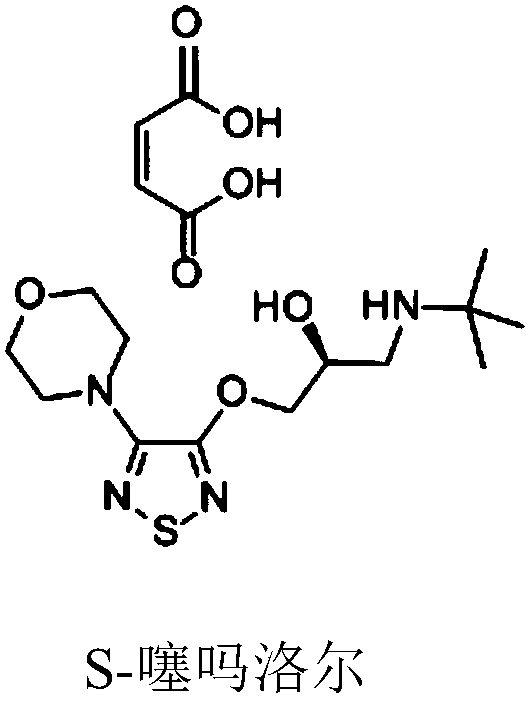
Method for separating timolol compound and timolol maleate related impurities and application of method
A technology of timolol maleate and timolol, which is applied to the separation of impurities related to timolol compounds and timolol maleate and its application field, can solve the problems of difficult separation of impurities and solvent volatility. Large, high-performance liquid chromatography damage and other problems, to achieve high sensitivity, strong specificity, to solve the effect of interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] 1. Instrument: Shimadzu LC-20AT, the detector model is SPD-M20A electronic analytical balance of Sartorius Scientific Instrument Co., Ltd., model is SECURA 125.
[0057] 2. High performance liquid chromatography conditions:
[0058] Mobile phase: methanol: acetonitrile = 10:90 (add 50mmol formic acid and 25mmol diethylamine to the mixed solution, accounting for 0.45% of the mobile phase volume)
[0059] Chromatographic column ZWIX(+)150×4mm, 4.0μm;
[0060] The detection wavelength is 298nm;
[0061] The column temperature is 25°C;
[0062] Flow rate 0.5ml / min.
[0063] 3. Measurement method:
[0064] 3.1 Accurately weigh the appropriate amount of S-timolol (manufacturer: EP, batch number: Batch3.0) and R-timolol (manufacturer: EP, batch number: Batch3.0) reference substance, add methanol to dissolve and dilute to each A solution containing 1 mg of S-timolol and 0.1 mg of R-timolol in 1 ml was used as a systemic suitability solution.
[0065] Experiments were ca...
Embodiment 2
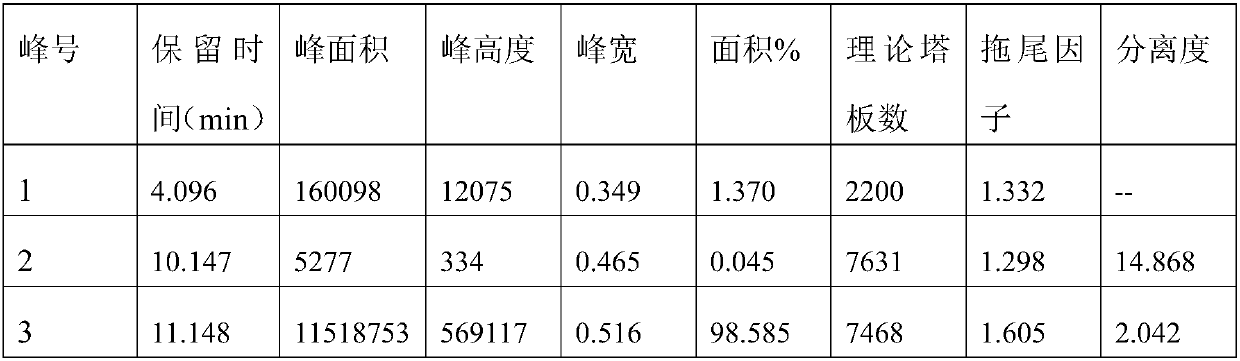
[0080] Carry out the experiment with reference to the sample and operating method of 3.1 of Example 1, the difference is that the mobile phase is methanol: acetonitrile=15:85 (add 50mmol formic acid and 25mmol diethylamine to the mixed solution, the percentage of the amount added is 0.45%) . Obtain efficient chromatogram, the result is as follows:
[0081]
[0082] Among them, peak number 1 is maleic acid; peak number 2 is R-timolol; peak number 3 is S-timolol.
[0083] The results show that the chromatographic conditions can effectively separate S-timolol, R-timolol and maleic acid, and the peak shape is better, the baseline is more stable, and the resolution is greater than 1.5.
[0084] 3.4 Sample recovery experiment
[0085] Accuracy is obtained by adding 80%, 100% and 120% of the recovery rate of three different concentrations of R-timolol stock solution (2μg / ml) in the test solution, adding a known amount of R-timolol Lol, then measure the ratio (recovery rate) bet...
Embodiment 3
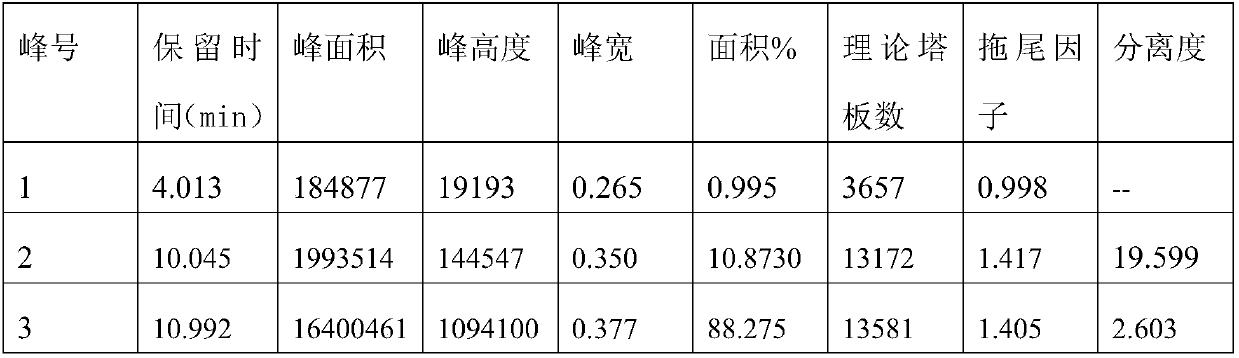
[0091] Carry out experiment with reference to the sample and operation method of 3.1 of embodiment 1, difference is that mobile phase is methyl alcohol: acetonitrile=5:95 (add 50mmol formic acid and 25mmol diethylamine in mixed solution, account for 0.45% of mobile phase volume ). Obtain high-efficiency chromatograms, the results are as follows:
[0092]
[0093] Among them, peak number 1 is maleic acid; peak number 2 is R-timolol; peak number 3 is S-timolol.
[0094] The results show that the chromatographic conditions can effectively separate S-timolol, R-timolol and maleic acid, and the peak shape is better, the baseline is more stable, and the resolution is greater than 1.5.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com