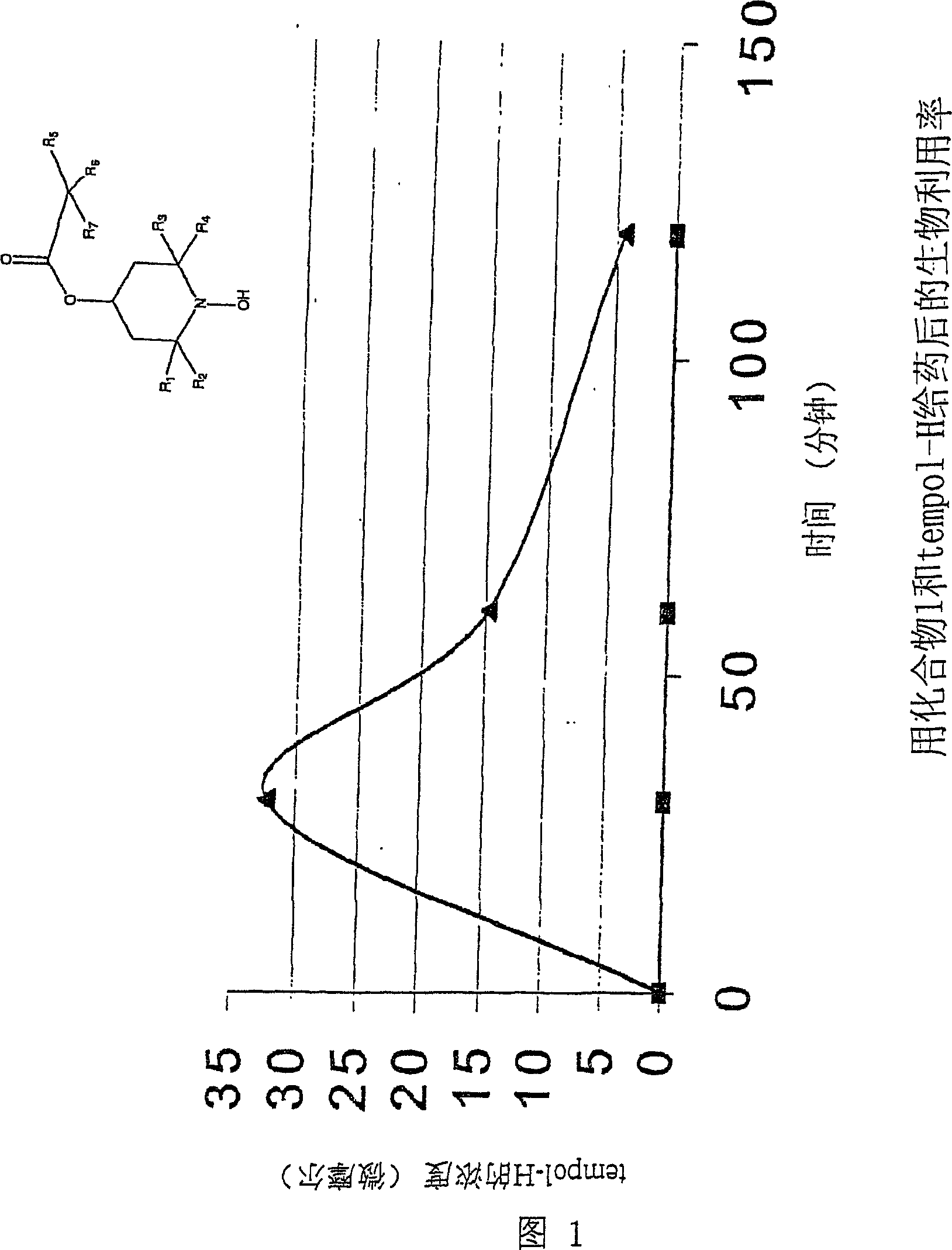
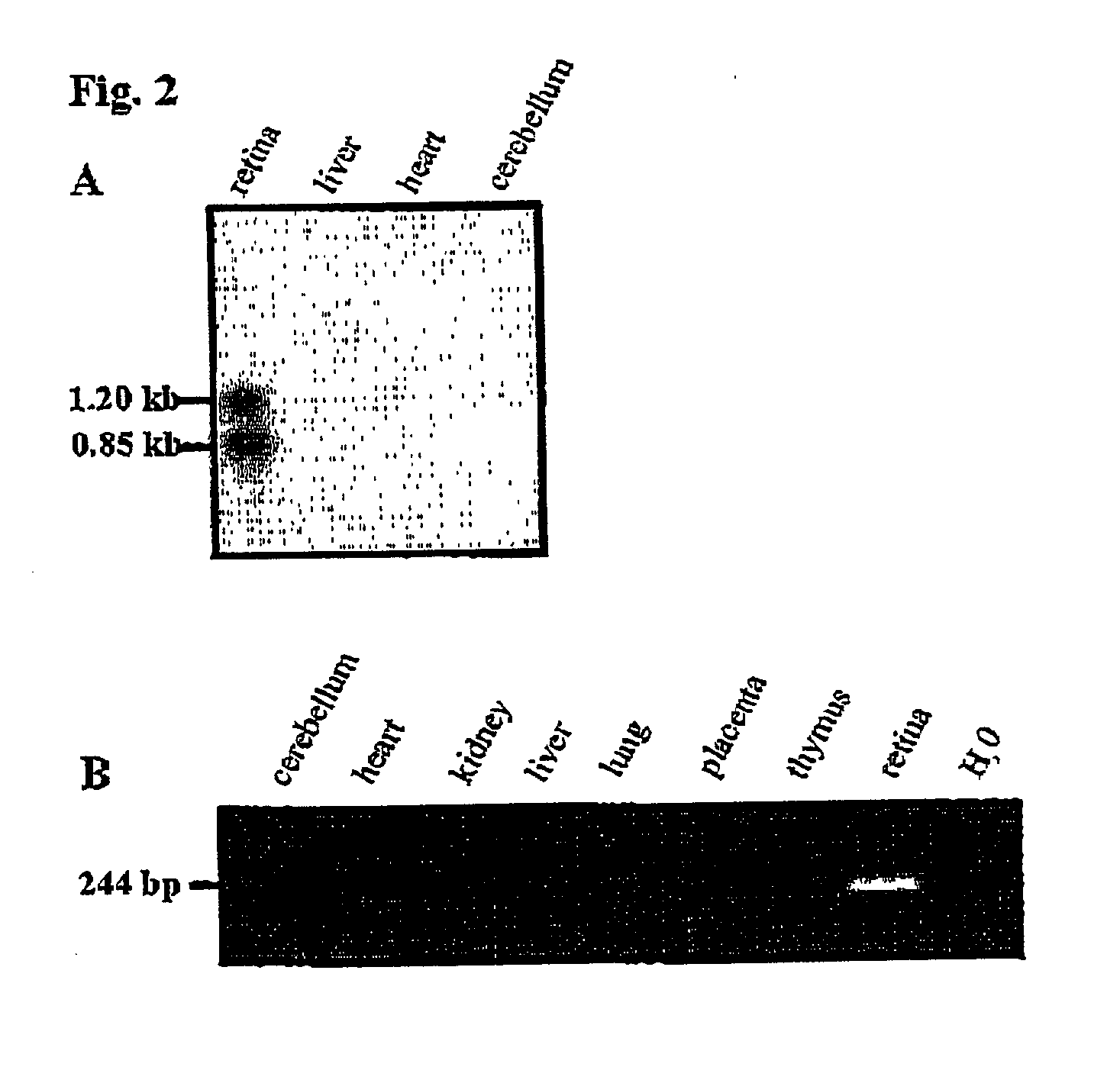
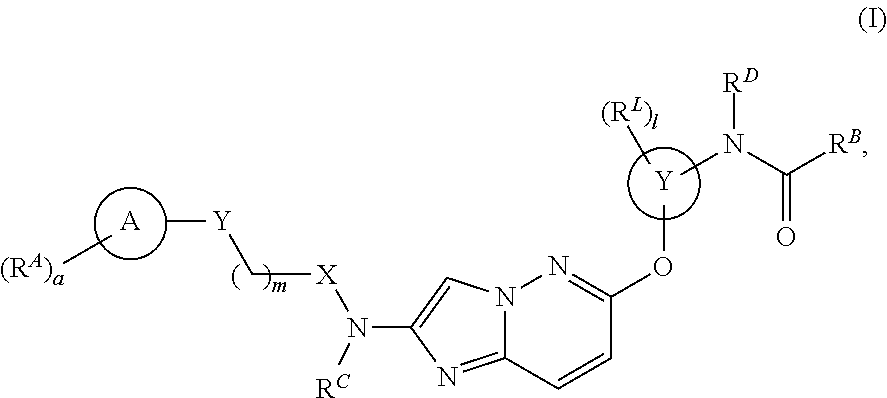
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
113 results about "Macula lutea degeneration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Deterioration of the eye part called macula lutea of the retina
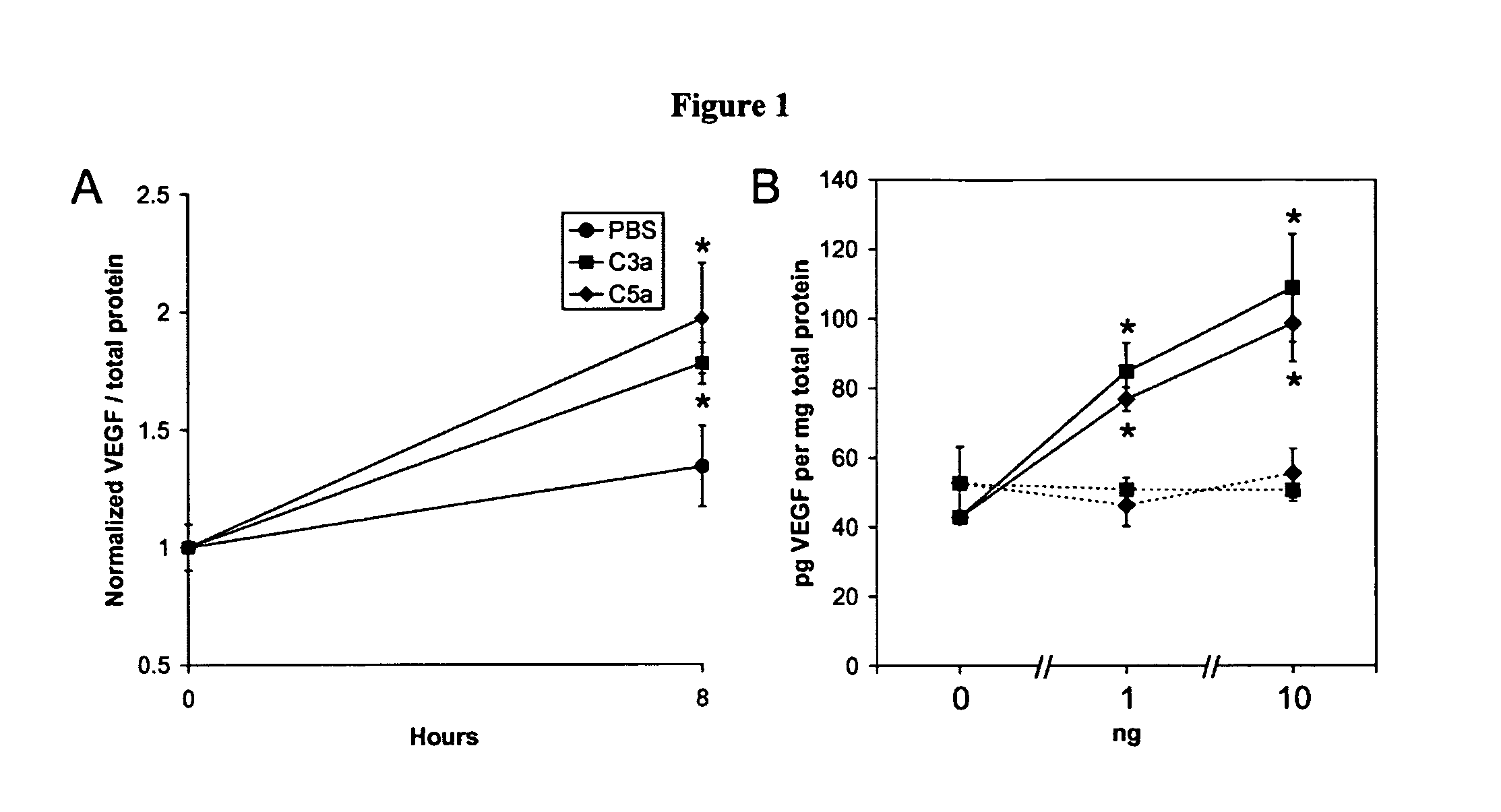
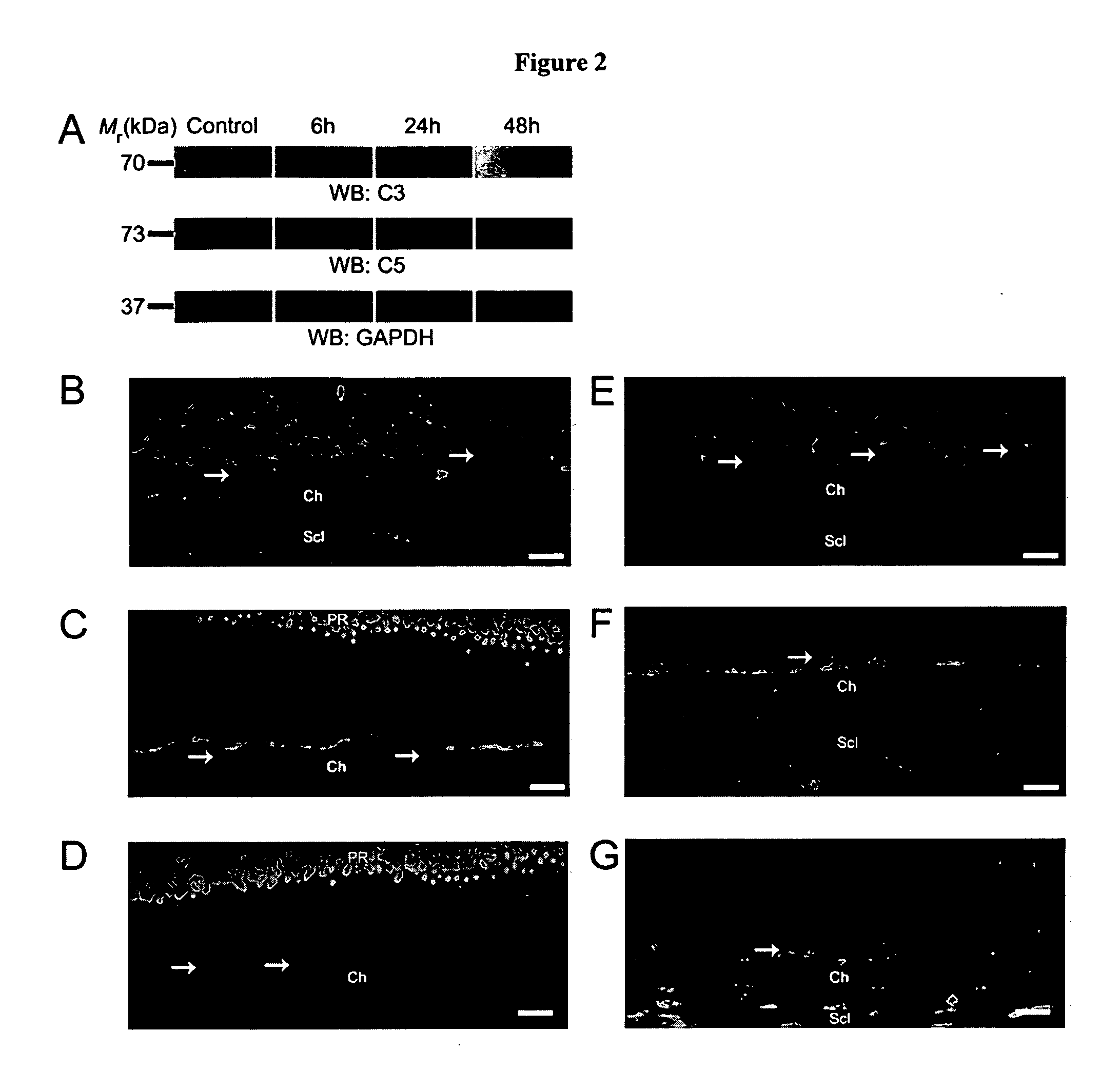
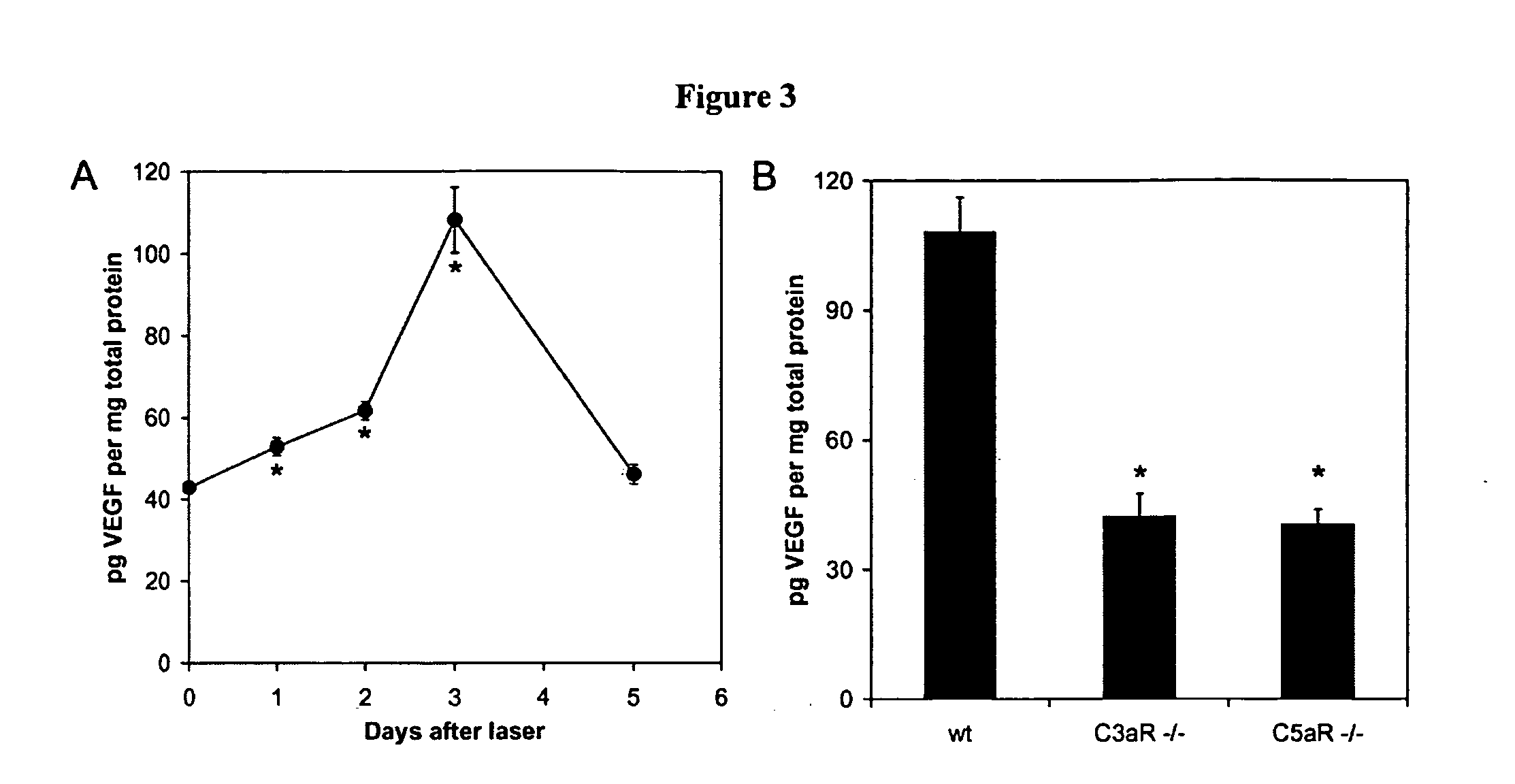
Compositions and methods for inhibiting drusen complement components C3a and C5a for the treatment of age-related macular degeneration
InactiveUS20060067935A1Reduces VEGF expressionReduce expressionGenetic material ingredientsAntibody ingredientsIn vivoBiology
Activated C3 (C3a) and its receptor (C3aR) and activated C5 (C5a) and its receptor (C5aR) have been shown to induce vascular endothelial growth factor (VEGF) expression in vitro and in vivo. Compositions and methods for inhibiting C3a, C3aR, C5a and C5aR for the treatment and / or prevention of neovascular disease are provided. Also provided are Novel therapeutic targets and diagnostic markers for choroidal neovascularization.
Owner:KENTUCKY UNIVERISTY OF
Treatment of macular degeneration-related disorders
InactiveUS20100093648A1Improve responseMinimize occurrenceBiocideSenses disorderDiseaseRelated disorder
The invention relates to compositions and methods for preventing or treating a macular degeneration-related disorder, comprising a scyllo-inositol compound or pharmaceutically acceptable salts thereof.
Owner:WARATAH PHARMA INC
Kinase inhibitors useful for the treatment of proliferative diseases
The present invention relates to novel kinase inhibitors and modulator compounds useful for the treatment of various diseases. More particularly, the invention is concerned with such compounds, kinase / compound adducts, methods of treating diseases, and methods of synthesis of the compounds. Preferrably, the compounds are useful for the modulation of kinase activity of Raf kinases and disease polymorphs thereof. Compounds of the present invention find utility in the treatment of mammalian cancers and especially human cancers including but not limited to malignant melanoma, colorectal cancer, ovarian cancer, papillary thyroid carcinoma, non small cell lung cancer, and mesothelioma. Compounds of the present invention also find utility in the treatment of rheumatoid arthritis and retinopathies including diabetic retinal neuropathy and macular degeneration.
Owner:DECIPHERA PHARMA LLC
Compositions and methods for treating ocular diseases
InactiveUS20150050277A1Dipeptide ingredientsNitro compound active ingredientsUveitisMacula lutea degeneration
Disclosed herein are compositions and methods for treating ocular diseases, inter alia, diabetic macular edema, age-related macular degeneration (wet form), choroidal neovascularization, diabetic retinopathy, retinal vein occlusion (central or branch), ocular trauma, surgery induced edema, surgery induced neovascularization, cystoid macular edema, ocular ischemia, uveitis, and the like. These diseases or conditions are characterized by changes in the ocular vasculature whether progressive or non-progressive, whether a result of an acute disease or condition, or a chronic disease or condition.
Owner:EYEPOINT PHARMA INC
Diagnostic and therapeutic target for macular degeneration
InactiveUS20070037183A1Reduce usageReduce dosageMicrobiological testing/measurementDiseaseComplement factor I
The present invention is based on the discovery of genetic polymorphisms that are associated with ocular diseases and disorders, such as age-related macular degeneration (AMD). In particular, the present invention relates to methods for determining an individuals susceptibility to ocular disorders such as AMD by screening for mutations and / or polymorphisms in the human complement factor H (CFH) gene or gene product that confer susceptibility to such disorders. Also encompassed in the present invention are nucleic acid molecules containing the polymorphisms, variant proteins encoded by such nucleic acid molecules, reagents for detecting the polymorphic nucleic acid molecules and proteins, and methods of treatment following detection of susceptibility.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
Alpha v integrin receptor antagonists
The present invention relates to novel chain-fluorinated alkanoic acid derivatives thereof, their synthesis, and their use as αv integrin receptor antagonists. More particularly, the compounds of the present invention are antagonists of the integrin receptors αvβ3 and / or αvβ5 and are useful for inhibiting bone resorption, treating and preventing osteoporosis, and inhibiting vascular restenosis, diabetic retinopathy, macular degeneration, angiogenesis, atherosclerosis, inflammation, inflammatory arthritis, viral disease, cancer, and metastatic tumor growth.
Owner:MERCK SHARP & DOHME CORP
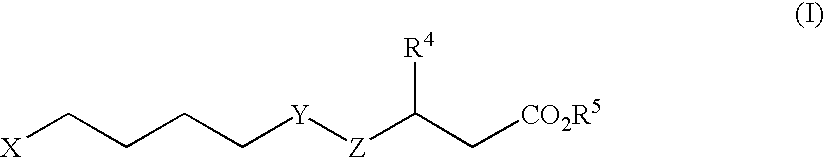
A non-retinoid rbp4 antagonist for treatment of age-related macular degeneration and stargardt disease
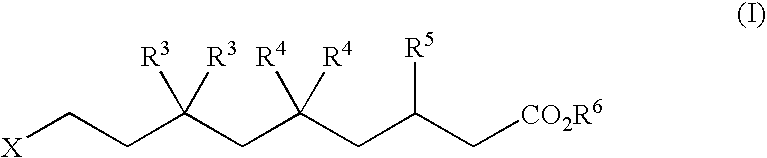
A method for treating bisretinoid-mediated macular degeneration in a mammal afflicted therewith comprising administering to the mammal an effective amount of a compound having the structure:or an ester or a pharmaceutically acceptable salt thereof.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Combinations of inhibitors of irak4 with inhibitors of btk
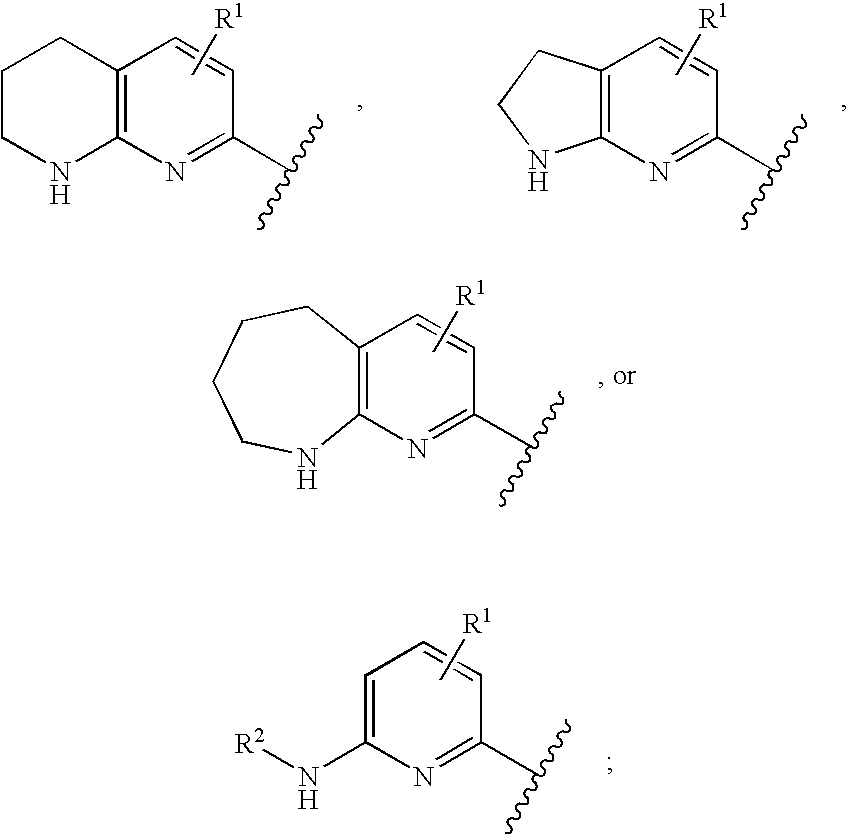
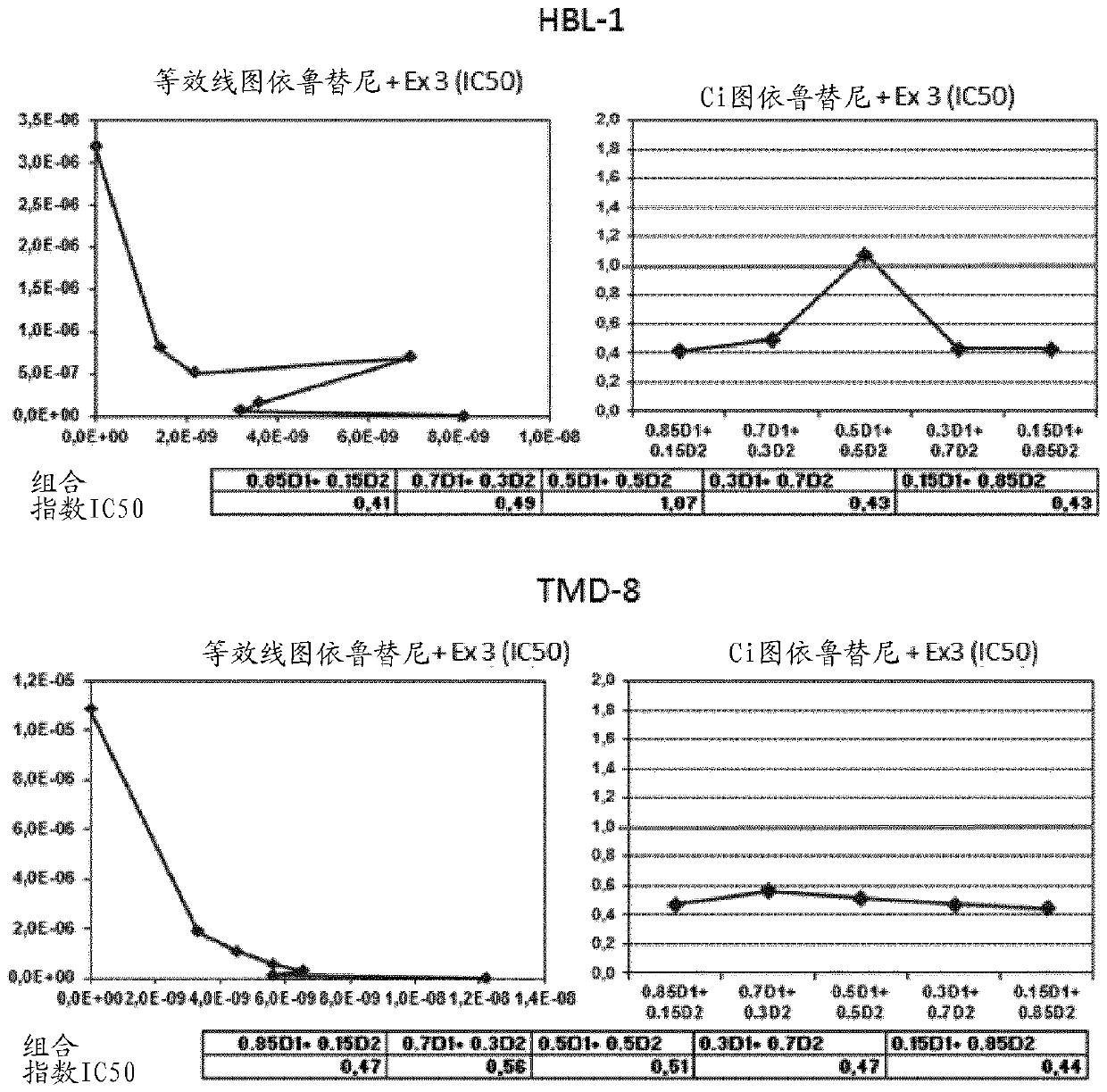
The present application relates to novel combinations of at least two components, component A and component B: component A is an IRAK4-inhibiting compound of the formula (I) as defined herein, or a diastereomer, an enantiomer, a metabolite, a salt, a solvate or a solvate of a salt thereof; component B is a BTK-inhibiting compound, or a pharmaceutically acceptable salt thereof; and, optionally, oneor more components C which are pharmaceutical products; in which one or two of the above-defined compounds A and B are optionally present in pharmaceutical formulations ready for simultaneous, separate or sequential administration, for treatment and / or prophylaxis of diseases, and to the use thereof for production of medicaments for treatment and / or prophylaxis of diseases, especially for treatment and / or prophylaxis of endometriosis, lymphoma, macular degeneration, COPD, neoplastic disorders and psoriasis.
Owner:BAYER PHARMA AG
Alphav integrin receptor antagonists
The present invention relates to novel alkanoic acid derivatives thereof, their synthesis, and their use as αv integrin receptor antagonists. More particularly, the compounds of the present invention are antagonists of the integrin receptors αvβ3 and / or αvβ5 and are useful for inhibiting bone resorption, treating and preventing osteoporosis, and inhibiting vascular restenosis, diabetic retinopathy, macular degeneration, angiogenesis, atherosclerosis, inflammatory arthritis, cancer, and metastatic tumor growth.
Owner:MERCK & CO INC
Effervescent Multi-Vitamin Formulation and Methods of Use Thereof
The present invention provides an effervescent dietary supplement formulation that may be beneficial to the management of symptoms related to ocular diseases. Also provided are methods of treating an ocular disease, such as macular degeneration, by administering the dietary supplement to a subject in need thereof.
Owner:RAFANELLI
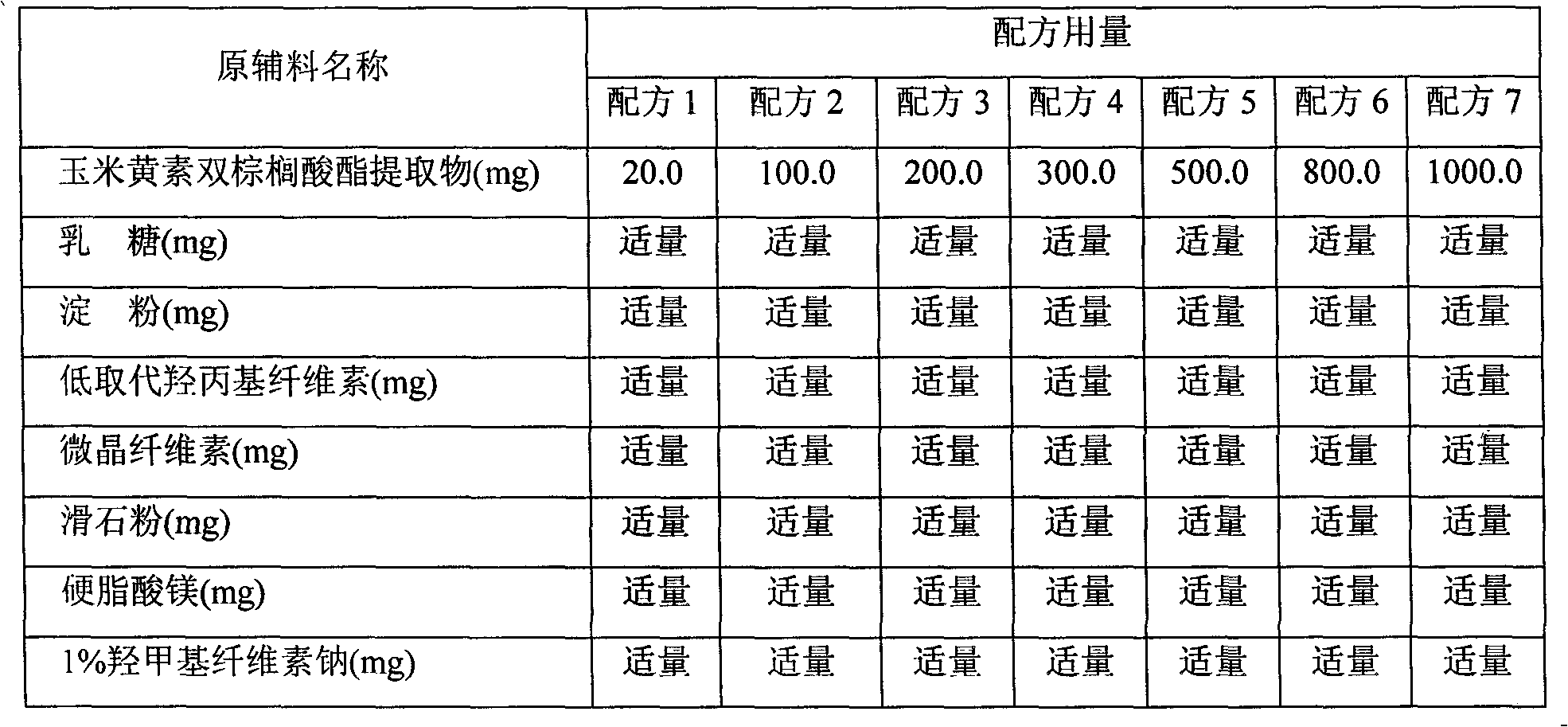
Preparation method of zeaxanthin dipalmitate extract in Chinese wolfberry and medicinal composition and application of the extract
The invention relates to a preparation method of a zeaxanthin dipalmitate extract in Chinese wolfberry and an application of the zeaxanthin dipalmitate extract in medicinal health-care products. Zeaxanthine can delay ageing, prevent cancers and treat age-related macular degeneration (ARMD or AMD), and is an ideal medicament for preventing and treating diseases caused by free radicals. The Chinese wolfberry is rich in zeaxanthin dipalmitate. According to the method disclosed by the invention, the zeaxanthin dipalmitate extract is extracted from the Chinese wolfberry by using a solvent, and the content of the extract is 1-100 percent; an oral liquid preparation prepared from the zeaxanthin dipalmitate extract is used for preventing cataract and delaying and treating age-related macular degeneration.
Owner:逯海龙 +1
Anti-c5a antibodies and methods for using the antibodies
The present disclosure relates to, inter alia, antibodies, or antigen-binding fragments thereof, that bind to C5a and to use of the antibodies in methods for treating or preventing complement-associated disorders such as, but not limited to, atypical hemolytic uremic syndrome, age-related macular degeneration, rheumatoid arthritis, sepsis, severe burn, antiphospho lipid syndrome, asthma, lupus nephritis, Goodpasture's syndrome, and chronic obstructive pulmonary disease.
Owner:ALEXION PHARMA INC
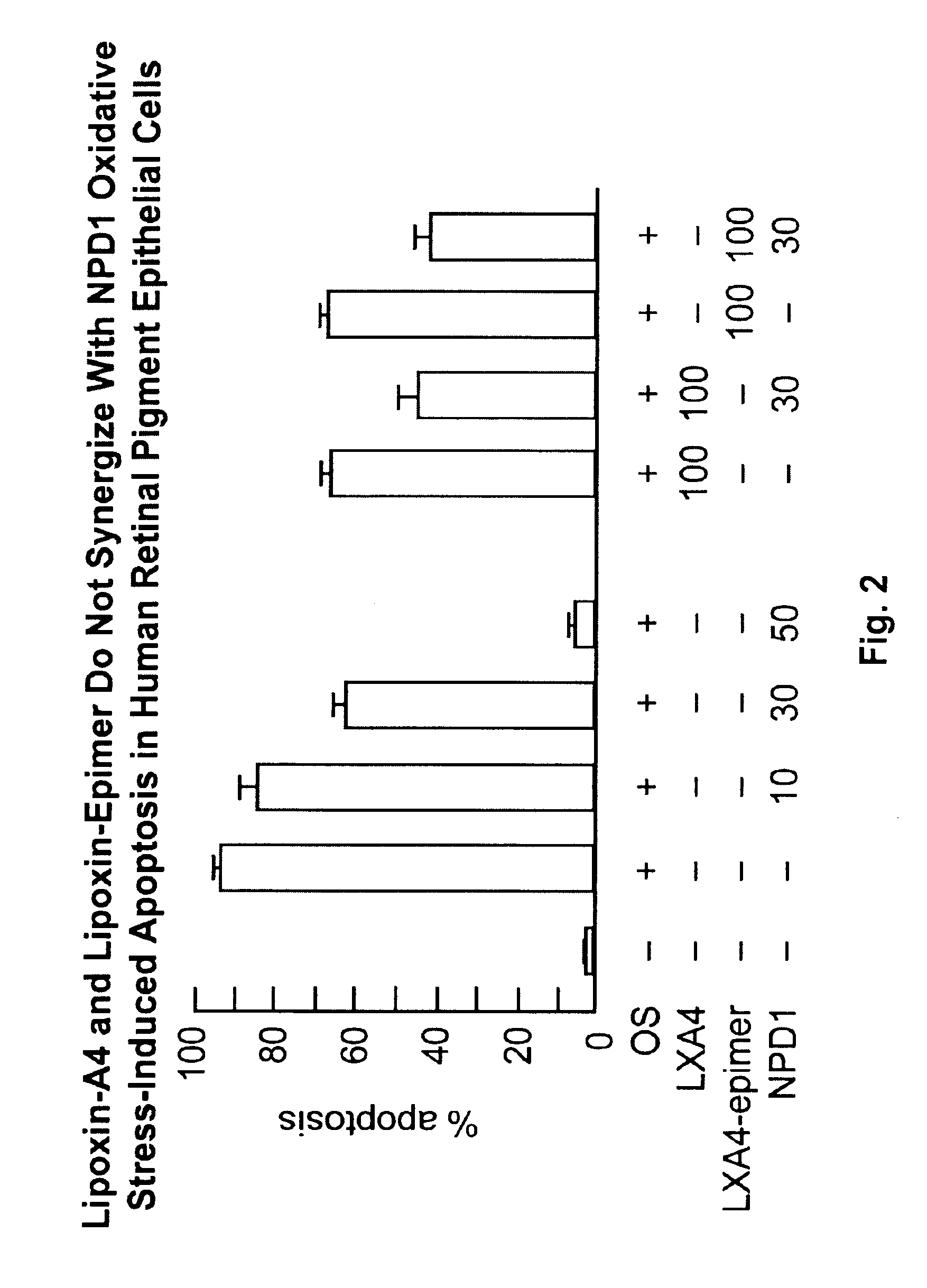
Lipoxin A4 Protection for Retinal Cells
InactiveUS20100324138A1Inhibit apoptosisBiocideSenses disorderNeuroprotective factorsOxidative Stress Induction
Lipoxin A4 and its analogs have been found to be effective in inhibiting apoptosis of retinal pigment epithelial cells induced by oxidative stress. Thus lipoxin A4 and its analogs, for example, lipoxin A4 epimer 15, can be used to prevent and treat retinal diseases due to the progressive degeneration of photoreceptors and retinal pigment epithelial cells (RPE cells), e.g., the dry form of age-related macula degeneration. They can also be combined with other compounds known to prevent apoptosis in retinal pigment epithelial cells, e.g., docosahexaenoic acid and neuroprotectin D1.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Transscleral delivery
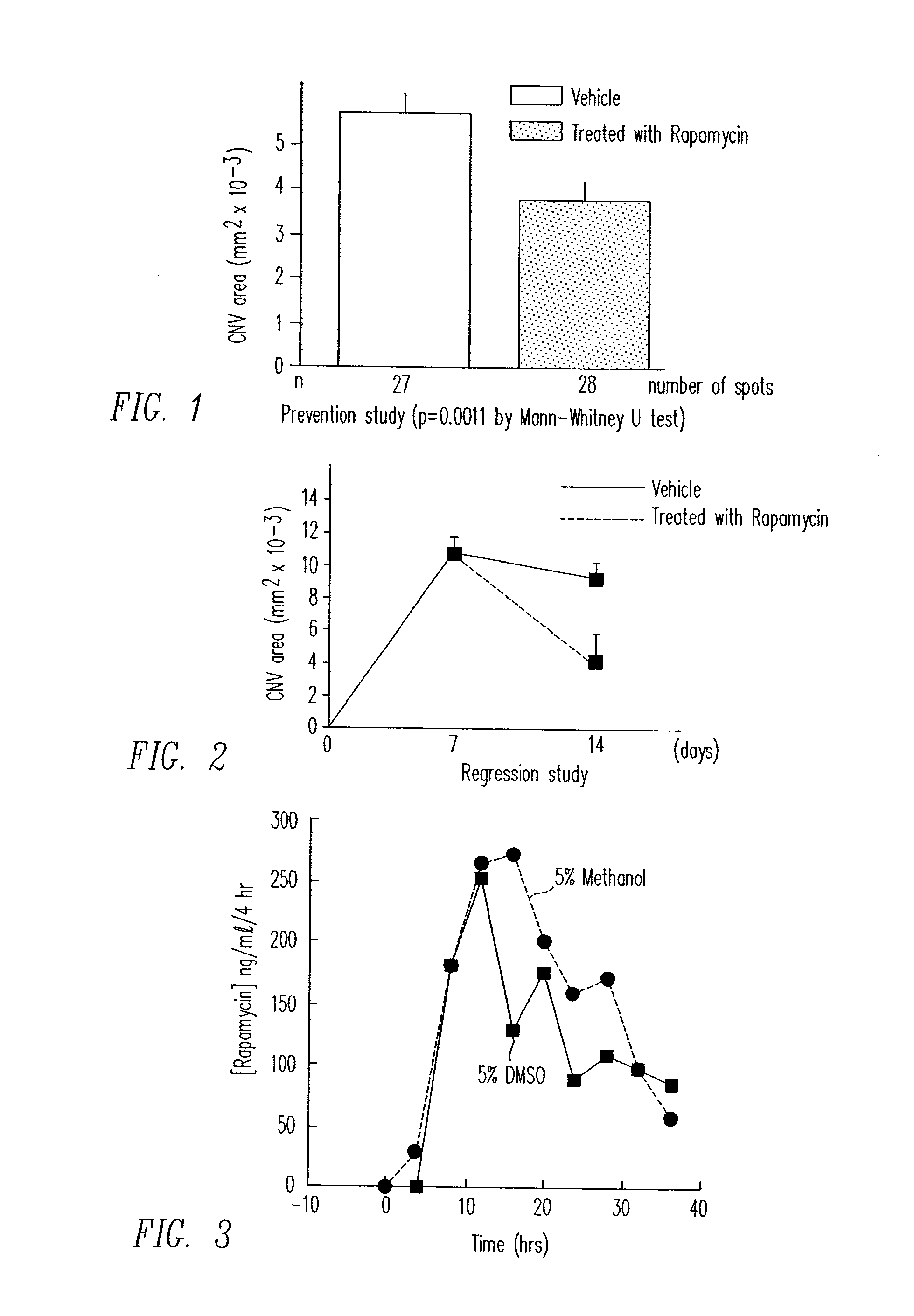
Diseases associated with the tissues in the posterior segment of the eye can be effectively treated by administering therapeutic agents transsclerally to those tissues. Compositions, devices, and methods for delivering therapeutic agents so that they cross the sclera and reach these tissues include injecting solutions or suspensions adjacent to or within the sclera and implanting solid structures containing the therapeutic agent adjacent to or within the sclera. These methods may be used for administering rapamycin or related compounds to treat choroidal neovascularization associated with age-related macular degeneration.
Owner:SANTEN PHARMA CO LTD
Rbp4 antagonists for the treatment of age-related macular degeneration and stargardt disease
InactiveUS20170258786A1Organic active ingredientsPharmaceutical delivery mechanismLipofuscin accumulationPhysiology
A method for treating a disease characterized by excessive lipofuscin accumulation in the retina in a mammal afflicted therewith, comprising administering to the mammal an effective amount of a compound having the structure of any one of Formulas I-IV described herein, or a pharmaceutically acceptable salt thereof.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Sunitinib formulations and methods for use thereof in treatment of ocular disorders
InactiveUS20170273901A1Avoid nerve damageIncreasing encapsulation and incorporationOrganic active ingredientsSenses disorderDiseaseCytotoxicity
Methods for increasing the encapsulation or incorporation of Sunitinib into polymeric matrices have been developed. The resulting formulations provide for more sustained controlled release of sunitinib or its analog or a pharmaceutically acceptable salt thereof. Increased loading is achieved using an alkaline solvent system. The pharmaceutical compositions can be administered to treat or prevent a disease or disorder in or on the eye of a patient associated with vascularization, such as corneal neovascularization and acute macular degeneration. Upon administration, the sunitinib or its analog or salt is released over an extended period of time at concentrations which are high enough to produce therapeutic benefit, but low enough to avoid unacceptable levels of cytotoxicity.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Compositions and methods for treating ocular diseases
ActiveUS20170319602A1Dipeptide ingredientsNitro compound active ingredientsUveitisMacula lutea degeneration
Owner:EYEPOINT PHARMA INC
Treatment of Ocular Diseases with Fully-Human Post-Translationally Modified Anti-VEGF Fab
Compositions and methods are described for the delivery of a fully human post-translationally modified (HuPTM) monoclonal antibody (“mAb”) or the antigen-binding fragment of a mAb against human vascular endothelial growth factor (“hVEGF”)—such as, e.g., a fully human-glycosylated (HuGly) anti-hVEGF antigen-binding fragment—to the retina / vitreal humour in the eye(s) of human subjects diagnosed with ocular diseases caused by increased neovascularization, for example, neovascular age-related macular degeneration (“nAMD”), also known as “wet” age-related macular degeneration (“WAMD”), age-related macular degeneration (“AMD”), and diabetic retinopathy.
Owner:REGENXBIO
Methods and Compositions for the Treatment of Angiogenesis and Macular Degeneration
InactiveUS20090259054A1Relieve symptomsInhibit neovascularizationOrganic active ingredientsOrganic chemistryAngiogenesis growth factorMacular degeneration
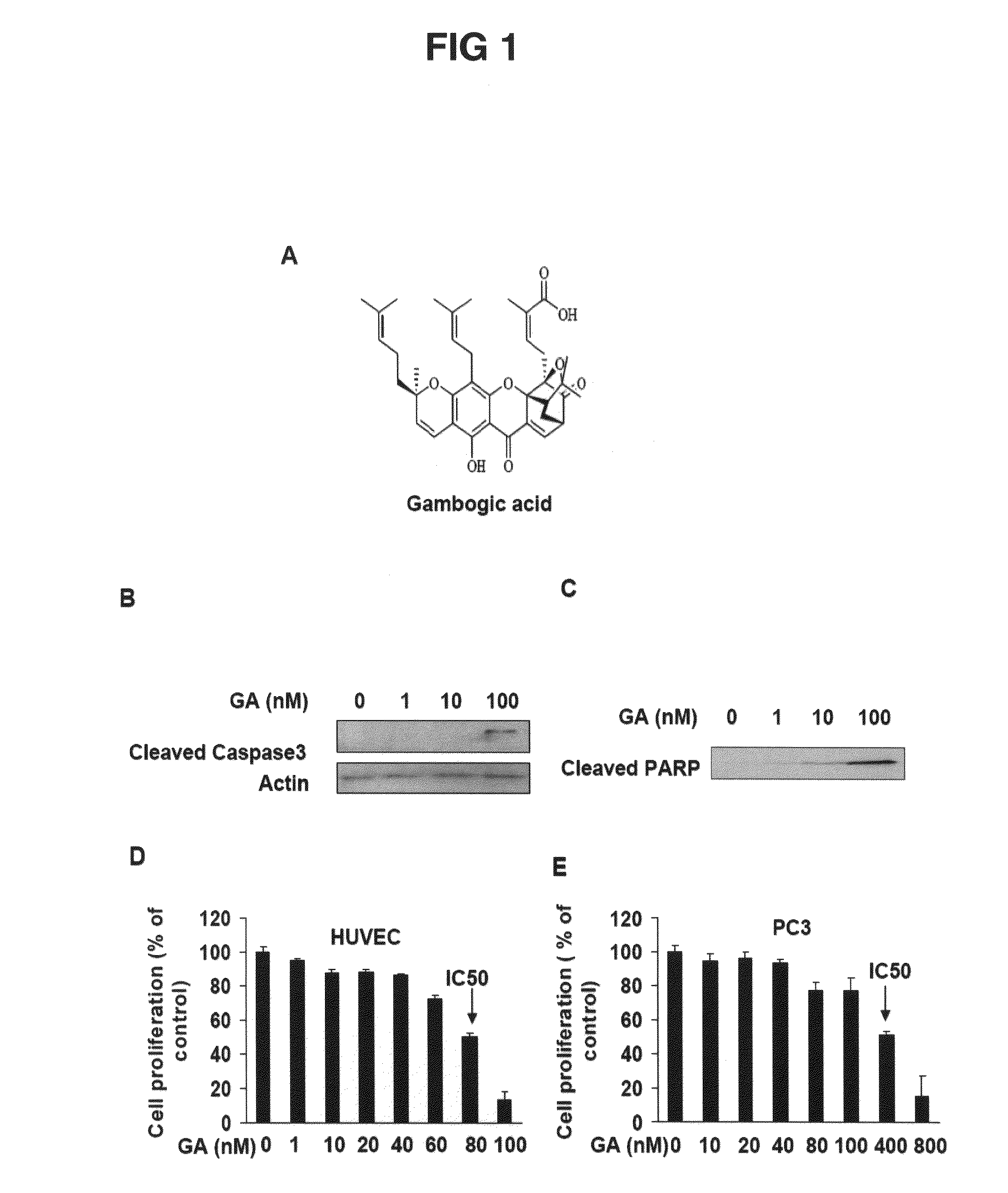
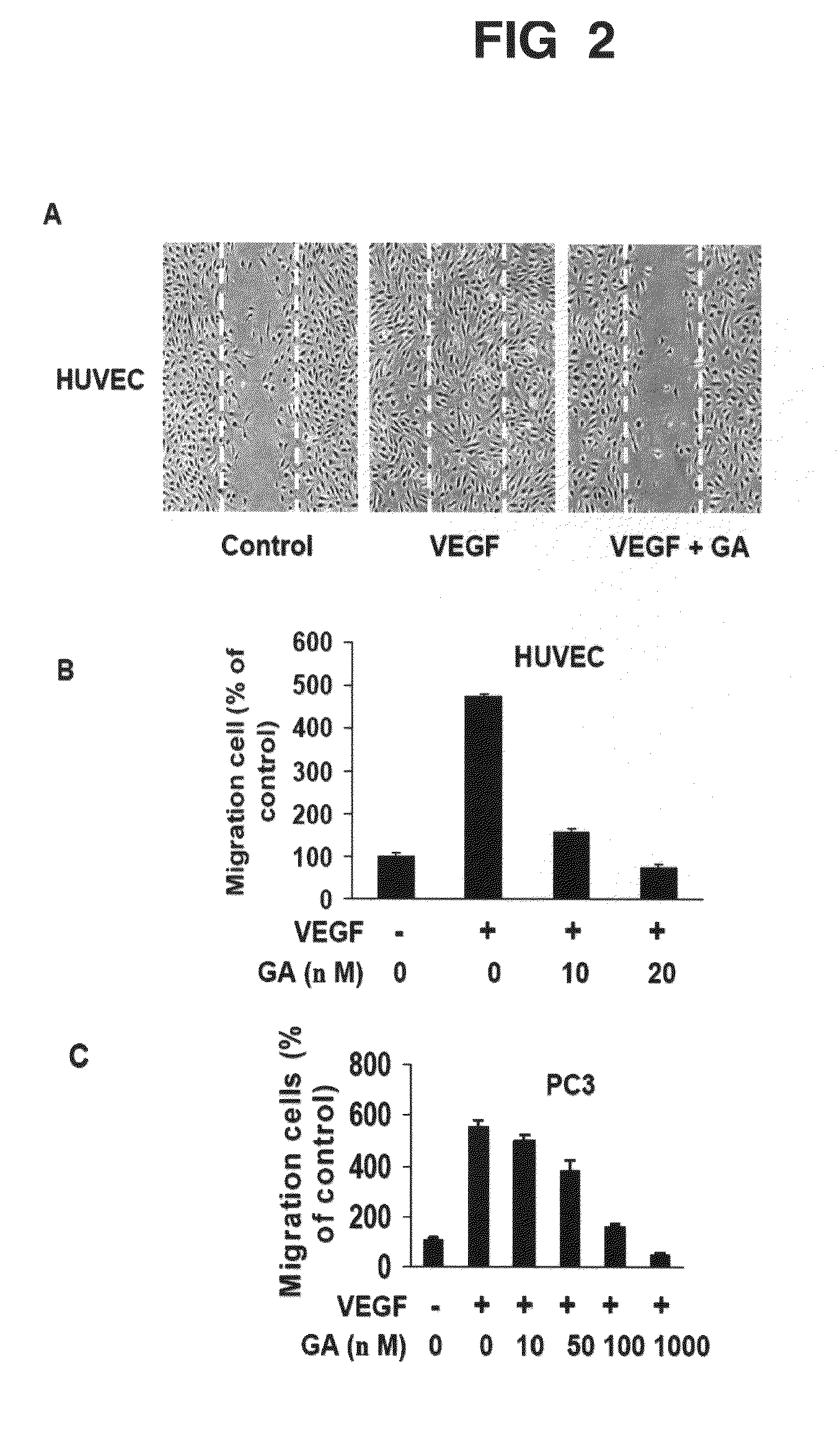
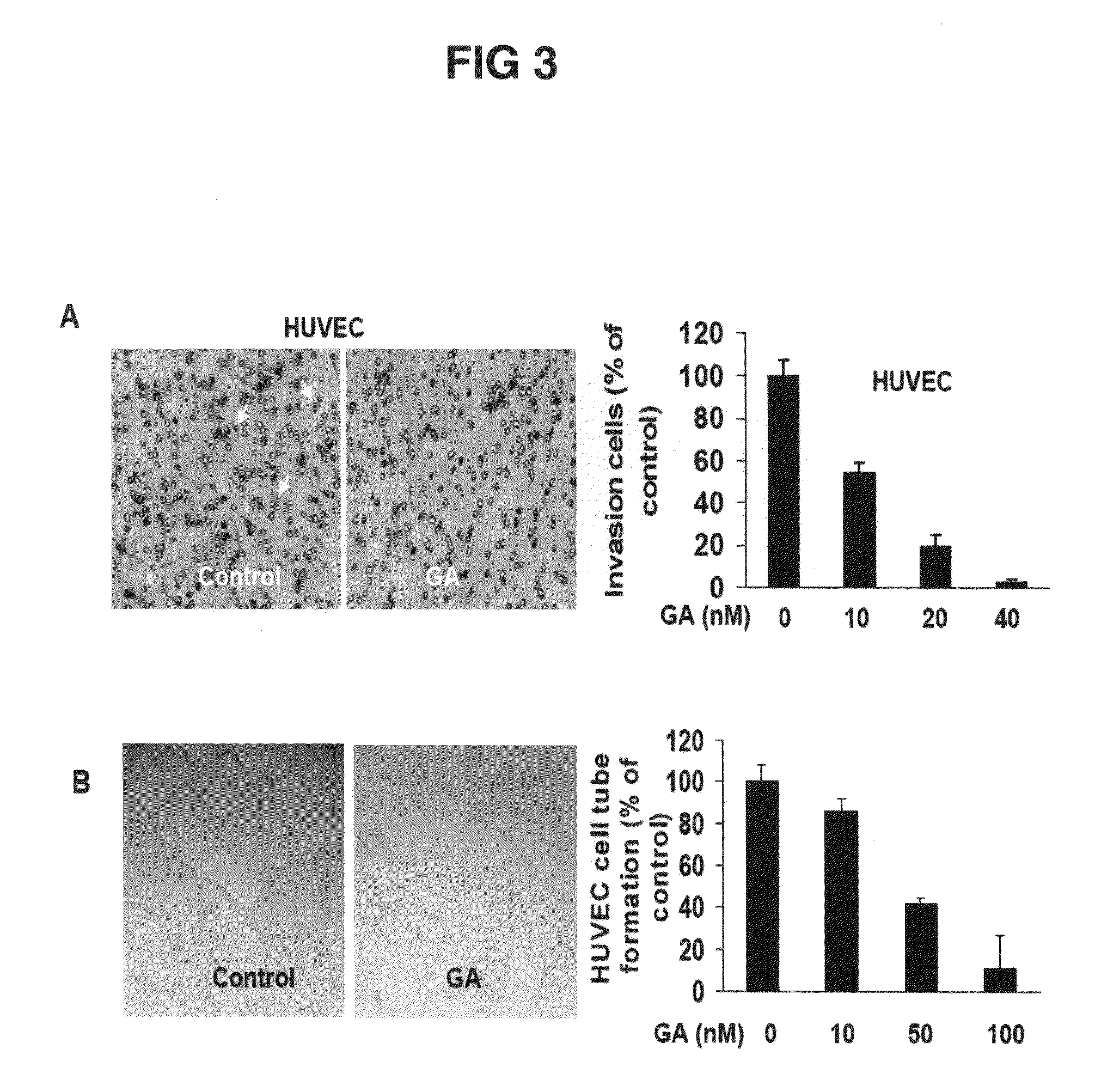
The present invention relates to methods and compositions for the treatment of angiogenesis and macular degeneration. In preferred embodiments, the invention relates to the field of eye health. In some embodiments, the invention relates to the prevention and treatment of angiogenesis by administering compounds disclosed herein. In further embodiments, the invention relates to the prevention and treatment of macular degeneration by administering compounds disclosed herein. In still further embodiments, the invention relates to methods and compositions comprising gambogic acid and gambogic acid derivatives.
Owner:TEXAS A&M UNIVERSITY
Compositions and method of administering tubulin binding agents for the treatment of ocular diseases
The present invention relates to the use of blood vessel target agent, especially tubulin binding agent, to treat diseases such as eye angiogenesis, eye tumor, diabetic retinopathy, premature retinopathy, retinoblastoma and macular degeneration.
Owner:OXIGENE
Antibodies specific for hyperphosphorlated tau for the treatment of ocular diseases
ActiveUS10995137B2Reduce accumulationNervous disorderPeptide/protein ingredientsRetinoidMacula lutea degeneration
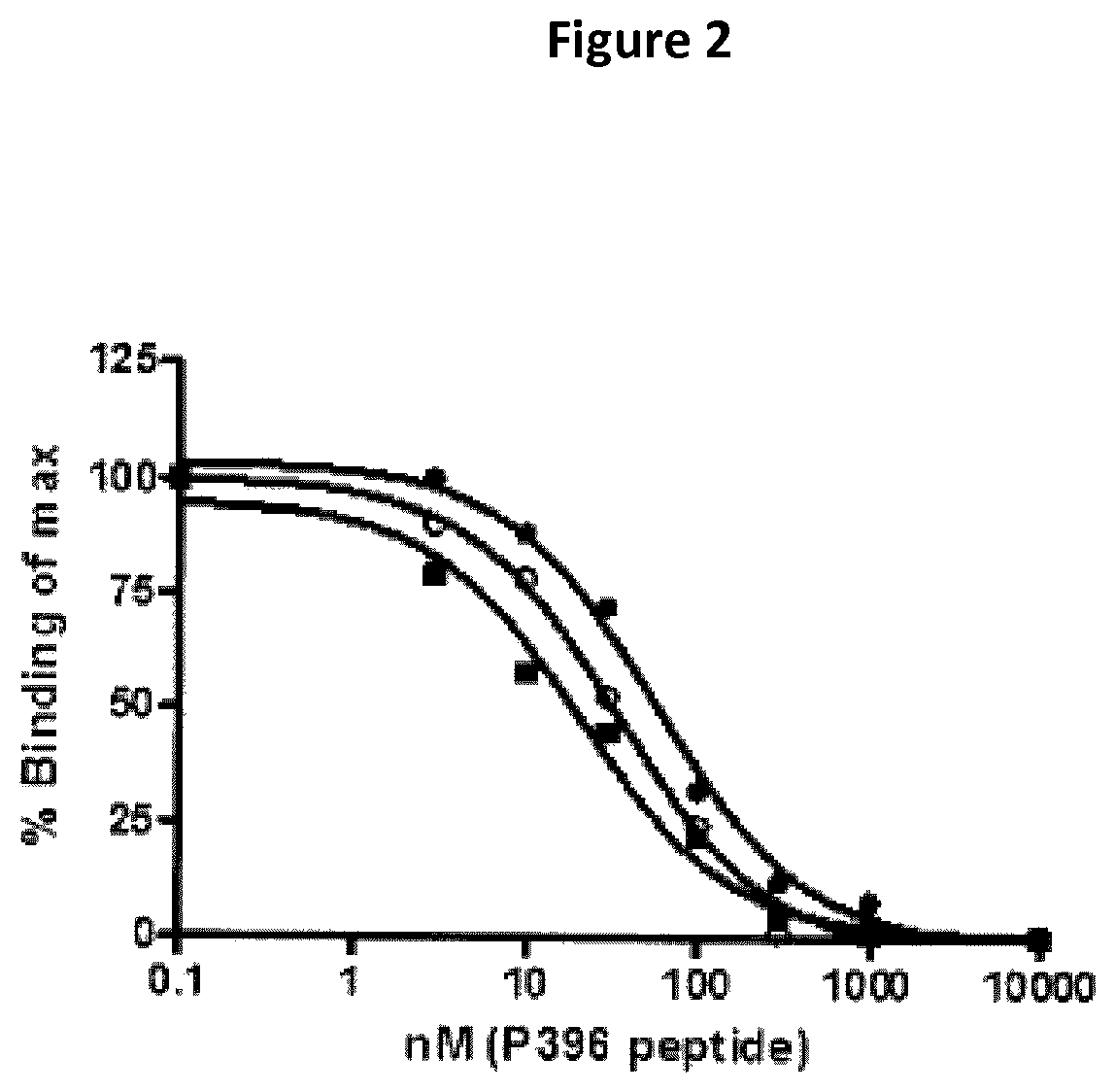
The present invention is based on antibodies which are both highly specific for hyperphosphorylated pathogenic P-S396 tau and highly specific for labelled sections of the human retina, as well as to methods of using these antibodies and their tau binding fragments in the treatment of retinoid amyloidosis, age related macular degeneration (ARMD), and glaucoma.
Owner:H LUNDBECK AS
Diphenyldihydroporphin compound, preparation method and application thereof
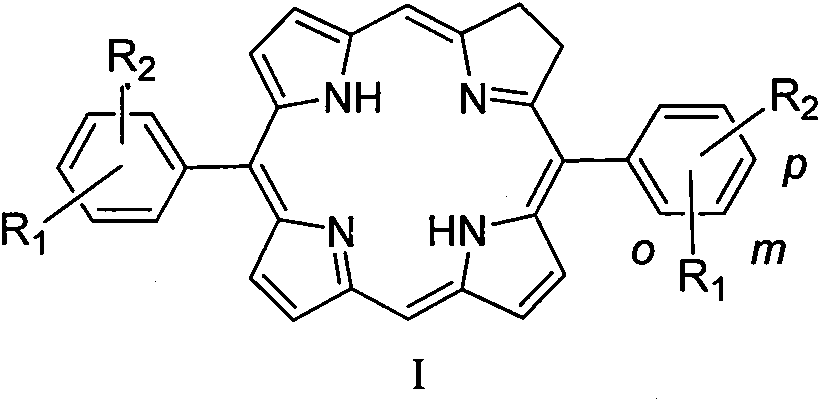
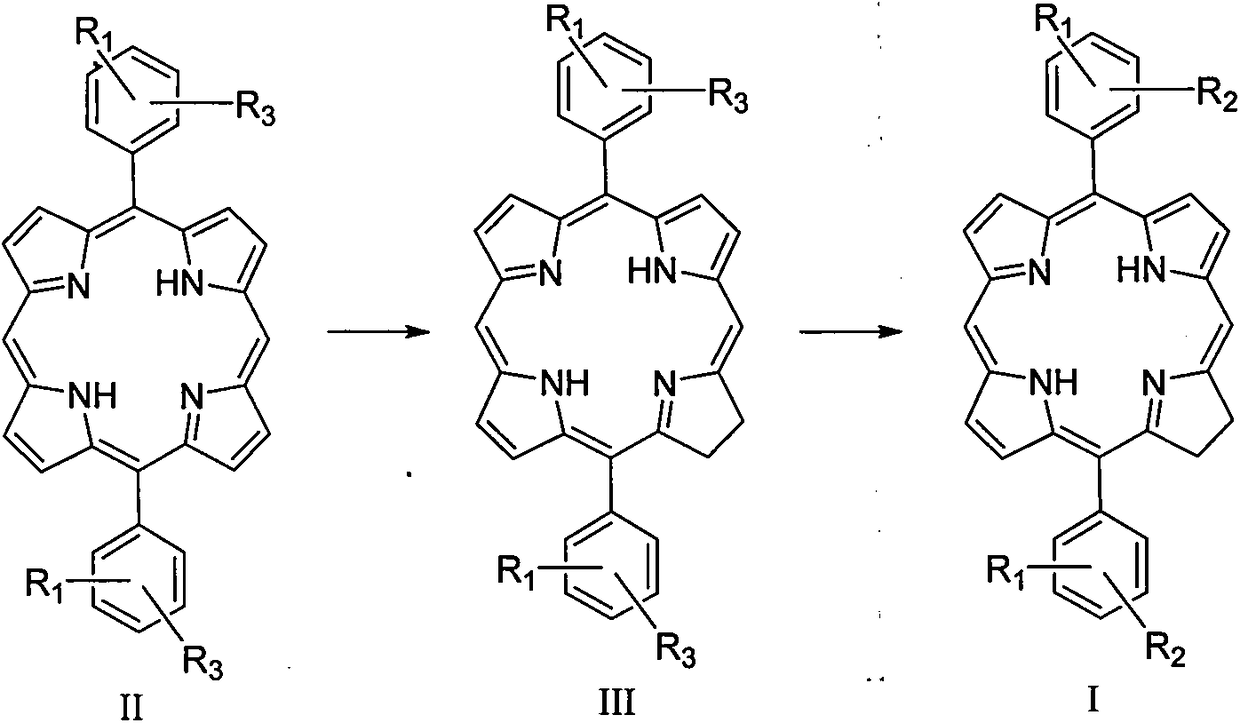
InactiveCN108864117AStable structureSimple structureSenses disorderOrganic chemistryDiseasePhotodynamic therapy
The invention relates to a diphenyldihydroporphin compound, a preparation method and application thereof. The compound has the following structure (I) which is shown in the description, wherein R1 andR2 are positioned at the ortho-position (o-), meta-position (m-) or para-position (p-) of a benzene ring; R1 is -H or -OMe; R2 is -OCH2COOH or -OCH2CH2CH2COOH. The invention relates to the fields ofphotosensitive drugs (also called as a photosensitizer or photodynamic drug) and photodynamic therapy, and particularly relates to a diphenyldihydroporphin photosensitizer, a preparation method and application in the field of medicines. The diphenyldihydroporphin compound prepared by the method provided by the invention is stable in chemical property, has strong photodynamic activity, and can be used as a drug for photodynamic diagnosis and treatment of diseases such as tumors, macula-retinae degeneration, actinic keratosis, nevus flammeus and condyloma acuminata and the like.
Owner:陈志龙
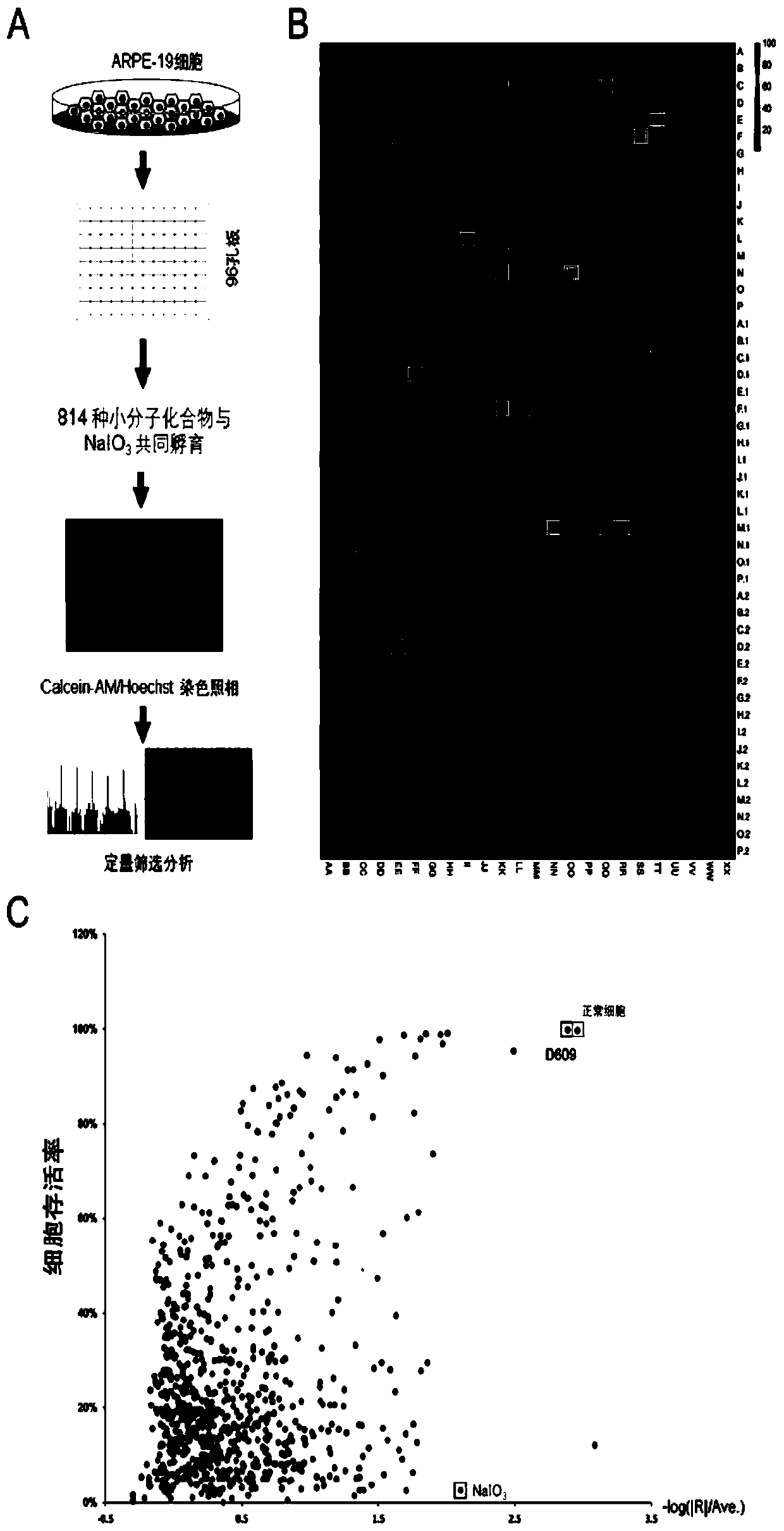
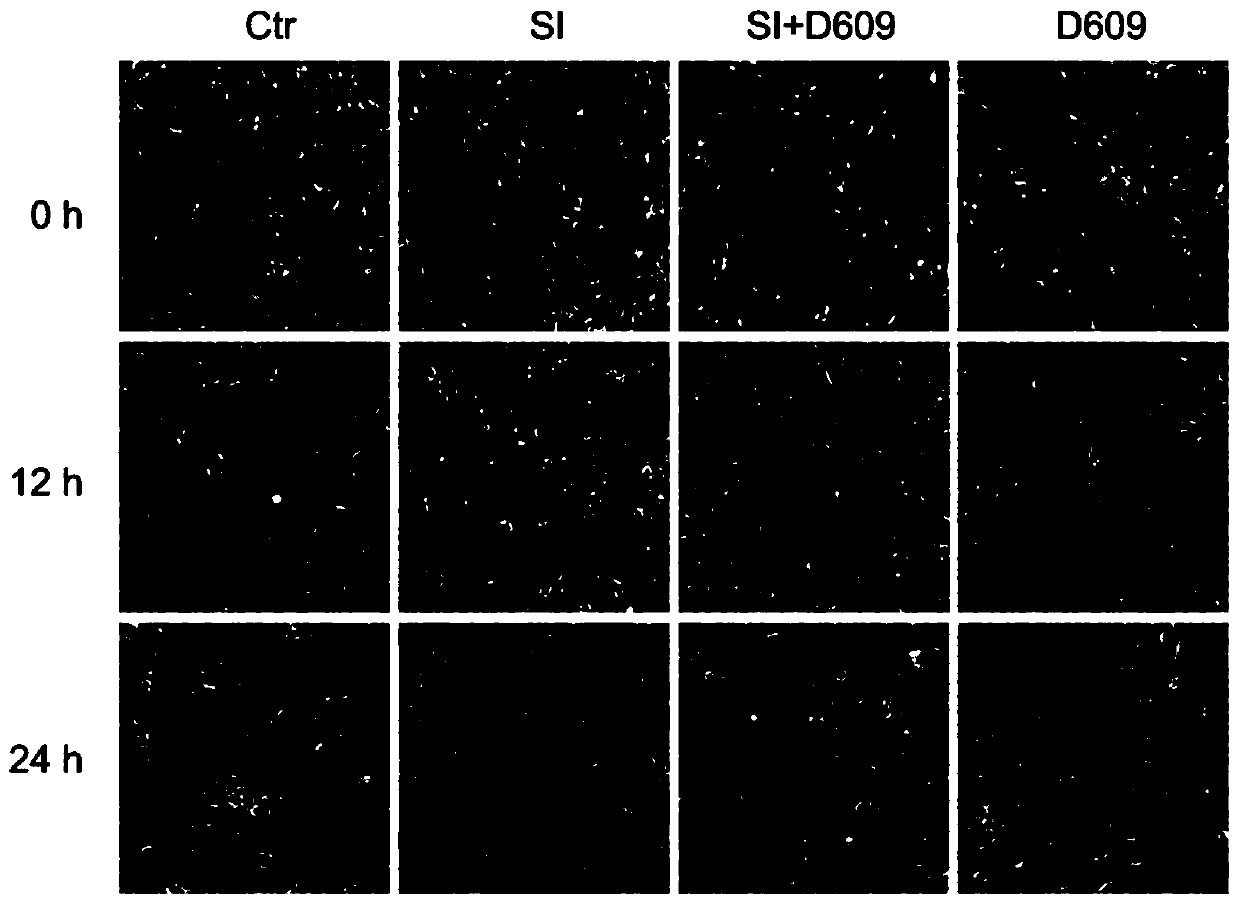
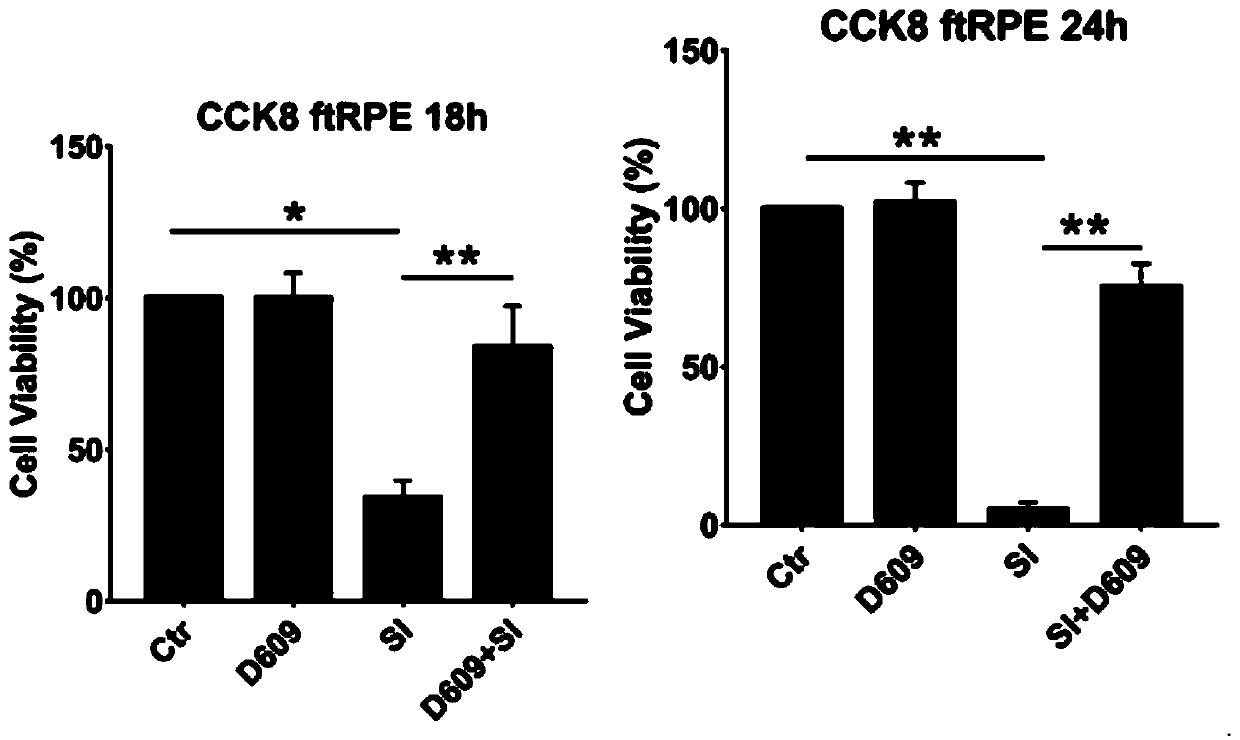
Application of D609 in preparation of medicine for preventing and treating retinal injury diseases
ActiveCN110787158AAvoid Oxidative DamageImprove survival rateOrganic active ingredientsSenses disorderPhospholipaseMacula lutea degeneration
The invention discloses an application of D609 in preparation of a medicine for preventing and treating retinal injury diseases. The Chinese name of D609 is tricyclic decane-9-yl-dithiocarbonate potassium salt, and the D609 is an antiviral and antitumor small molecular compound, which belongs to a selective inhibitor of phosphatidylcholine specific phospholipase (PC-PLC). According to the invention, the wide and deep research is carried out, the unexpected discovery is realized for the first time, the expression of metallothionein is up-regulated by the D609; the oxidative damage of RPE cellscan be effectively inhibited; therefore, the compound can be used for preventing or treating retinal injury diseases caused by oxidative stress injury of retinal pigment epithelial cells, especially,the compound can be extremely effectively used for preventing or treating macular degeneration diseases of retina, has no obvious drug toxicity, can effectively control the occurrence and developmentof injury diseases of retina, and provides a new theoretical support for developing unknown biological activity of D609 and future clinical treatment effects.
Owner:广州因明生物医药科技股份有限公司
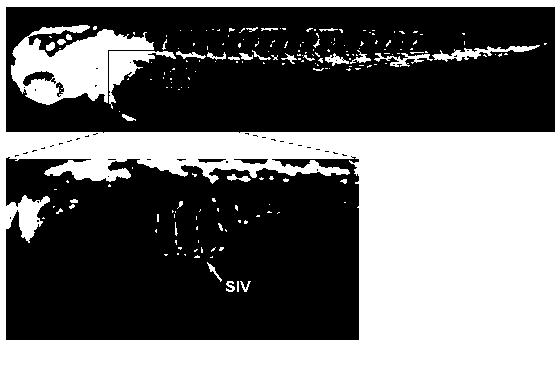
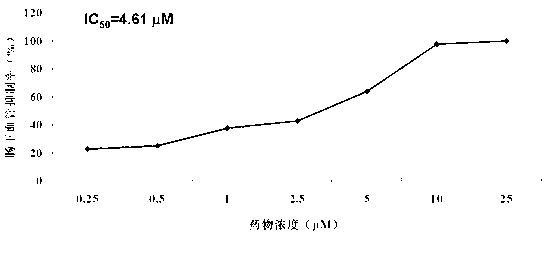
Application of chloroxine in preparing anti-angiogenesis medicine
The invention relates to an application of chloroxine in preparing anti-angiogenesis medicine. Until now no relevant reports about the anti-angiogenesis activity of the chloroxine are available. The invention provides the application of chloroxine in preparing anti-angiogenesis medicines, anti-tumor medicines and medicines for preventing wet age-related macular degeneration. The in-vivo pharmacodynamic experiment using a zebra fish angiogenesis model shows that the chloroxine can remarkably suppress zebra fish angiogenesis, remarkably suppress the growth of transplanted human tumor cells and has a therapeutic effect to wet age-related macular degeneration. Therefore, the chloroxine can be used for preparing anti-angiogenesis medicines, anti-tumor medicines and medicines for preventing wet age-related macular degeneration.
Owner:HANGZHOU LEISUO PHARMA
Compounds for treatment of diseases of abnormal angiogenesis or aberrant growth factors and uses thereof
ActiveUS9988386B2Good effectMechanism is preventedSenses disorderOrganic chemistryDiabetic retinopathyUveitis
The present invention provides novel compounds of any one of Formulae (I)-(IV), and pharmaceutical compositions thereof. Also provided are particles (e.g., nanoparticles) comprising compounds of any one of Formulae (I)-(IV) and pharmaceutical compositions thereof that are mucus penetrating. The invention also provides methods and kits for using the inventive compounds, and pharmaceutical compositions thereof, for treating and / or preventing diseases associated with abnormal or pathological angiogenesis and / or aberrant signaling of a growth factor signaling pathway (e.g., vascular endothelial growth factor (VEGF)), such as proliferative diseases (e.g., cancers, benign neoplasms, inflammatory diseases, autoimmune diseases) and ocular diseases (e.g., macular degeneration, glaucoma, diabetic retinopathy, retinoblastoma, edema, uveitis, dry eye, blepharitis, and post-surgical inflammation) in a subject in need thereof.
Owner:KALA BIO INC
Efficient process for the preparation of lycopene containing oleoresin and lycopene crystals for human consumption
InactiveUS20150004236A1Efficiently preparedEfficient processingPowder deliveryBiocideChemistryAntioxidant
The present invention provides a process for the preparation of lycopene containing oleoresin and lycopene crystals for human consumption. The present invention provides an efficient process for the preparation of lycopene crystals from lycopene containing oleoresin with at least 85% by weight lycopene, containing at least 90% by weight trans-lycopene and trace amounts of cis-lycopene and other carotenoids. The production of commercial grade lycopene crystals with high content of trans-lycopene makes it ideal and suitable for human consumption, use as an anti-oxidant, for applications in prevention of cancer and macular degenerative diseases, as an anti-oxidant, and as a food / feed colorant. The process is simple, convenient, economical and commercially feasible.
Owner:OMNIACTIVE HEALTH TECH
Amelioration of cataracts, macular degeneration and other ophthalmic diseases
InactiveCN101102770AImprovement of cataract developmentProtection from photooxidative damageSenses disorderOrganic chemistryPresbyopiaDisease
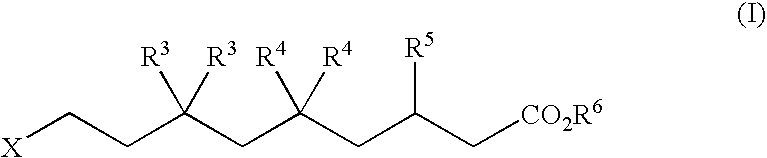
Ophthalmically acceptable compositions used in arresting the development of cataract, presbyopia, macular degeneration and other retinopathies, glaucoma, uveitis and various corneal disorders are disclosed. The compositions are also useful as a prophylactic treatment to prevent or delay development of age-related ocular disorders, which include cataracts, presbyopia, glaucoma and macular degeneration. The compositions comprise a pharmaceutically acceptable carrier or diluent and at least one compound having the formula where R1 and R2 are, independently, H or C1 to C3 alkyl ; R3 and R4 are, independently C1 to C3 alkyl; and where R1 and R2, taken together, or R3 and R4, taken together, or both may be cycloalkyl; R5 is H, OH, or C1 to C6 alkyl ; R6 is or C1 to C6 alkyl, alkenyl, alkynyl, or substituted alkyl or alkenyl ; R7 is C1 to C6 alkyl, alkenyl, alkynyl, or substituted alkyl or alkenyl or where R6 and R7, or R5, R6 and R7, taken together, form a carbocycle or heterocycle having from 3 to 7 atoms in the ring.
Owner:OTHERA HLDG
Novel retina-specific human proteins C7orf9, C12orf7, MPP4 and F379
The present invention relates to the novel human retina-specific proteins called C7orf9, C12orf7, MPP4 and F379, and isolated nucleic acid molecules encoding said proteins. Also provided are vectors, host cells, antibodies and recombinant methods for producing these human proteins. The invention further relates to diagnostic and therapeutic methods useful for diagnosing and treating macular degeneration, e.g. AMD.
Owner:MULTIGENE BIOTECH
Novel Compounds and Uses Thereof
ActiveUS20160137646A1Good effectMechanism is preventedSenses disorderOrganic chemistryDiabetic retinopathyUveitis
The present invention provides novel compounds of any one of Formulae (I)-(IV), and pharmaceutical compositions thereof. Also provided are particles (e.g., nanoparticles) comprising compounds of any one of Formulae (I)-(IV) and pharmaceutical compositions thereof that are mucus penetrating. The invention also provides methods and kits for using the inventive compounds, and pharmaceutical compositions thereof, for treating and / or preventing diseases associated with abnormal or pathological angiogenesis and / or aberrant signaling of a growth factor signaling pathway (e.g., vascular endothelial growth factor (VEGF)), such as proliferative diseases (e.g., cancers, benign neoplasms, inflammatory diseases, autoimmune diseases) and ocular diseases (e.g., macular degeneration, glaucoma, diabetic retinopathy, retinoblastoma, edema, uveitis, dry eye, blepharitis, and post-surgical inflammation) in a subject in need thereof.
Owner:KALA BIO INC
Doxorubicin structure-containing star-shaped polymer and preparation method and use thereof
InactiveCN103910873AImprove solubilityExtended half-lifeOrganic active ingredientsSenses disorderMedicinePolymer
The invention discloses a doxorubicin structure-containing star-shaped polymer and a preparation method and use thereof. The doxorubicin coupled star-shaped polymer has a better effect on treatment of age-related macular degeneration compared with drugs or medicaments in other forms.
Owner:张雅珍
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com