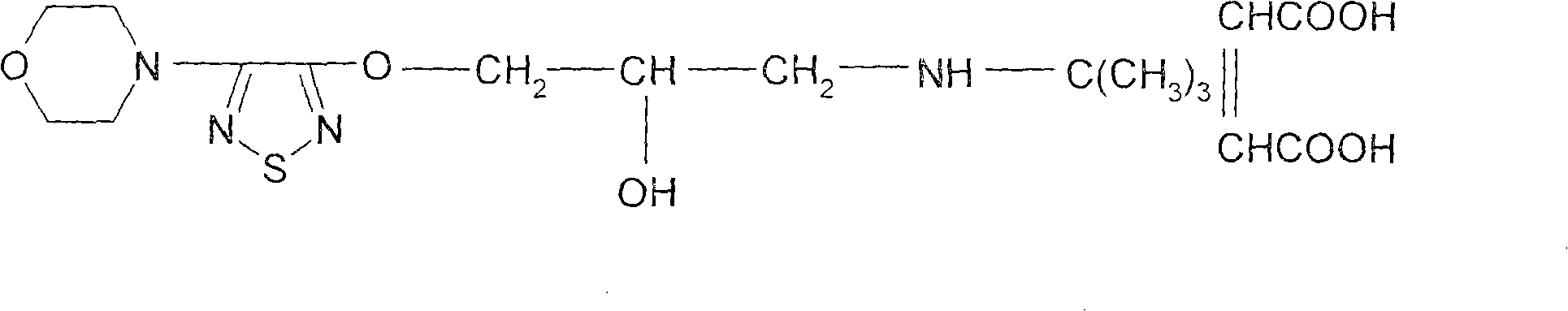
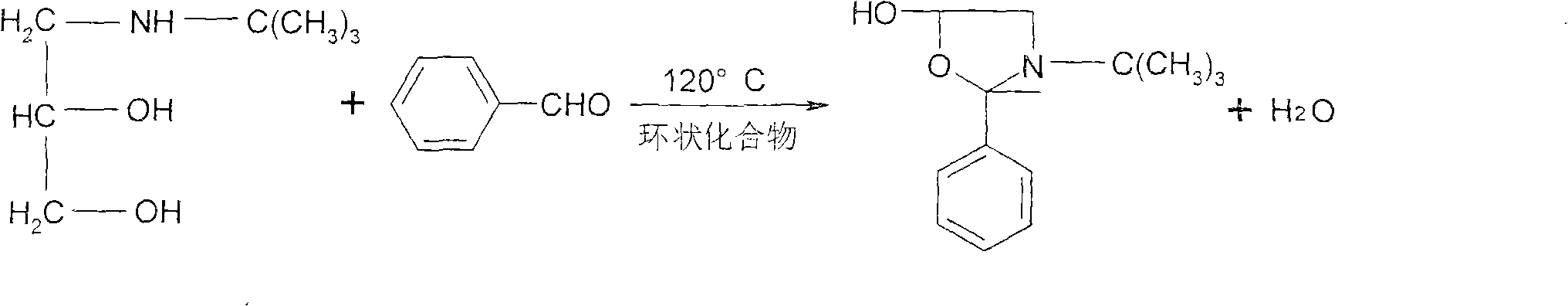
Synthesis method of timolol maleate intermediates
A technique for synthesizing timolol maleate and a synthetic method, which is applied in the field of synthesizing timolol maleate intermediates, can solve problems such as difficult separation of products, and achieve the benefits of large-scale industrial production and stable quality of high-quality products , the effect of reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0029] Put 30g diol amine (0.2mol), 94ml benzaldehyde (3.28 times, 0.91mol), 70ml cyclic compound (dioxane 0.65mol, 1.82 times), put into reaction bottle, reaction temperature 113-115 ℃, reflux After 13 hours, it was evaporated to 130°C under reduced pressure to obtain 47.9 g of oxazole, with a yield of 99.89%.
preparation Embodiment 2
[0031]30g diol amine (0.2mol), 94ml benzaldehyde (3.28 times, 0.91mol), 62ml cyclic compound (toluene, 0.57mol, 1.6 times), put into reaction bottle, reaction temperature 120 ℃, reflux 10 hours, reduce Pressure steamed to 130°C, 48g of oxazole was obtained with a yield of 100.3%.
preparation Embodiment 3
[0033] Put 30g of diolamine (0.2mol), 94ml of benzaldehyde (3.28 times, 0.91mol), 57ml of cyclic compound (1,4-cyclohexadiene, 1.5 times, 0.52mol) into the reaction flask, and the reaction temperature was 125 -130°C, reflux for 8 hours, and evaporate to 130°C under reduced pressure to obtain 48.3 g of oxazole, with a yield of 100.3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com