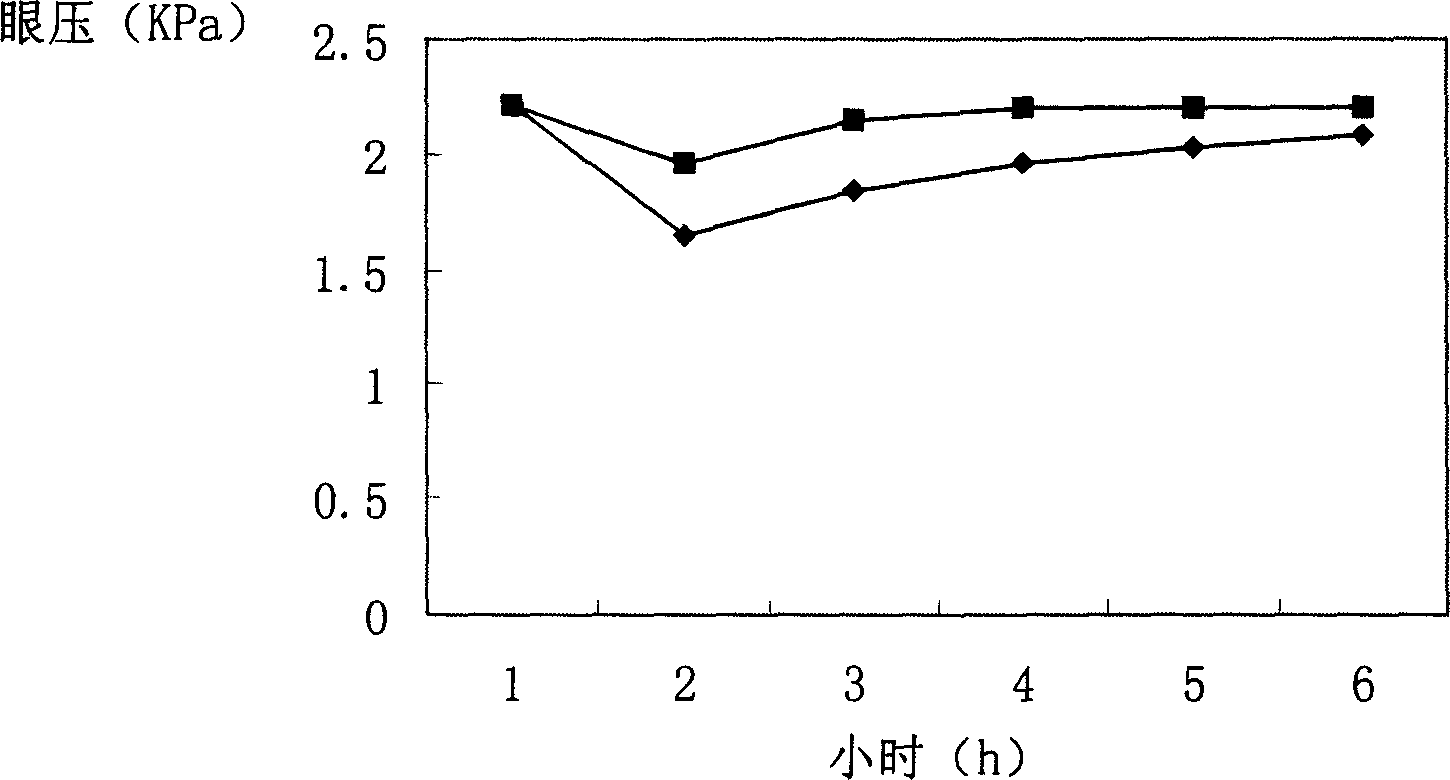
Timolol maleate cubic liquid crystal nanoparticle eye drops and preparation method thereof
ActiveCN106619573AImprove permeabilityExtended stayOrganic active ingredientsSenses disorderIntra ocular pressureRetention time
The invention relates to timolol maleate cubic liquid crystal nanoparticle eye drops and a preparation method thereof. The imolol maleate cubic liquid crystal nanoparticle eye drops are mainly prepared from, by weight, 0.4-1.0 part of timolol maleate, 1.0-18.0 parts of liquid crystal material, 0.2-3.0 parts of surface active agent and 100 parts of water. The liquid crystal material is glycerol monoolein. The surface active agent is poloxamer. According to the timolol maleate cubic liquid crystal nanoparticle eye drops, the cornea infiltration capacity of timolol maleate can be remarkably improved, intra-ocular pressure can be remarkably and stably reduced, the retention time of a drug in the eyes can be prolonged, no preservative is added, and the problems that in the prior art, the retention time of timolol maleate eye drops in the eyes is short and irritation is caused by long-time use can be effectively solved.
Owner:GUANGZHOU NEWORLD PHARMA CO LTD
Timolol maleate pharmaceutical composition and preparation method thereof
ActiveCN107281094AImprove securityImprove efficacyOrganic active ingredientsAerosol deliveryWhole bodyBiocompatibility Testing
The invention belongs to the field of preparations and relates to a timolol maleate pharmaceutical composition and a preparation method thereof. The timolol maleate pharmaceutical composition contains timolol maleate, Carbopol, triethanolamine, propylene glycol, ethylparaben and water. The pharmaceutical composition has the advantages of good biocompatibility, easiness in coating, no greasiness and the like, and the use compliance of a patient is greatly improved; the timolol maleate pharmaceutical composition can directly act on a focus of infection, and the local drug concentration is increased, so that the drug effect is furthest exerted, and the untoward effect of the whole body caused due to oral medication or intravenous medication is avoided; and the pharmaceutical composition is suitable for industrial large-scale production, is used for treating hemangioma and is particularly suitable for treating superficial type infantile hemangioma.
Owner:SHANGHAI AUSON PHARM CO LTD
Maleic acid timolol preparation for treating infant superficial hemangioma and application of maleic acid timolol preparation
InactiveCN106214624AIncrease viscosityKeep dryOrganic active ingredientsAerosol deliveryTimololWater content
The invention provides a maleic acid timolol preparation for treating infant superficial hemangioma and application of the maleic acid timolol preparation. The maleic acid timolol preparation adopts water as a dispersing medium and comprises the following components by mass percent with the total percent of 100%: 0.1-1% of maleic acid timolol and 0.5-2% of hyaluronic acid or hyaluronate. The maleic acid timolol preparation provided by the invention is relatively good in viscosity and convenient for medical operators to rub on patients, a film can be formed on the skin surface, the adhesion time is greatly prolonged, and active components can play roles continuously; the maleic acid timolol preparation has a moisturizing function and is not dried within a short time, so that the skin surface can be kept in a wet state for a long time; as the keratoderma of an infant is thin and the water content is higher than that of an adult, the moisturizing function is particularly important for infant skin, however, hyaluronic acid or hyaluronate is not used in infant preparation dressing yet at present; in addition, application of hyaluronic acid or hyaluronate to the dressing shows by accident that the function of promoting wound healing of infant skin can be achieved.
Owner:北京中燕瑞康医药科技开发有限公司
Gel agent for treating glaucoma and its preparing process
InactiveCN1634071AExtended stayImprove bioavailabilityOrganic active ingredientsSenses disorderTreatment effectMedicine
The invention discloses a gel agent for treating glaucoma and its preparing process which comprises Timolol Maleate, macromolecular material, glycerin, Ethylparabenum, Disodium Edetate, anti-oxidant agent, and distilled water, the preparation consists of steeping the macromolecular material into water, dissolving the Ethylparabenum with hot-water, charging Ethylparabenum solution, Timolol Maleate, glycerin, anti-oxidant agent, Disodium Edetate into macromolecular solution, agitating homogeneously.
Owner:WUHAN UNIV
Instant gel rubber of timolol maleate for eyes
InactiveCN101342176AExtended stayVarious dosage formsOrganic active ingredientsSenses disorderPreservativeGram
The invention discloses an immediate type ophthalmic gelatin of timolol maleate and a preparation method thereof. 100 milliliters of the immediate type ophthalmic gelatin of timolol maleate contains 1 to 1.5 grams of the timolol maleate, 0.05 to 1 gram of preservative, 1.0 to 7.5 grams of permeation pressure conditioning agent and 30 to 150 grams of gelatin substrate. Remaining components are acid-base buffer regent and water for injection. The invention enriches the dosage forms of the timolol maleate by optimizing auxiliary materials and improving technology so that lag time in eyes is greatly prolonged and a curative effect is improved without adverse stimulus reaction.
Owner:肖正连
Timolol hydrogenmaleate gel and preparation method thereof
ActiveCN106727278AEasy to useGood treatment effectOrganic active ingredientsAerosol deliveryMedicineTimolol
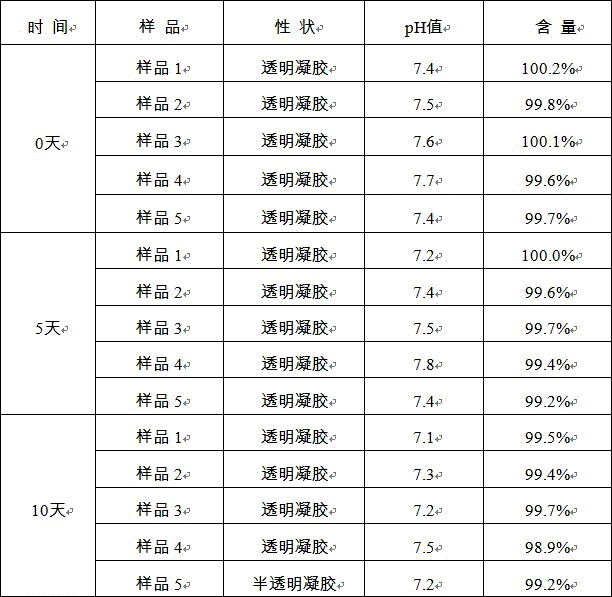
The invention provides timolol hydrogenmaleate gel and a preparation method thereof. The content of the timolol hydrogenmaleate gel is 95.0 to 105.0 percent of the labeled amount, and the timolol hydrogenmaleate gel is transparent or semi-transparent hydrogel and has the pH value between 6.0 and 8.0. The timolol hydrogenmaleate gel disclosed by the invention is high in stability and non-stimulating to the skin and can achieve the medicinal effect through the skin.
Owner:BEIJING MERSON PHARMA CO LTD
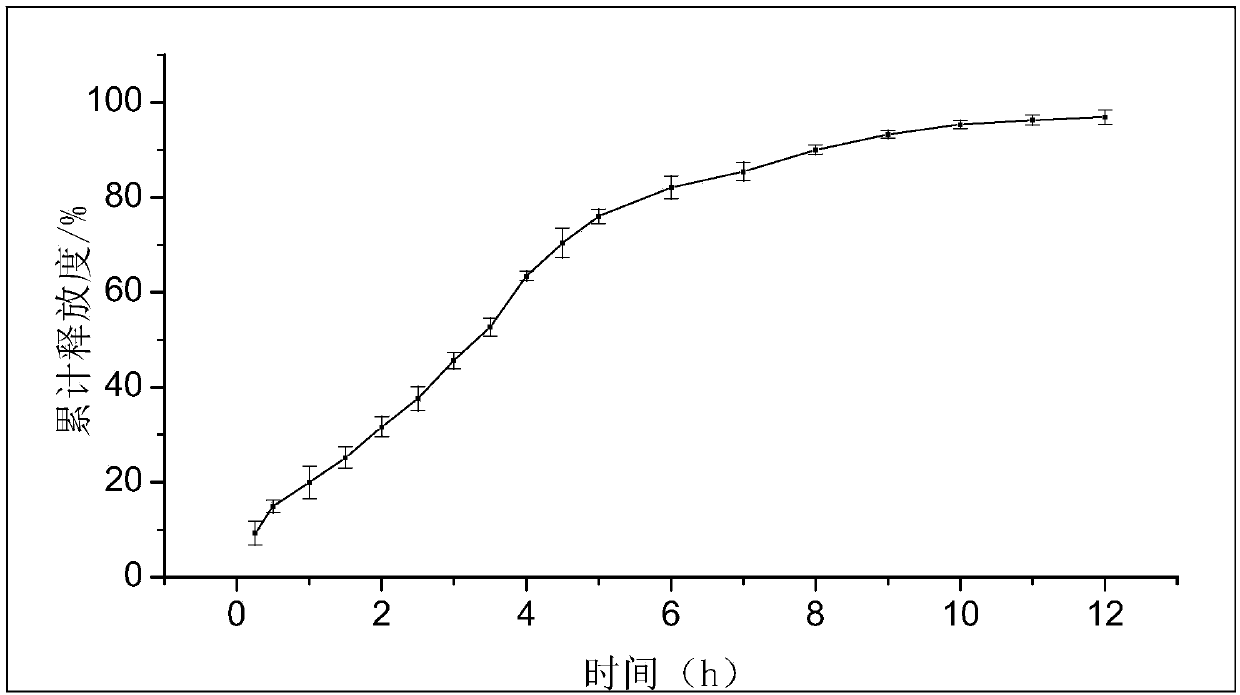
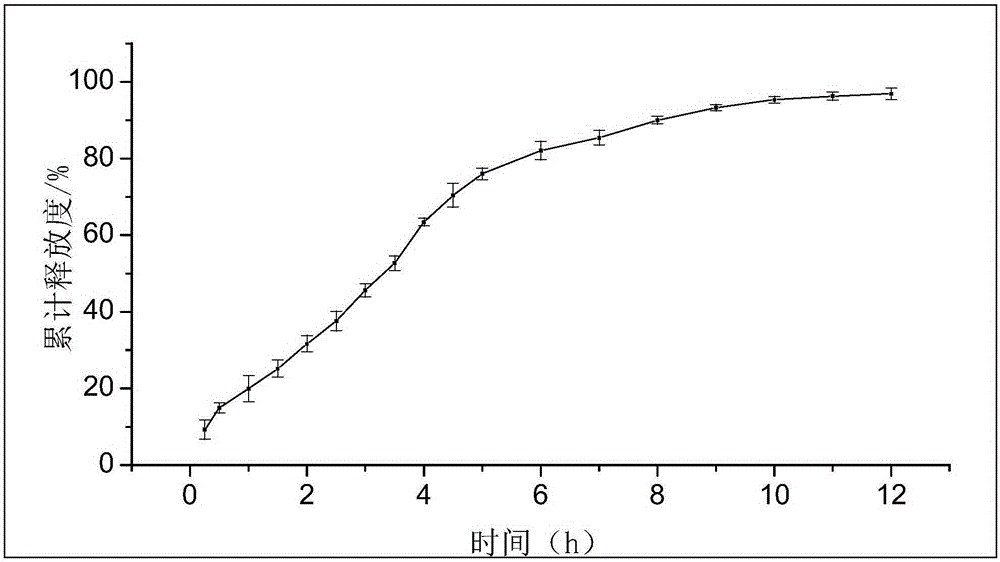
Preparation method of timolol maleate sustained release microspheres
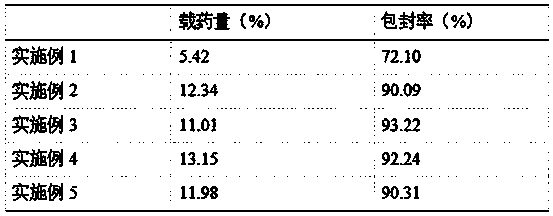
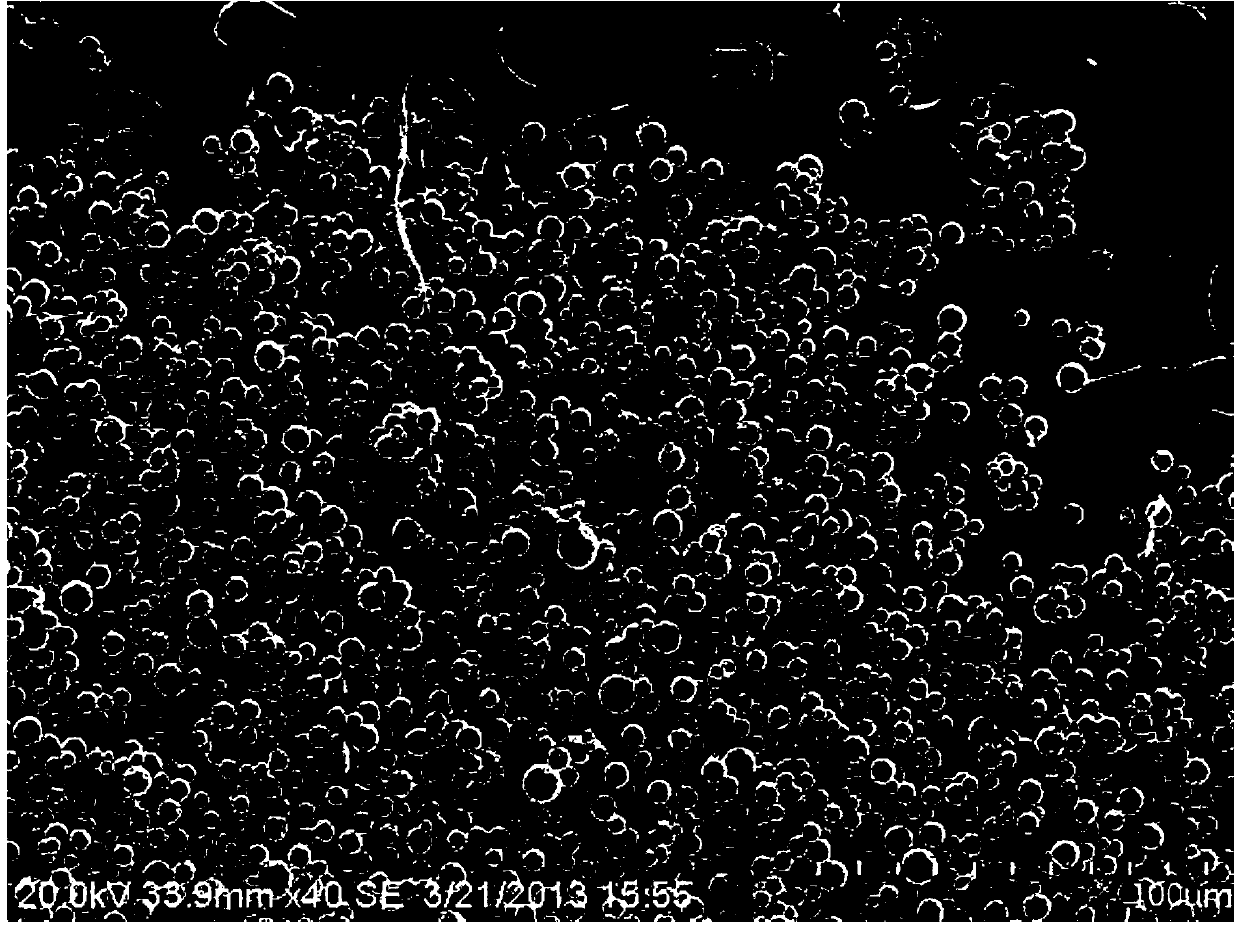
ActiveCN103735518AMeet the requirementsHigh encapsulation efficiencyOrganic active ingredientsSenses disorderIntra ocular pressureVegetable oil
The invention discloses a preparation method of timolol maleate sustained release microspheres. The preparation method comprises the following steps: by adopting an oil-in-oil technology, preparing an internal phase from timolol maleate, modified montmorillonite, acrylic resin, tween and a plasticizer and preparing an external phase from vegetable oil and span; carrying out ultrasonic treatment on an obtained oil-in-oil emulsion; then, electromagnetically stirring to evaporate the internal phase to obtain the timolol maleate sustained release microspheres. The grain diameter of the timolol maleate sustained release microspheres can be controlled in a range of 10 mu m, so that the demand of eye-drops preparations is met. The medicine encapsulation rate reaches up to 80-99%, and the in vitro releasing time can be prolonged to 10-12 hours. The timolol maleate sustained release microspheres prepared by the method serving as an ocular hypotensive agent can reduce intraocular discomfort and reduce the medication frequency, so that the purpose of reducing intraocular pressure for a long time by one-time delivery is realized.
Owner:广州铂思雅生物医药科技有限公司
Timolol hydrogenmaleate eye drops and preparation method thereof
The invention provides timolol hydrogenmaleate eye drops, comprising timolol hydrogenmaleate, an isotonizing agent, a thickener, and a stabilizing agent, wherein glycerol is used as the isotonizing agent, the thickener and the stabilizing agent and plays a stabilizing role in the timolol hydrogenmaleate eye drops since glycerol has osmosis and thickening functions. According to the invention, glycerol is used as the isotonizing agent, the thickener and the stabilizing agent, and the effect that one raw material has multiple functions is achieved, so that the timolol hydrogenmaleate eye drops can be produced conveniently and can be applied comfortably without irritation; moreover, since glycerol which is used as the isotonizing agent, the thickener and the stabilizing agent in the timolol hydrogenmaleate eye drops, the stability of the medicine is increased and the clinical functions of the timolol hydrogenmaleate eye drops are ensured. In addition, the invention also provides a preparation method of the timolol hydrogenmaleate eye drops.
Owner:ANHUI GLOBAL PHARM CO LTD
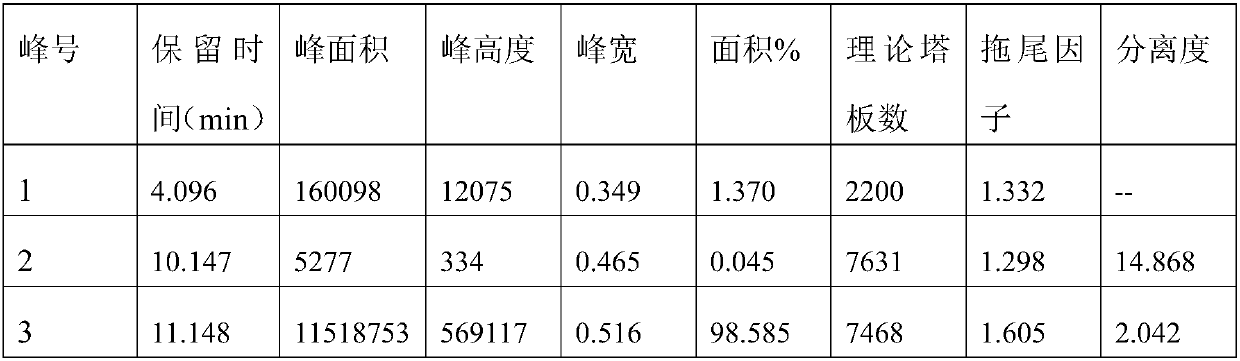
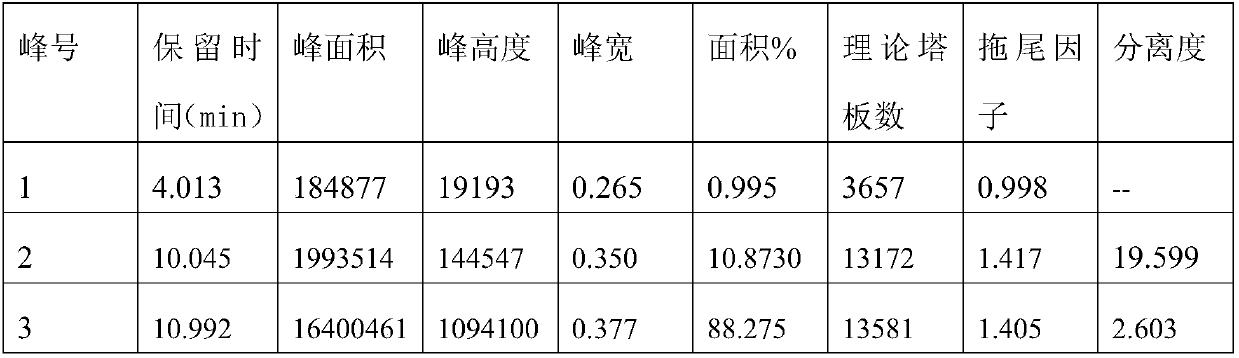
Method for separating timolol compound and timolol maleate related impurities and application of method
ActiveCN109991319AAchieve separationQuality improvementComponent separationSilica gelChromatographic column
The invention discloses a method for separating a timolol compound and / or timolol maleate related impurities and application of the method. According to the method for separating the timolol compoundand / or the timolol maleate related impurities and the application of the method, a chromatographic column of which filler is silica gel bonded with quinine derivatives on the surface is applied to high performance liquid chromatography conditions to separate the timolol compound, a timolol maleate compound and the related impurities of timolol maleate. The method adopts a reversed-phase high-performance liquid phase system, the timolol compound and the related impurities of timolol maleate can be effectively separated, the accuracy is high, the sensitivity is high, and the method is environmentally friendly and can be used for quantitative detection of the timolol compound.
Owner:WUHAN CONFORM PHARMA CO LTD
Sustained-release suspension for treating glaucoma and preparation method thereof
InactiveCN103432071AImprove bioavailabilityMedication safetyOrganic active ingredientsSenses disorderIrritationSuspending Agents
The invention relates to a sustained-release suspension for treating glaucoma and a preparation method thereof, effectively solving the problem of low bioavailability in the prior art. According to the technical scheme, the sustained-release suspension comprises the following components in percentage by weight: 1.0-5.0 percent of timolol maleate, 1.0-5.0 percent of IRP-69 cation exchange resin, 0.1-10 percent of a micro-capsule coating material, 0.1-10 percent of plasticizer, 3-10 percent of impregnant, 0.1-20 percent of suspending agent, 0.1-10 percent of osmotic pressure regulator, 0.001-0.02 percent of preservative, 0.1-10 percent of pH regulator, 1.0-20 percent of sorbitan monooleate, 5-50 percent of liquid paraffin, 1.0-50 percent of micro-capsule coating material solvent, and 30-70 percent of super-pure water. The sustained-release suspension is safe in administration and definite in curative effect, does not have irritation, can remarkably improve the bioavailability of timolol maleate, and is an innovation in the field of medicine for treating glaucoma.
Owner:ZHENGZHOU UNIV
Ophthalmologic intraocular pressure reduction drug for external use
InactiveCN103040837AImprove efficacyGood curative effectOrganic active ingredientsSenses disorderAdditive ingredientTanshinone IIA
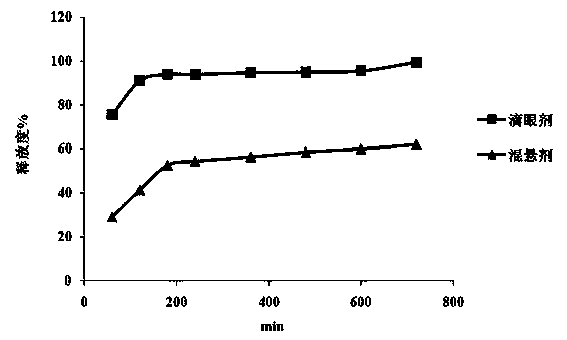
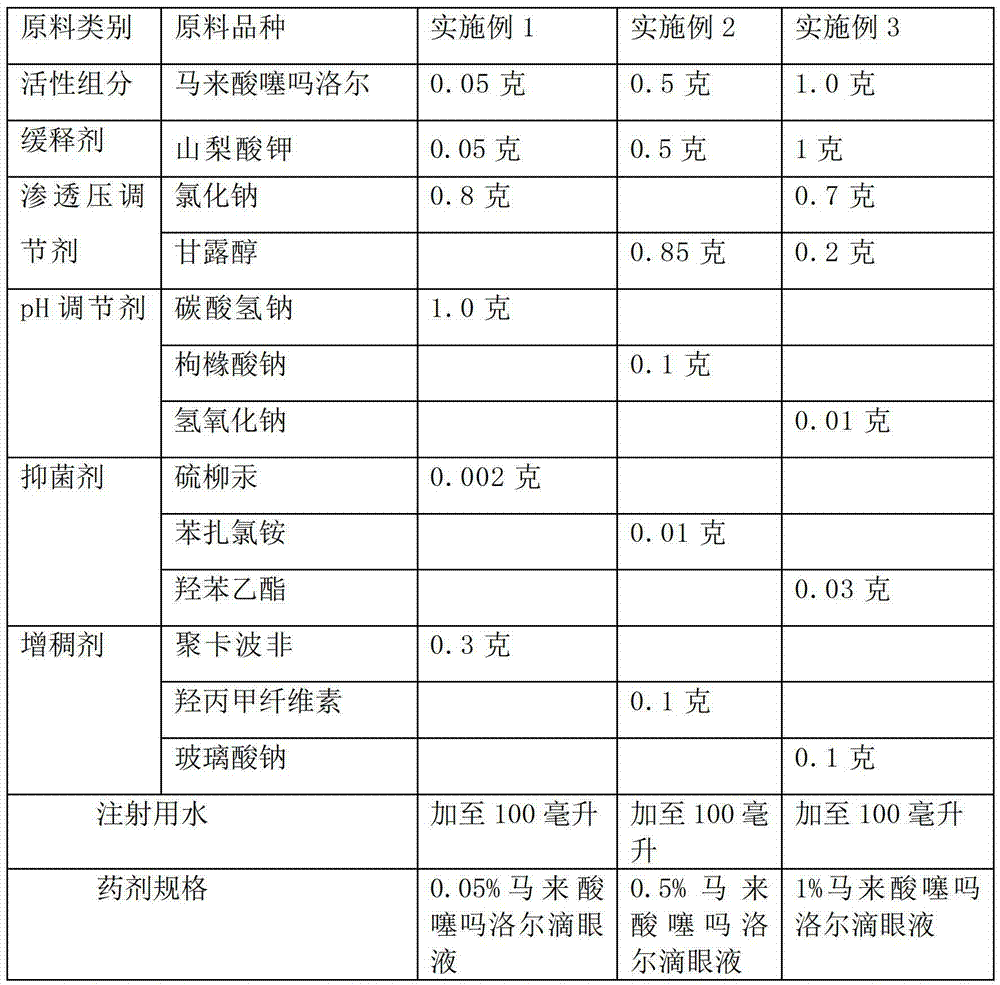
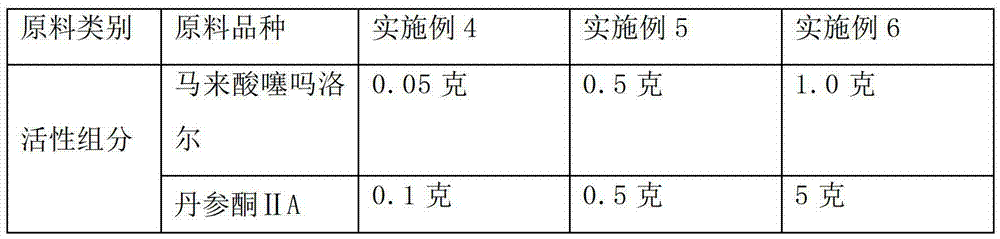
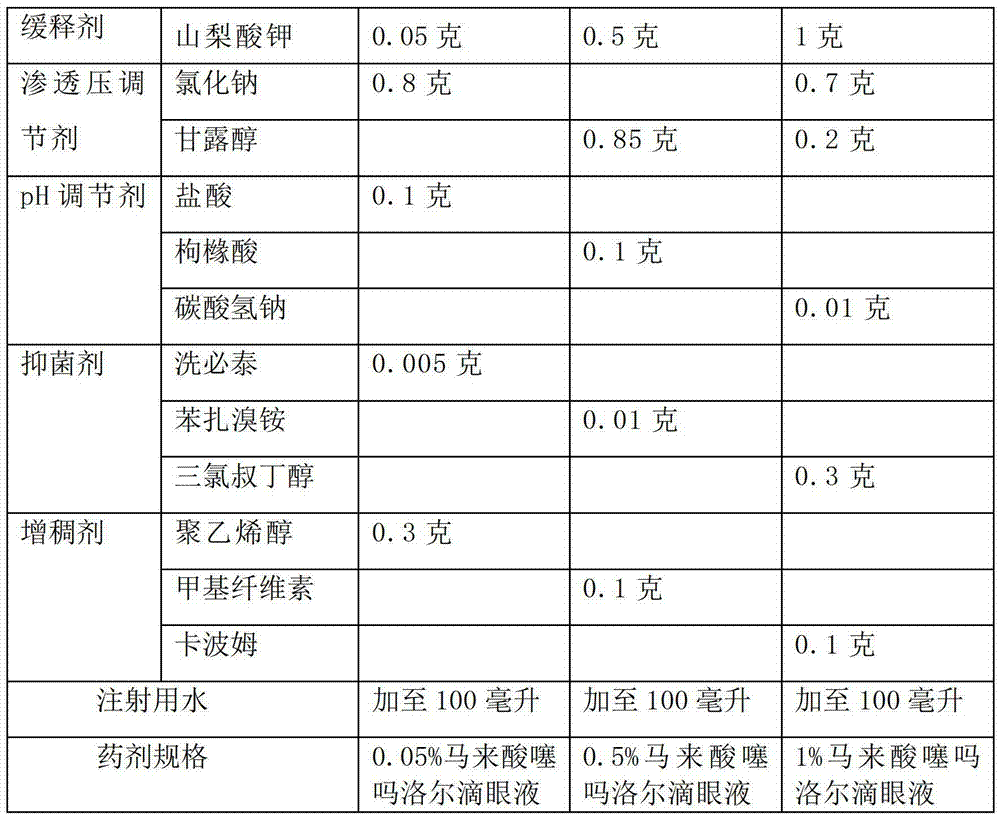
The invention discloses an ophthalmologic intraocular pressure reduction drug for external use. The drug takes timolol maleate as an active pharmaceutical ingredient; and the drug further comprises potassium sorbate, and the potassium sorbate is added according to the ratio that timolol maleate:potassium sorbate equals to 1:(0.1-5.0). The drug also takes tanshinone IIA as the active pharmaceutical ingredient, and the tanshinone IIA is added according to the ratio that timolol maleate:tanshinone IIA equals to 1:(0.1-5.0). The potassium sorbate-containing timolol maleate eye drops have a lasting medicinal effect, and the frequency for dropping the eye drops can be reduced.
Owner:GUANGDONG WHOLEWIN TECH
Timolol maleate cubic liquid crystal nanoparticle eye drops and preparation method thereof
ActiveCN106619573BImprove permeabilityExtended stayOrganic active ingredientsSenses disorderIntra ocular pressureIrritation
The invention relates to timolol maleate cubic liquid crystal nanoparticle eye drops and a preparation method thereof. The imolol maleate cubic liquid crystal nanoparticle eye drops are mainly prepared from, by weight, 0.4-1.0 part of timolol maleate, 1.0-18.0 parts of liquid crystal material, 0.2-3.0 parts of surface active agent and 100 parts of water. The liquid crystal material is glycerol monoolein. The surface active agent is poloxamer. According to the timolol maleate cubic liquid crystal nanoparticle eye drops, the cornea infiltration capacity of timolol maleate can be remarkably improved, intra-ocular pressure can be remarkably and stably reduced, the retention time of a drug in the eyes can be prolonged, no preservative is added, and the problems that in the prior art, the retention time of timolol maleate eye drops in the eyes is short and irritation is caused by long-time use can be effectively solved.
Owner:GUANGZHOU NEWORLD PHARMA CO LTD
Timolol maleate liposome gel and preparation method thereof
PendingCN113244171AImprove stabilityImprove applicabilityOrganic active ingredientsAerosol deliveryInfantile haemangiomaDrug administration
The invention provides a timolol maleate liposome gel and a preparation method thereof. The gel is prepared from timolol maleate, a penetration enhancer, a humectant, a preservative, a liposome forming agent, a gel matrix, a pH regulator and medical purified water. The prepared timolol maleate liposome gel is uniform and delicate, suitable in viscosity and easy to spread, the drug release time is prolonged through the liposome technology, the concentration of the drug at the diseased region is kept, the gel is used for treating infantile hemangioma, drug administration is convenient, meanwhile, the first-pass effect of the liver is avoided, and meanwhile the gel is convenient to use, high in safety, simple in production process and good in application prospect.
Owner:周斌
Method for determining impurities in timolol maleate
PendingCN114354818AStrong targetingImprove accuracyComponent separationLiquid chromatography mass spectroscopySolvent
The invention discloses a method for determining impurities in timolol maleate. The method comprises the following steps: taking a timolol maleate sample homologous with a timolol maleate sample to be detected, and reacting to obtain a destroying solution containing an impurity X; the method comprises the following steps: taking a timolol maleate sample to be detected, and preparing a test solution by using a solvent; diluting the test solution as a contrast solution; respectively carrying out liquid chromatography detection on the destroyed solution, the test solution and the contrast solution so as to calculate the content of the impurity X in the to-be-detected sample. According to the method, impurities G, B, D, E, C and 5 can be directionally damaged, the interference impurity is small, the positioning of each impurity is not influenced, an impurity reference substance is not used, an ion pair reagent and a peak shape improver are removed from a chromatographic system, the loss of a chromatographic column is relatively small, the method can be simultaneously used for liquid chromatography and liquid chromatography-mass spectrometry detection, the qualitative analysis on the impurities can be conveniently carried out at the same time, and the detection accuracy is high. The economical efficiency and the universality of the method are improved.
Owner:广东省药品检验所
Synthesis method of timolol maleate intermediates
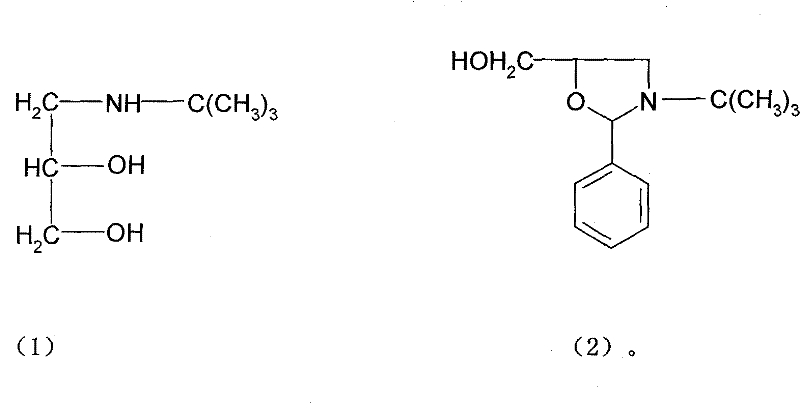
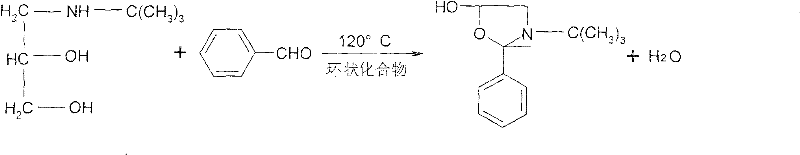
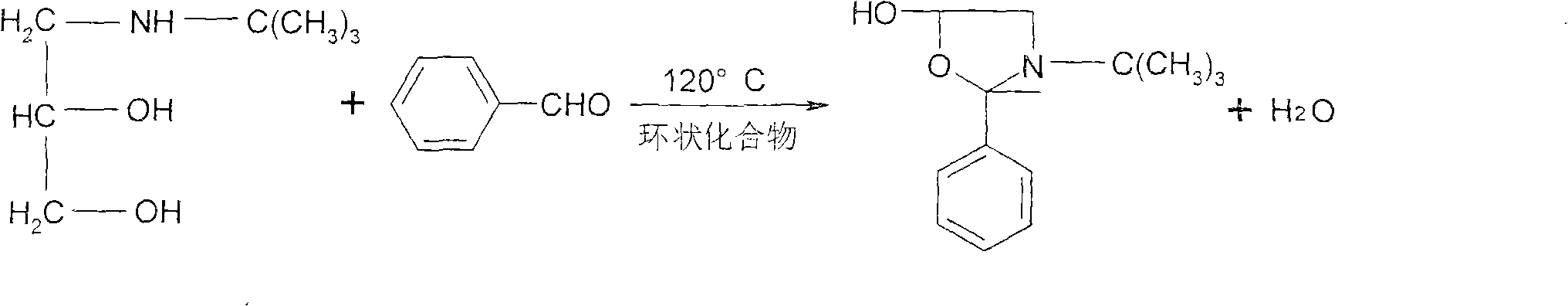
ActiveCN101774977BSimple and fast operationConducive to large-scale industrial productionOrganic chemistryDistillationBenzaldehyde
The invention discloses a synthesis method of timolol maleate intermediates, which takes a cyclic compound as a dissolvent. The synthesis method is characterized in that the cyclic compound is taken as the dissolvent, and amine diol and benzaldehyde generates oxazole product by cyclization; and the timolol maleate intermediate is obtained after distillation, wherein the molar ratio of the cyclic compound as the dissolvent to the amine diol is 1:2-6.5. The invention adopts the cyclic compound as the dissolvent to replace a highly toxic benzene dissolvent so as to obtain high-quality and high-yield timolol maleate intermediates. The synthesis method of the invention has simple operation in industrial production, repeatedly recycled dissolvent, and environment protection, and is more favorable for large-scale industrial production.
Owner:TIANJIN CENT PHARM CO LTD
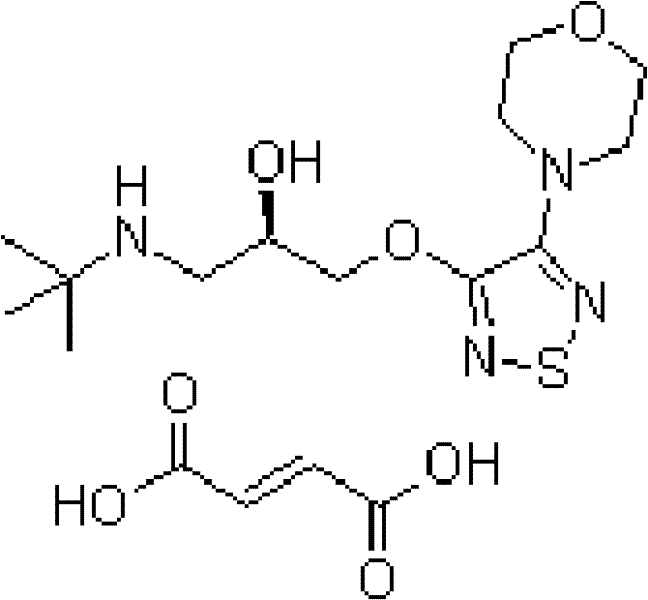
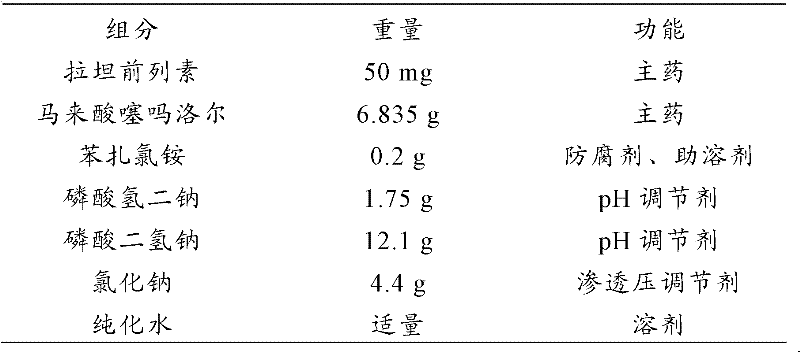
Pharmaceutical composition and compound preparation thereof
InactiveCN102389433AReduce generationPromote excretionOrganic active ingredientsSenses disorderSide effectPatient compliance
The invention discloses a pharmaceutical composition consisting of latanoprost and timolol maleate, with weight percentages of 0.004-0.007% and 0.6-0.8% respectively. The invention also provides a compound preparation of the pharmaceutical composition, wherein the mass content of the timolol maleate is 0.6-0.8%, and the mass content of the latanoprost is 0.004-0.007%; and auxiliary materials include benzalkonium chloride or RH40 hydrogenated castor oil, sodium chloride, sodium dihydrogen phosphate, disodium hydrogen phosphate and purified water, with the pH of 5.0-6.5. The compound preparation can be used for treating glaucoma with small side effect, so that patient compliance is higher.
Owner:ZHAOKE PHARMA HONG KONG COMPANY
Timolol maleate eye drops and preparation process
PendingCN112451478AExtended stayImprove bioavailabilityOrganic active ingredientsSenses disorderEye dropPhotochemistry
The invention discloses timolol maleate eye drops and a preparation process. The timolol maleate eye drops are prepared from the following raw materials in parts by weight: 10 to 12 parts of dispersion liquid C, 5 to 7 parts of timolol maleate nanoparticles, 20 to 30 parts of a water-based solvent, 4 to 6 parts of a buffering solution, 100 to 200 parts of a diluting agent and 0.01 to 0.03 part ofbenzalkonium chloride. The timolol maleate eye drops are prepared through the following steps: step 1, preparing the dispersion liquid C; step 2, adding the timolol maleate nanoparticles into the dispersion liquid C prepared by step 1 to prepare a nano mixed solution; and step 3, mixing a glucose solution with the mass percent of 8 percent and the nano mixed solution obtained by step 2, and carrying out vacuum freeze-drying for 8h to 10h; after freeze-drying is finished, adding a solid obtained by the freeze-drying into the buffering solution and the diluting agent; raising the temperature to80 DEG C, and keeping the heat for 30min; then cooling to 40 to 50 DEG C; adding the benzalkonium chloride and stirring and dissolving; filtering by a filter element; filling and sealing.
Owner:JIANGSU YUANHENG PHARMA
Composition for treating ocular disorders such as macular degeneration, retinopathy and glaucoma
InactiveUS20200188405A1Effective controlReduce in quantitySenses disorderInorganic non-active ingredientsMacula lutea degenerationRetinal
A method for treating choroidal neovascularization in a patient includes administrating as eye drops into the eye of the patient having choroidal neovascularization a therapeutically effective amount of an aqueous ophthalmic composition that is formulated for topical administration as eye drops. The aqueous ophthalmic composition consists essentially of Timolol Maleate at a concentration of about 0.1% to 0.5%, Dorzolamide at a concentration of about 0.5% to 2.5%, Prednisolone at a concentration of about 1.0% to 3.0%, Ketorolac Tromethamine at a concentration of about 0.4% to 1.2%, and sterile water at a concentration of at least about 90.0%.
Owner:RHEOSTASIS LLC
Preparation method of timolol maleate sustained-release microspheres
ActiveCN103735518BMeet the requirementsHigh encapsulation efficiencyOrganic active ingredientsSenses disorderVegetable oilOil emulsion
The invention discloses a preparation method of timolol maleate slow-release microspheres. The present invention adopts oil-in-oil technology, prepares inner phase with timolol maleate, modified montmorillonite, acrylic resin, Tween, and plasticizer, prepares outer phase with vegetable oil and Span, and the obtained oil-in-oil After the emulsion is ultrasonically treated, the internal phase is evaporated by electromagnetic stirring to obtain timolol maleate sustained-release microspheres, whose particle size can be controlled within the range of 10 μm, which meets the requirements of ophthalmic preparations. Its drug encapsulation rate is as high as 80-99%, and the release time in vitro can be extended to 10-12 hours. The timolol maleate sustained-release microspheres prepared by the method are used as an intraocular pressure-lowering drug, which can reduce discomfort in the eye, reduce the number of medications, and realize the purpose of lowering the intraocular pressure for a long time by one administration.
Owner:广州铂思雅生物医药科技有限公司
Timolol maleate external preparation and preparation method thereof
PendingCN114748451AImprove stabilityPlay a synergistic roleOrganic active ingredientsSenses disorderPolymer scienceActive agent
The invention discloses a timolol maleate external preparation and a preparation method thereof. The timolol maleate external preparation is prepared from the following components in percentage by mass: 4 to 8 weight percent of timolol maleate, 18 to 25 weight percent of polylactic acid, 3 to 8 weight percent of nanofiber, 10 to 15 weight percent of gel matrix, 4 to 10 weight percent of phospholipid, 1 to 2 weight percent of surfactant, 0.8 to 1.2 weight percent of water-retaining agent and the balance of deionized water. The invention provides the timolol maleate external preparation in a gel form, a thin film can be formed on the surface of the external preparation after the external preparation is smeared, the external preparation is slowly hardened into a soft sheet, timolol maleate is gradually and slowly released in the hardening process, and the hardening time is basically matched with the effective drug release time; the external preparation is not easy to erase by clothes and the like, is easy to tear off after being hardened, and is convenient to use.
Owner:WUHAN UNIV
Synthesis method of timolol maleate intermediates
ActiveCN101774977ASimple and fast operationConducive to large-scale industrial productionOrganic chemistryDistillationBenzaldehyde
The invention discloses a synthesis method of timolol maleate intermediates, which takes a cyclic compound as a dissolvent. The synthesis method is characterized in that the cyclic compound is taken as the dissolvent, and amine diol and benzaldehyde generates oxazole product by cyclization; and the timolol maleate intermediate is obtained after distillation, wherein the molar ratio of the cyclic compound as the dissolvent to the amine diol is 1:2-6.5. The invention adopts the cyclic compound as the dissolvent to replace a highly toxic benzene dissolvent so as to obtain high-quality and high-yield timolol maleate intermediates. The synthesis method of the invention has simple operation in industrial production, repeatedly recycled dissolvent, and environment protection, and is more favorable for large-scale industrial production.
Owner:TIANJIN CENT PHARM CO LTD
Timolol maleate (TM) eye gel and preparation method thereof
ActiveCN102178644BImprove complianceLess irritatingOrganic active ingredientsSenses disorderTimolol maleateChemistry
The invention provides timolol maleate (TM) eye gel and a preparation method thereof. One hundred grams of the eye gel comprises the following components by weight: 0.10-0.70g of TM, 0.10-2.0g of gel matrix, 0.2-5.0g of osmotic pressure regulator and the balance of water, wherein the gel matrix is a mixture of cellulose derivatives and carbopol and the weight ratio of cellulose derivatives to carbopol is 1:0.02-1, the cellulose derivatives comprise more than one of methylcellulose, hydroxymethyl cellulose, hydroxypropyl methylcellulose (HPMC) and carboxymethyl cellulose (CMC), and the carbopol comprises one of polyacrylic acid (PAA), polyacrylate or crosslinking polyacrylic resin. Patients with glaucoma and ocular hypertension use the controlled-release TM eye preparation once a day; and the prescription of the eye preparation is free of preservatives, thus lowering stimulation to eyes and improving medication safety if the gel is used for a long term.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com