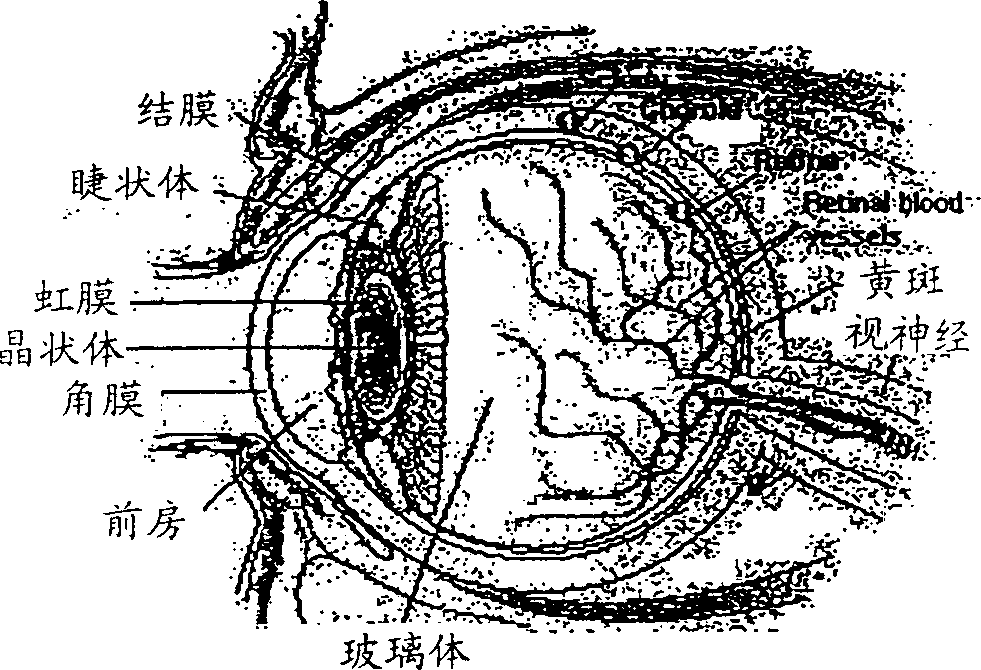
Compositions and method of administering tubulin binding agents for the treatment of ocular diseases
A technology of tubulin and binding agent, which is applied in the direction of drug combination, antiviral agent, medical preparation containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0114] Example 1. Topical administration with three routes of administration, study
[0115] Eye Irritation and Determination of Average Tolerance of CA4P
[0116] (i) Intravitreal medication
[0117] After intravitreal injection in rats, the test article CA4P was evaluated for possible intraocular irritation. After general anesthesia, 0.2 ml dose of CA4P was administered to the right eyes of 8 mice. Then, 0.2 ml dose of 0.9% sodium chloride USP solution was administered to the left eye of the rat as a negative control. Four different concentrations of CA4P were measured. Each of 4 concentrations (0.1 mg / ml, 1 mg / ml, 10 mg / ml, 100 mg / ml) was administered to the right eyes of 2 mice. Approximately 48 hours after administration, the eye was examined with a biomicroscopy slit lamp and an indirect ophthalmoscope. After recording the results, the experimental mice were euthanized. Immediately after euthanasia, a sample of the vitreous humor was aspirated and the number of...
example 2
[0129] Example 2. When using different routes of administration for topical administration in the eye
[0130] Evaluation of the in vivo distribution of CA4P
[0131] To effectively treat ocular neovascularization, extrasystemic drug delivery must pass through the relevant ocular structures to deliver effective doses of the drug to the diseased site. In order to confirm that different extrasystemic injections can produce effective distribution of CA4P in vivo, radioactive isotope tracer drug experiments were carried out.
[0132] method:
[0133] Unless stated below, each in vivo distribution experiment followed the protocol described below.
[0134] Will 14 C-CA4P (OxiGENE Inc. Watertown, MA) was suspended in 100 μl of saline solution, and then injected into anesthetized male New Zealand rats (4 months old, 1.8-2.5 kg, 3 vials per sample) with a 30G injection needle in the right eye. Corresponding to the use of 1, 5 and 5 μCi radioactive reagents, respectively, to de...
example 3
[0158] Example 3 adopts iontophoresis eye drug CA4P
[0159] CA4P can be ionized at physiological pH conditions, so it can be delivered by iontophoresis. Iontophoresis delivery (transcleral) of CA4P (10 mg / ml) was evaluated using the Vision Rabbit Eye Applicator (IOMED Inc, Salt Lake City, UT), which consisted of a 180 μl silicone container body. , connecting wires, and a monolayer hydrogel-impregnated polyvinyl acetal matrix, the 180 μl silicone container body is supported by a silver foil current distribution assembly covered with silver chloride. The contact surface area of the applicator was 0.54 square centimeters. It was placed in the blind canal above the sclera of the right eye of New Zealand white rats (3-3.5 kg, n=6 per treatment), 1-2 mm above the edge of the distal front of the corneal-scleral junction. Adopt trademark Phoresor II TM A PM700 (IOMED Inc. Salt Lake City, UT) power supply, using 2, 3, and 4 mA of each applicator, was subjected to DC anodic ion...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com