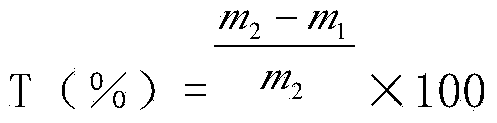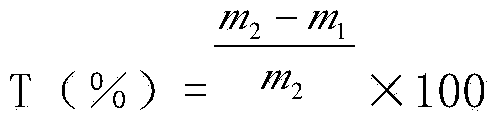Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
34 results about "Flow chemistry" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
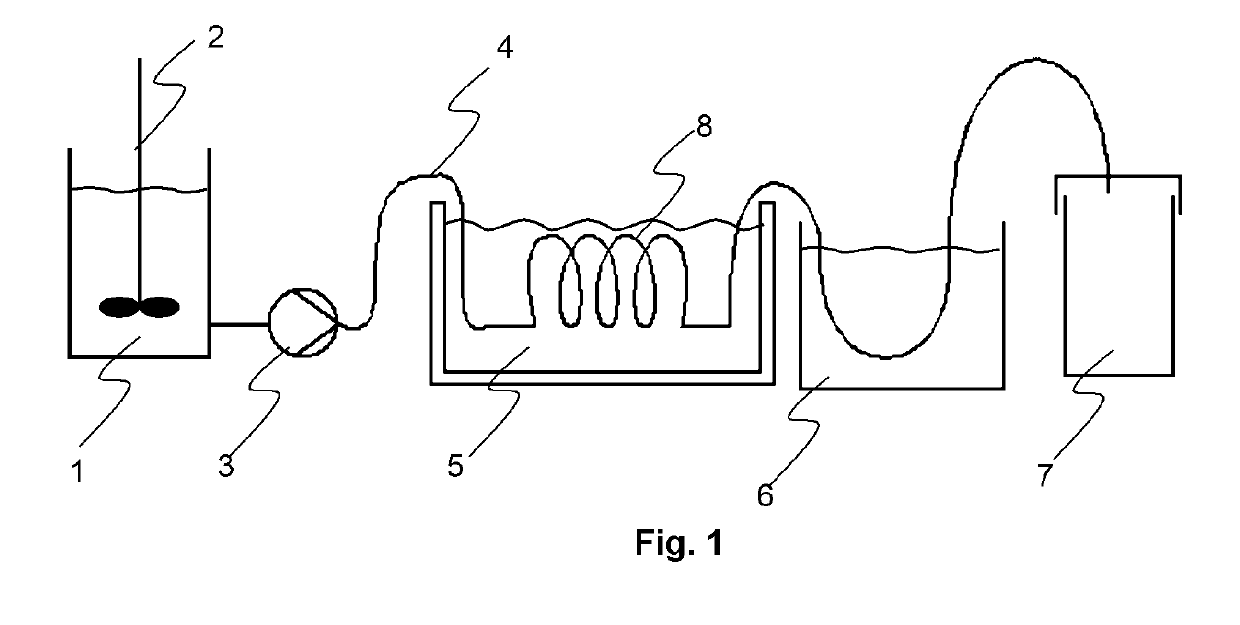
In flow chemistry, a chemical reaction is run in a continuously flowing stream rather than in batch production. In other words, pumps move fluid into a tube, and where tubes join one another, the fluids contact one another. If these fluids are reactive, a reaction takes place. Flow chemistry is a well-established technique for use at a large scale when manufacturing large quantities of a given material. However, the term has only been coined recently for its application on a laboratory scale. Often, microreactors are used.
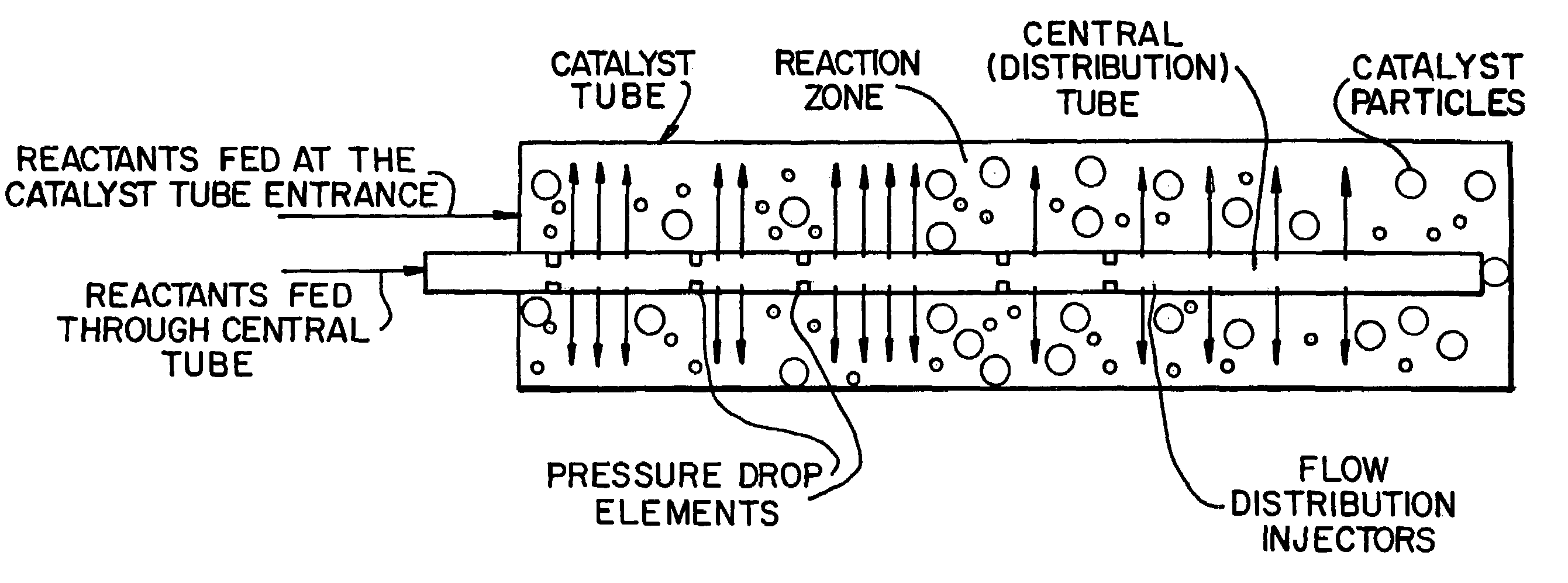
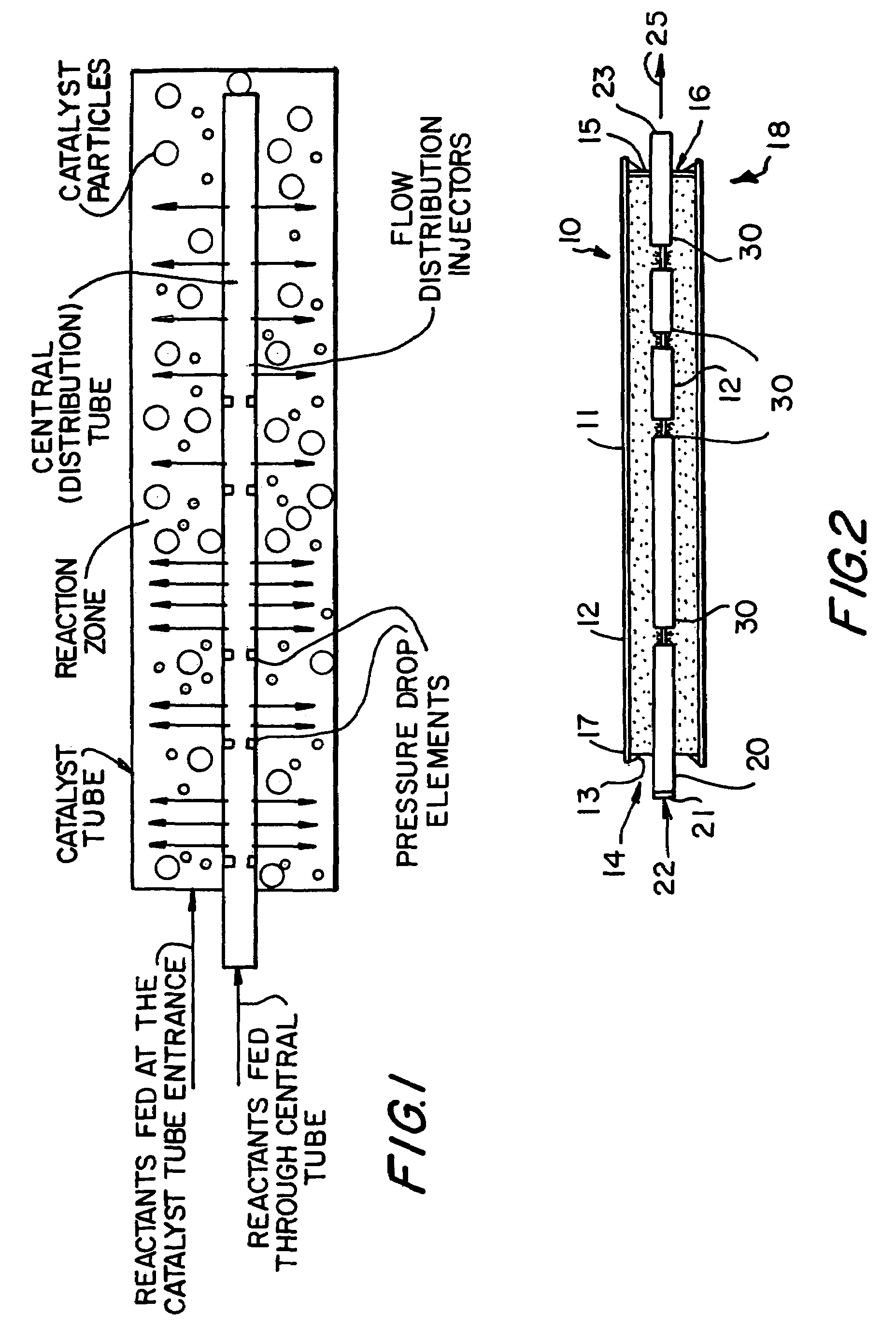
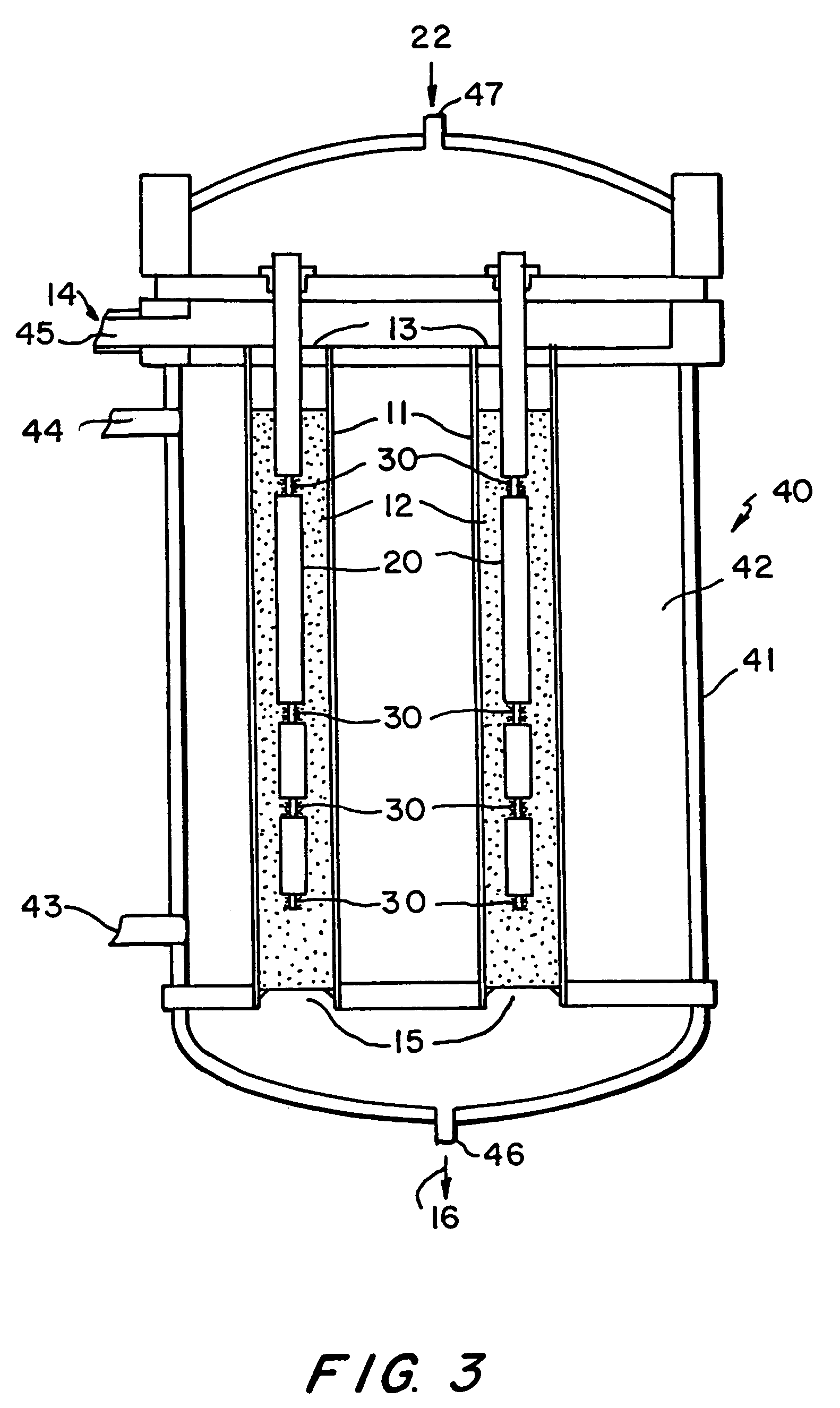
Apparatus for the controlled optimized addition of reactants in continuous flow reaction systems
InactiveUS6977064B1Improve catalytic performanceProcess control/regulationCombination devicesChemical reactionPartial oxidation
An apparatus for performing continuous flow chemical reactions such as oxidation, oxidative dehydrogenation and partial oxidation processes involving a reactor design characterized by controlled / optimized addition of a reactant with the objective of: (i) avoiding the explosion regime of the reactant mixture (e.g., hydrocarbon / oxidant mixture); (ii) maximizing the selectivity of the reaction to the desired product; (iii) limiting the reactor temperature gradient and therefore the threat of reaction runaway; and (iv) controlling the operating temperature of the reaction zone so that desirable temperature range is maintained over the entire zone.
Owner:SAUDI BASIC IND CORP SA
Measuring method for spreading rate in paper-making reconstituted tobacco production process
ActiveCN103439466AObjective accuracyMeet process technology control needsMaterial analysisFlow chemistryChemistry
The invention relates to a measuring method for a spreading rate in a paper-making reconstituted tobacco production process, belonging to the technical field of paper-making reconstituted tobacco production. The measuring method for the spraying rate in the paper-making reconstituted tobacco production process comprises the following steps: (1) measuring chlorine content c1 of approached stock in the production process by adopting a flow chemical analyzer; (2) measuring the chlorine content c2 in coating liquor in the production process by adopting the flow chemical analyzer; (3) measuring the chlorine content c3 of a reconstituted tobacco finished product by adopting the flow chemical analyzer; (4) calculating the spreading rate x of paper-making reconstituted tobacco according to the following formula: x=(c3-c1) / (c2-c1)*100%. The measuring method for the spreading rate in the paper-making reconstituted tobacco is higher in degree of accuracy and capable of objectively representing the spreading rate of the paper-making reconstituted tobacco.
Owner:FUJIAN JINMIN RECONSTITUTED TOBACCO DEV
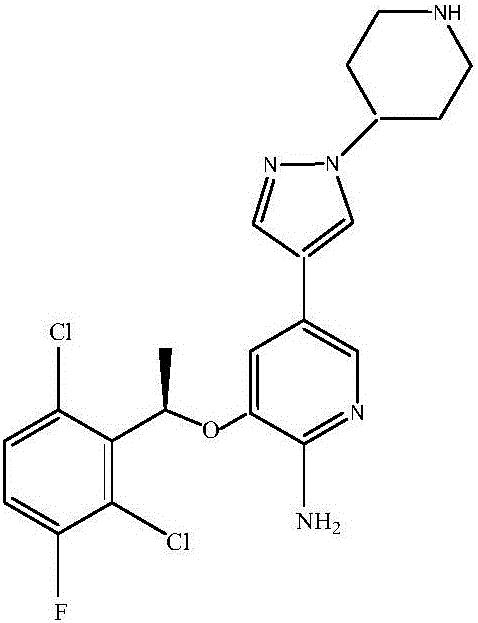
Synthetic method of crizotinib intermediate
ActiveCN105906656AImprove efficiencyHigh yieldGroup 3/13 element organic compoundsFermentationLeaving groupBoric acid
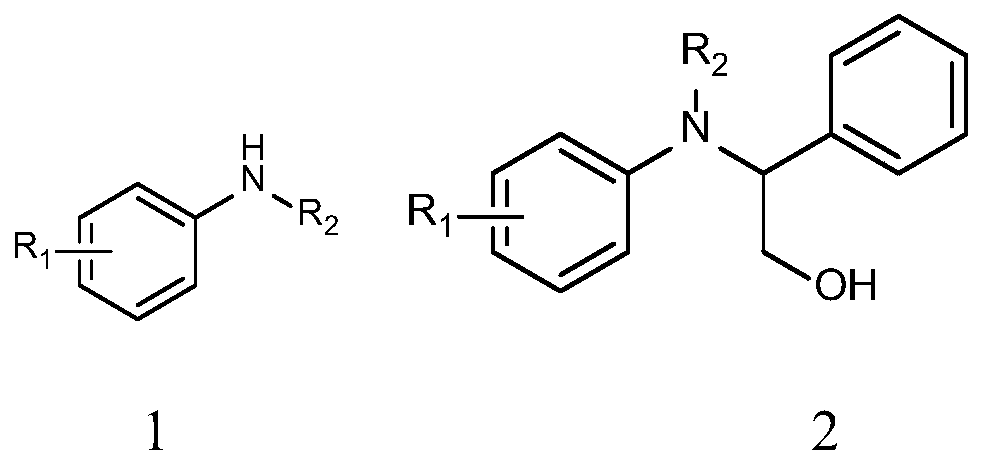
The invention relates to the technical field of small-molecule chemical drug, and particularly relates to a preparation method of a crizotinib intermediate. The preparation method comprises: (1) synthesizing a compound 3 from a compound 1 and a compound 2 by flow chemistry; (2) synthesizing the crizotinib intermediate I from the compound 3 obtained in the step (1) and a boric acid ester compound 4 by flow chemistry. The preparation method of the crizotinib intermediate, which is provided by the invention, is high in yield, can greatly reduce energy consumption and cost in the preparation process of crizotinib, is environmental-friendly, is high in safety and high in automation degree, and is suitable for industrial amplification production. A reaction route is as follows: (with reference to the specification), wherein Y represents a leaving group, Z represents an amino protection group, and X is selected from F, Cl, Br and I.
Owner:ASYMCHEM LAB TIANJIN +5
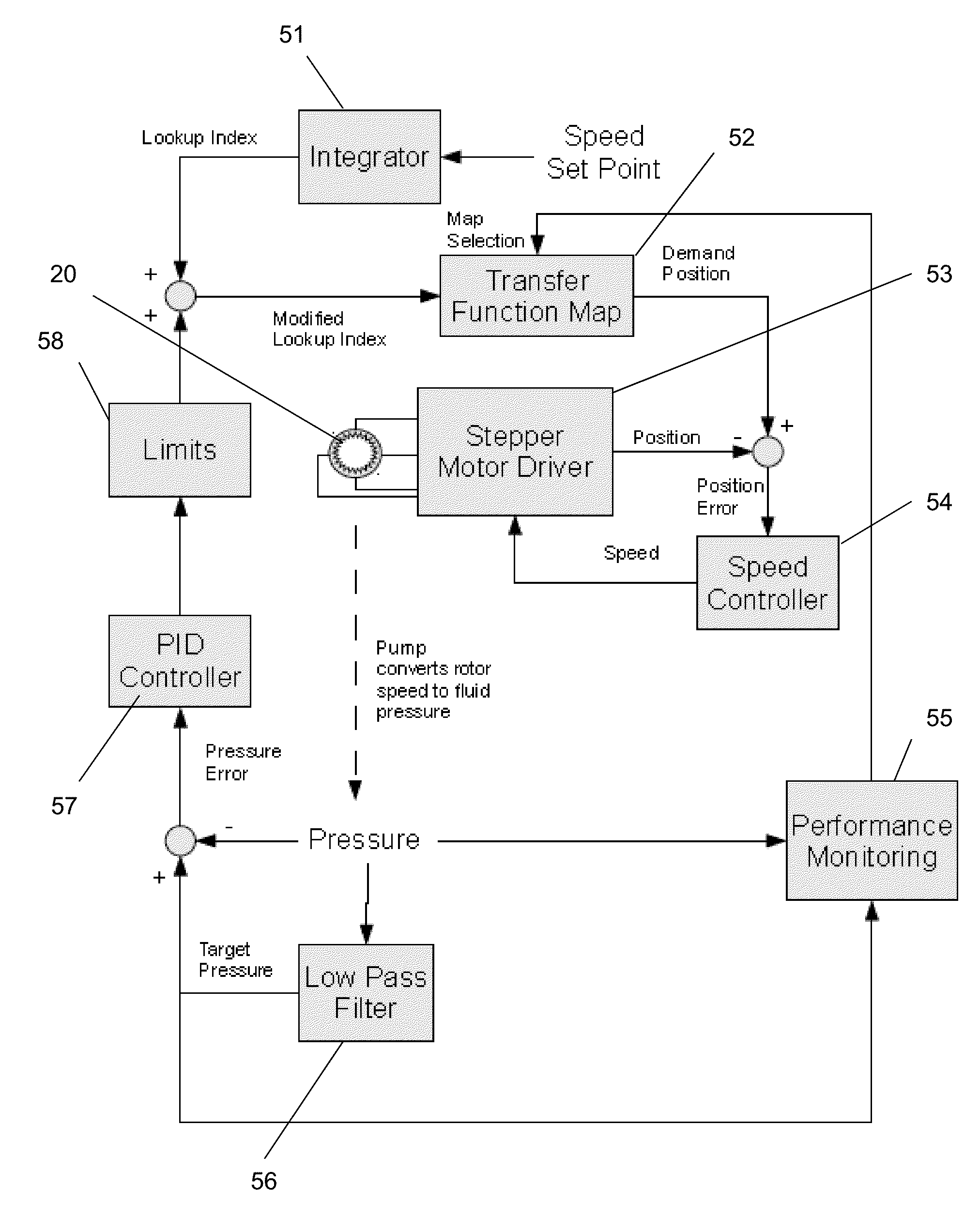
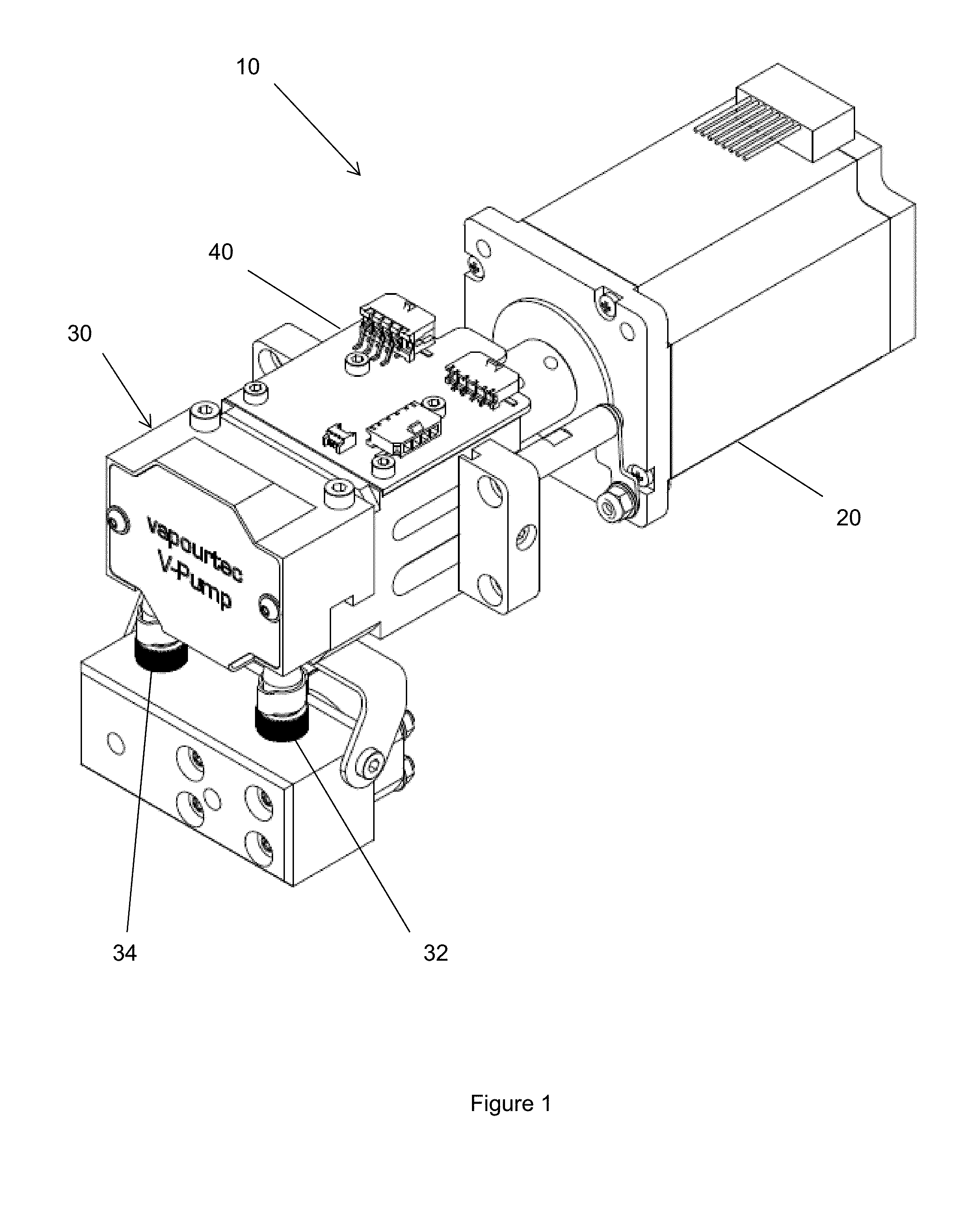
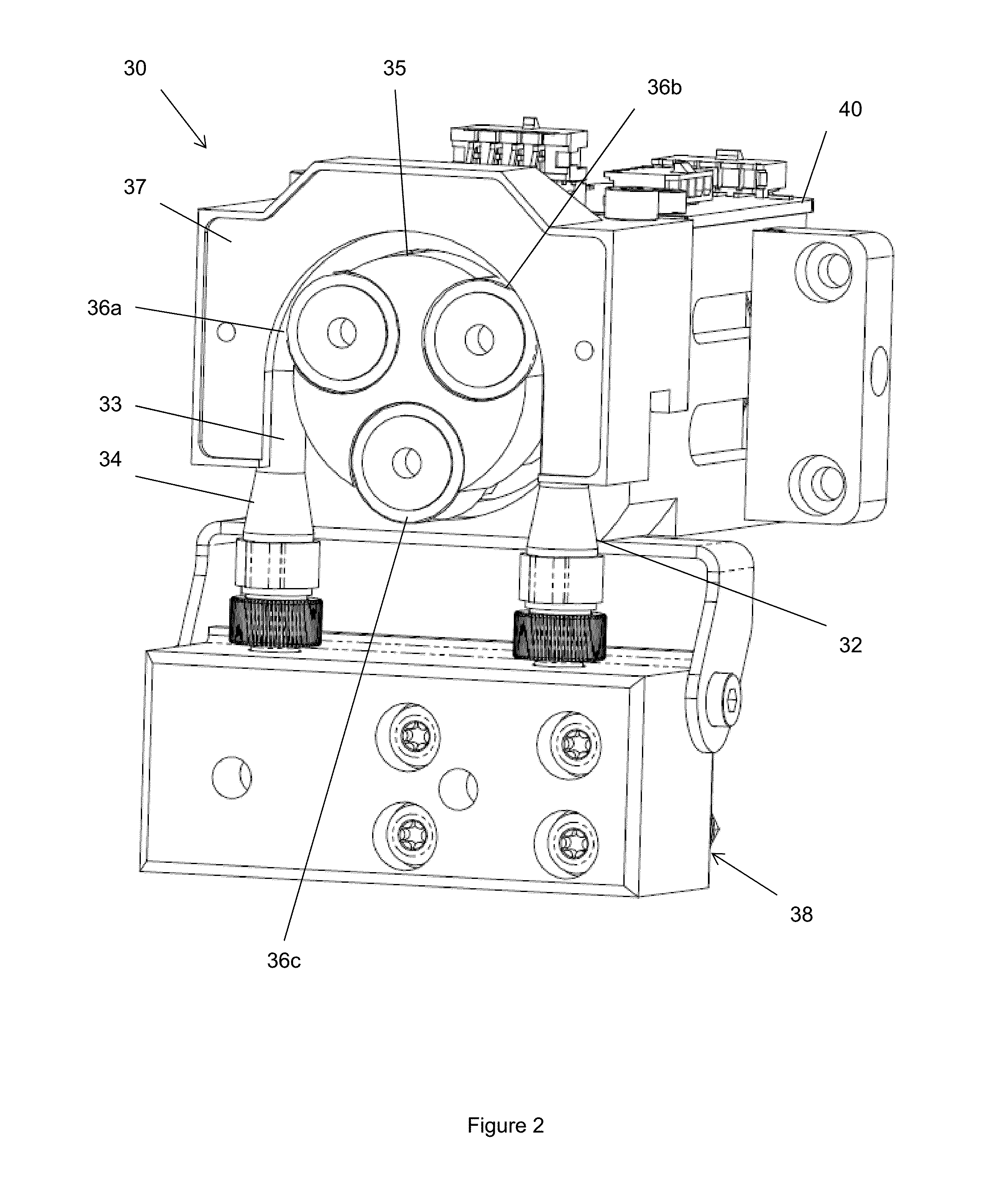
Pump
InactiveUS20150240802A1Accurate and rapid feedbackEliminate needFluid parameterFlexible member pumpsPeristaltic pumpEntry point
Provided is a pump and a method of controlling a pump. The pump and method are particularly for use in dispensing reagents, for example in flow chemistry, and more particularly on a laboratory scale. The pump aims to provide a substantially constant output flow of fluid. The pump comprises: a motor; a peristaltic pump having a rotor driven by the motor; a pressure sensor monitoring the pressure of the pumped fluid downstream of the pump; and a control unit which controls the motor by adjusting the standard operating speed of the motor according to the pressure detected by the pressure sensor, such that the pump operates continuously at a rate set by an operator. Embodiments of the pump make use of lookup tables to determine a desired position of the pump rotor at each point in the cycle with the entry point into the lookup table being determined by the feedback from the pressure sensor. Long term changes in the performance of the pump can also be accounted for by changing the entry in the lookup table which is consulted.
Owner:VAPORTEC
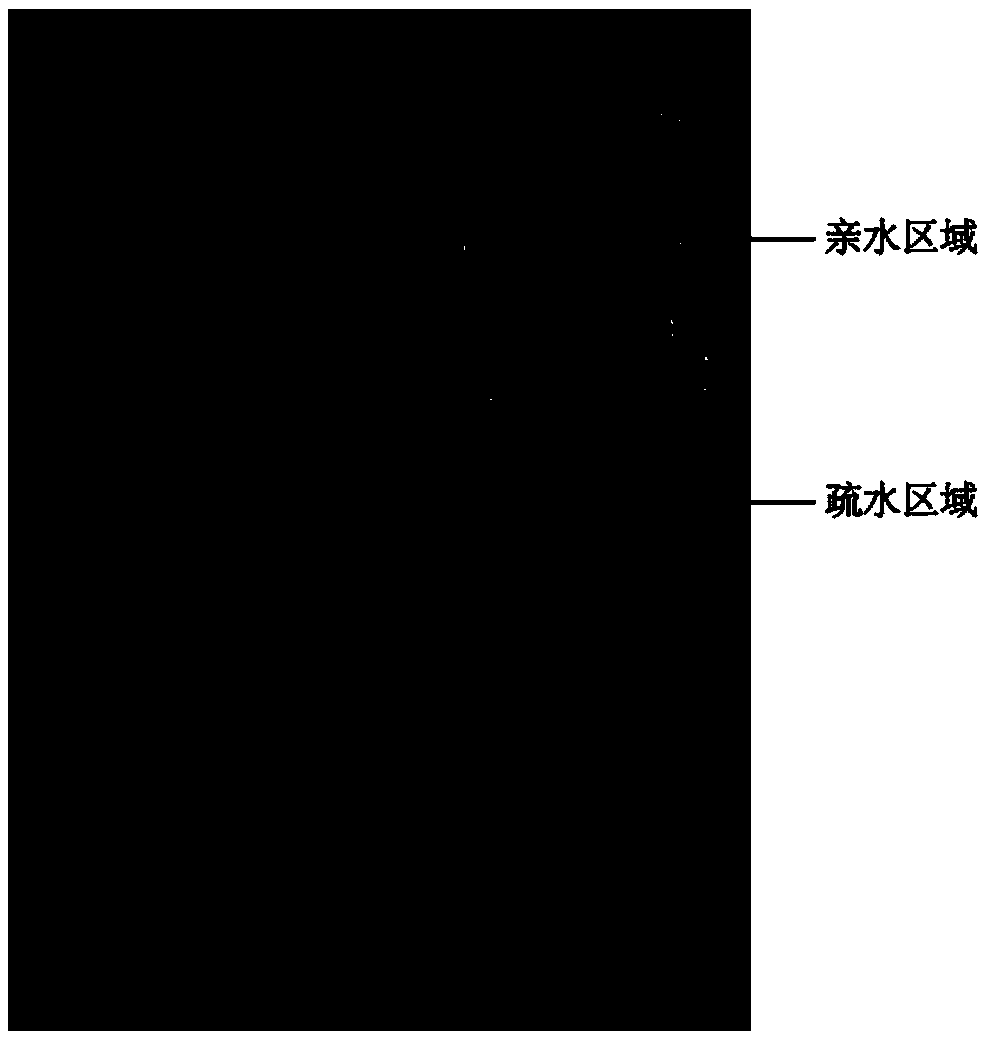
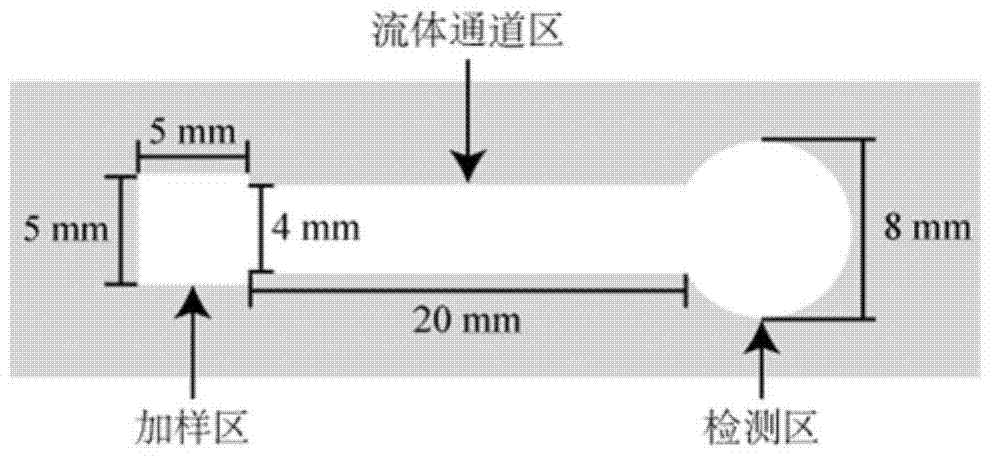
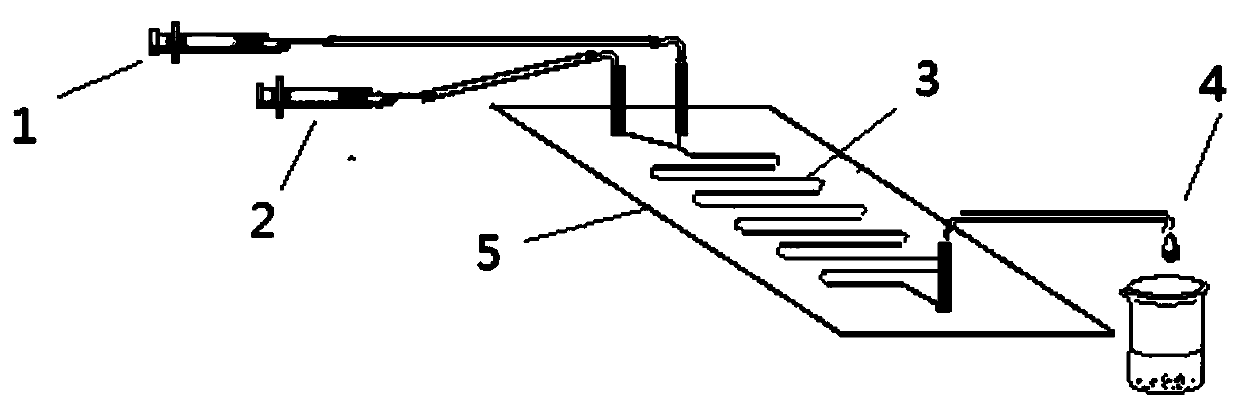
Paper chip gravity / capillary flow chemiluminescence method
InactiveCN105372233AEasy to useGood biocompatibilityChemiluminescene/bioluminescencePeristaltic pumpFiber
The present invention discloses a paper chip gravity / capillary flow chemiluminescence method, and specifically discloses a paper chip for driving fluid by coupled gravity / capillary force, and a preparation method and application thereof in detection of heavy metal ions. The paper chip of the invention is divided into a hydrophobic region and a hydrophilic region; and the hydrophilic region is divided into a loading zone, a detection zone and a fluid passage zone. For usage, the paper chip is closely placed on a stand, and the slanted surface of the stand and the horizontal plane form an angle; and after placement, the loading zone and the fluid passage zone are on an inclined surface of the stand, and the detection zone is on the horizontal portion of the stand. Compared with the conventional flow chemiluminescence, the paper chip and detection method do not use any expensive pumping unit (precision syringe pump, peristaltic pump, etc.) to drive the liquid flow. The method only uses the natural gravity of liquid and capillary force of paper fiber voids to drive the flow of liquid.
Owner:SOUTH CHINA NORMAL UNIVERSITY
Methods of evolutionary synthesis including embodied chemical syntheses
ActiveUS20150133306A1Maximize chanceLarge in compositionSequential/parallel process reactionsDirected macromolecular evolutionChemical synthesisGenetics algorithms
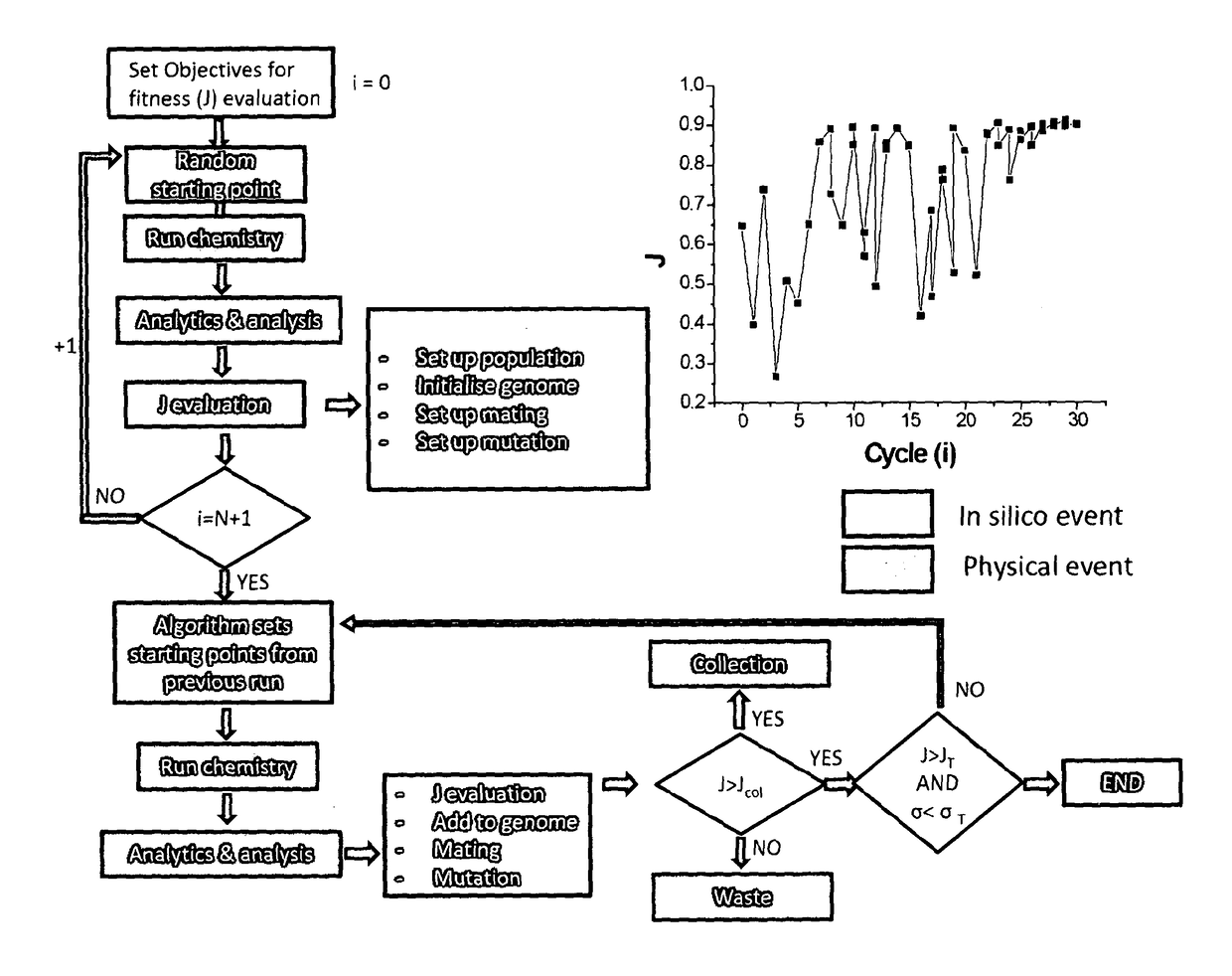
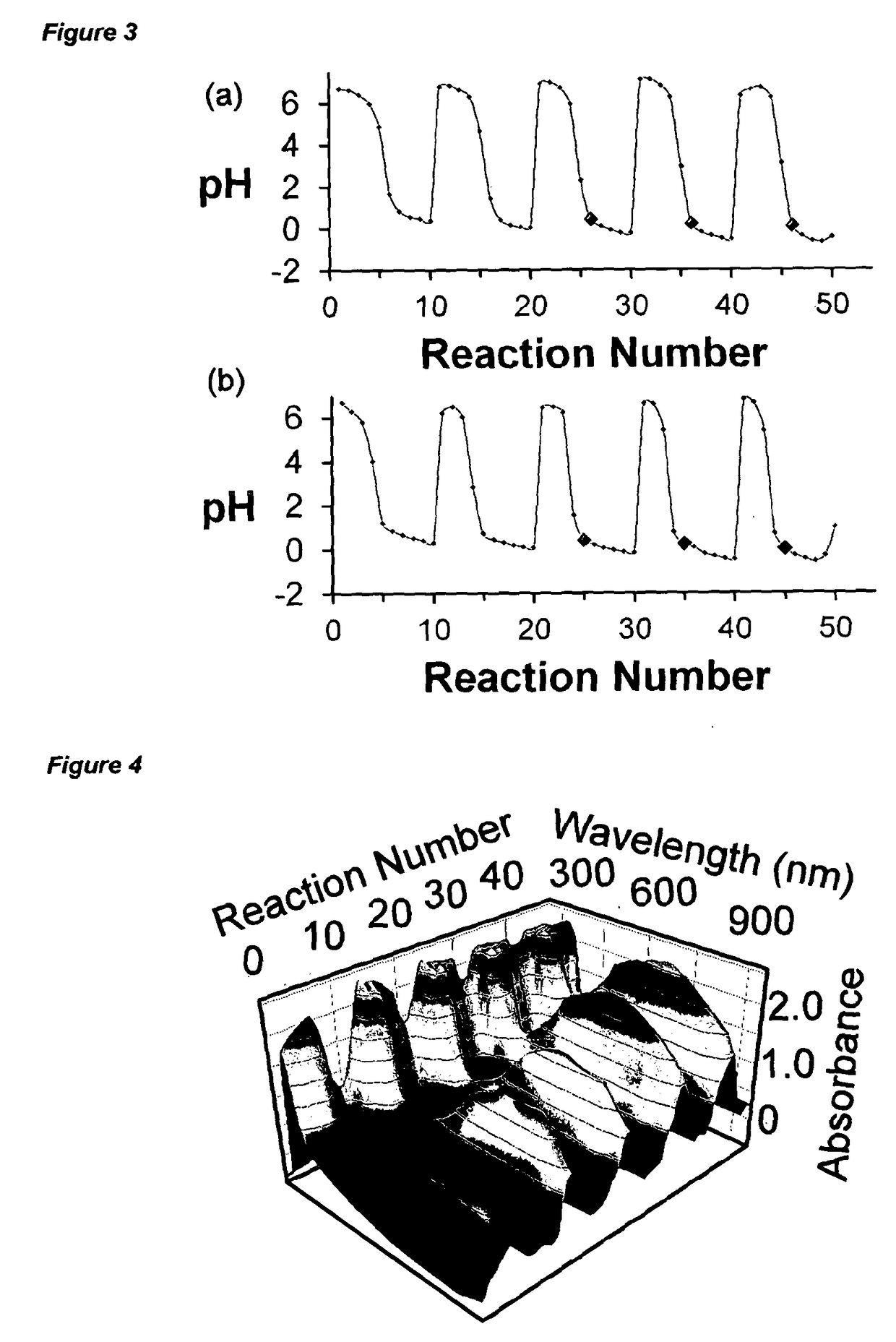
The invention provides a method for preparing a compound or a product having one or more characteristics that meet or exceed a user specification, the process comprising the step of selecting a first combination of chemical inputs, optionally together with physical inputs, and supplying those inputs to a reaction space, thereby to generate a first product; analysing one or more characteristics of the product generated; comparing the one or more characteristics against a user specification; using a genetic algorithm selecting a second combination of chemical inputs, optionally together with physical inputs, wherein the second combination differs from the first combination, and supplying those inputs to the reaction space, thereby to generate a second product; analysing one or more characteristics of the second product generated; comparing the one or more characteristics generated against the user specification; repeating the selecting and analysing steps for further individual combinations of chemical and / or physical inputs, to provide an array of products wherein the flow chemistry system operates continuously to provide the first, second and further products, thereby to identify one or more products meeting or exceeding the user specification.
Owner:DEEPMATTER LTD
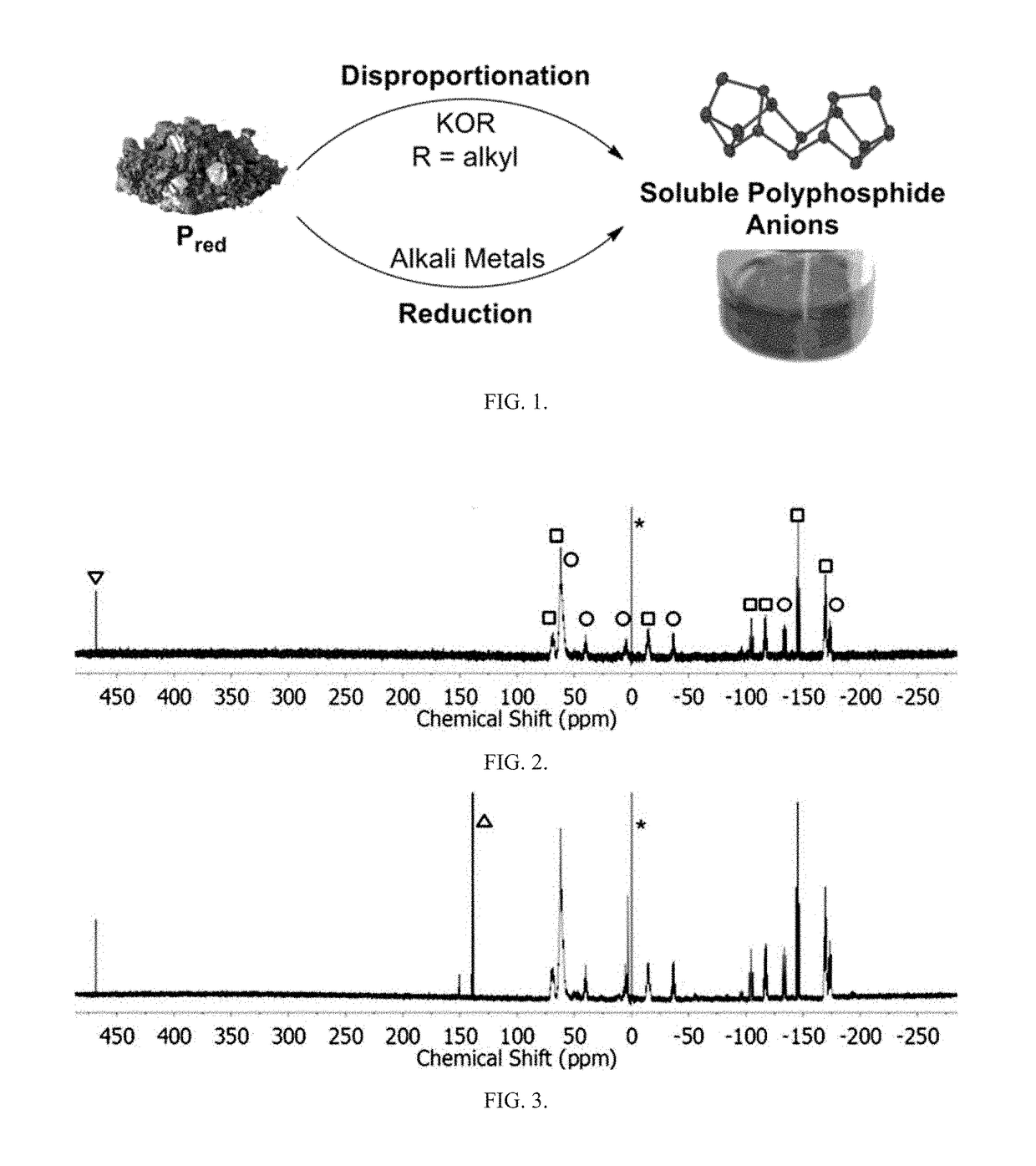
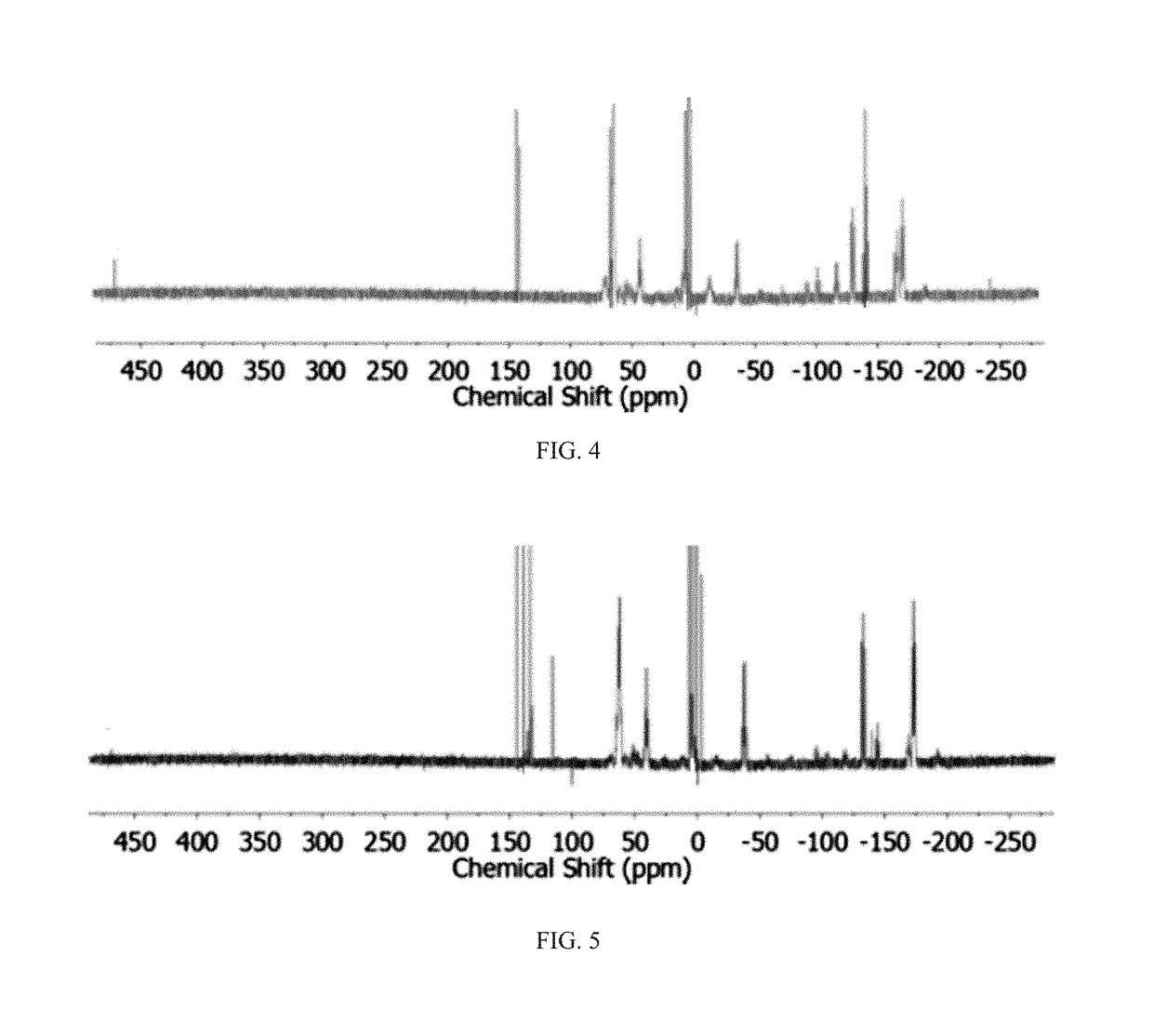
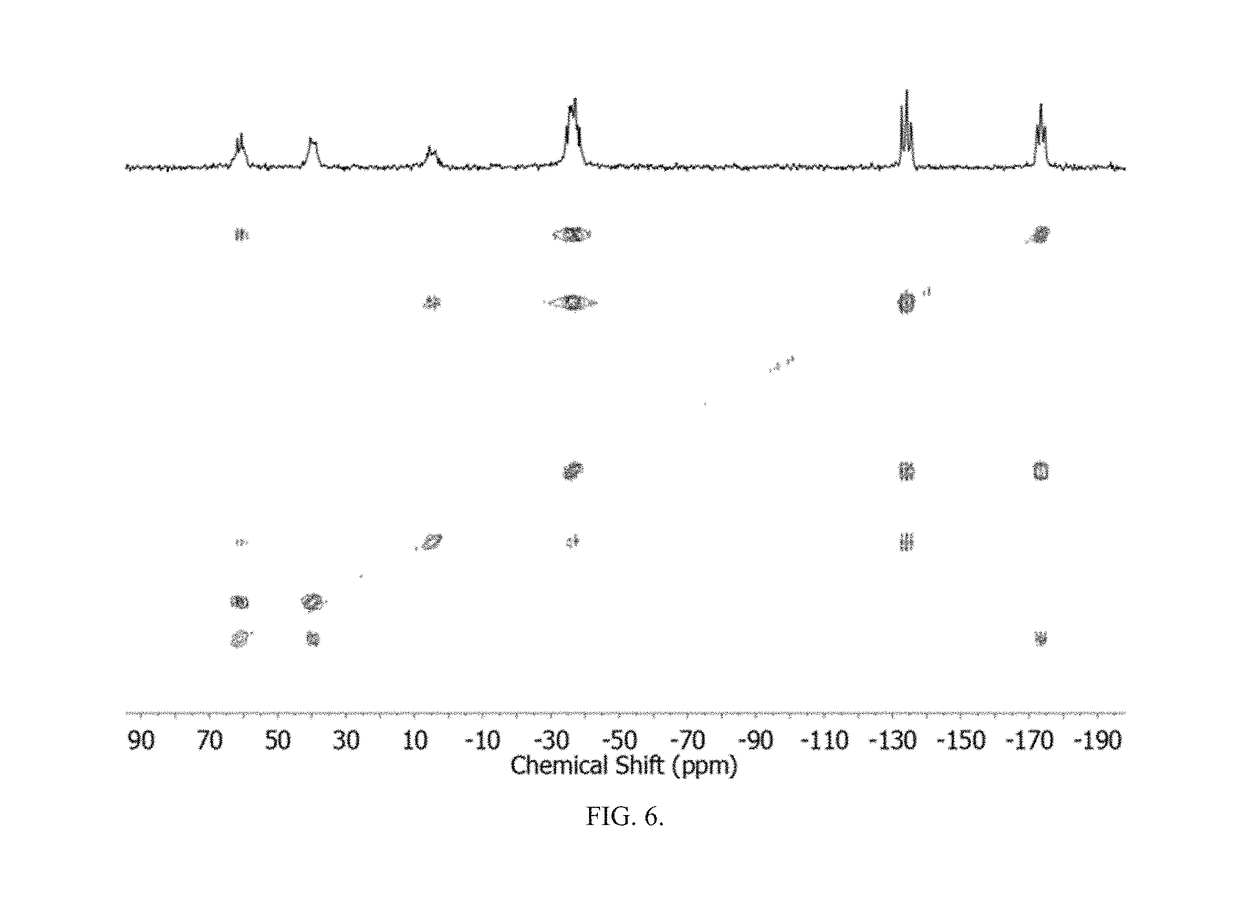
Method of conversion of red phosphorous to soluble polyphosphides
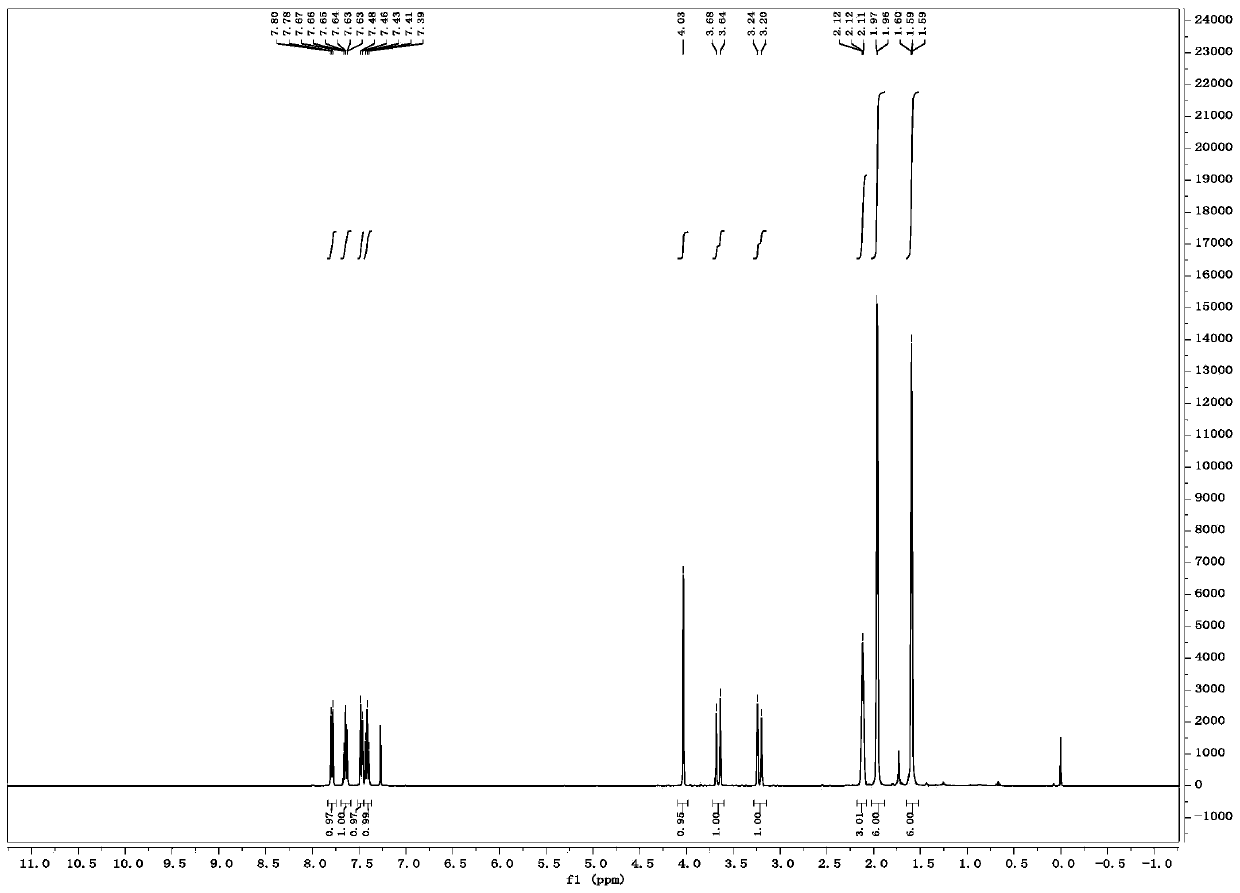
Soluble polyphosphides were successfully generated in a number of organic solvents by a reaction between shelf-stable red phosphorus and potassium ethoxide or other nucleophilic activators. The species were identified by 31P-NMR in solution. The reaction was scaled up to gram quantities by using a flow-chemistry process.
Owner:FLORIDA STATE UNIV RES FOUND INC
Novel method for realizing visible light catalytic asymmetric oxidation by using micro reactor
ActiveCN110372506ANo safety hazardEliminate the effects ofOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic oxidationReaction temperature
The invention belongs to the field of flow chemistry, and provides a novel method for realizing visible light catalytic asymmetric oxidation by using a micro reactor. According to the method, a chiralbeta-hydroxy-beta-dicarbonyl compound is prepared continuously by the asymmetric photocatalytic oxidation of a beta-dicarbonyl compound with oxygen as an oxidizing agent under the conditions of visible light, a phase transfer catalyst derived from cinchona base and without additional photosensitizers or photosensitizer group introduction. The reaction temperature is -15-30 DEG C, the reaction residence time is 5-200 min, and the 100% substrate conversion rate can be immediately achieved and enantioselectivity greater than 70% can further be achieved. The method has the advantages of mild reaction conditions, continuous reaction, the catalyst provided with dual functionalization of chiral catalyst center and photosensitive center, no amplification effect and easy industrialization.
Owner:DALIAN UNIV OF TECH
Methods of evolutionary synthesis including embodied chemical syntheses
ActiveUS9757706B2Maximize chanceLarge in compositionSequential/parallel process reactionsLibrary creationChemical synthesisGenetic algorithm
The invention provides a method for preparing a compound or a product having one or more characteristics that meet or exceed a user specification, the process comprising the step of selecting a first combination of chemical inputs, optionally together with physical inputs, and supplying those inputs to a reaction space, thereby to generate a first product; analyzing one or more characteristics of the product generated; comparing the one or more characteristics against a user specification; using a genetic algorithm selecting a second combination of chemical inputs, optionally together with physical inputs, wherein the second combination differs from the first combination, and supplying those inputs to the reaction space, thereby to generate a second product; analyzing one or more characteristics of the second product generated; comparing the one or more characteristics generated against the user specification; repeating the selecting and analyzing steps for further individual combinations of chemical and / or physical inputs, to provide an array of products wherein the flow chemistry system operates continuously to provide the first, second and further products, thereby to identify one or more products meeting or exceeding the user specification.
Owner:DEEPMATTER LTD
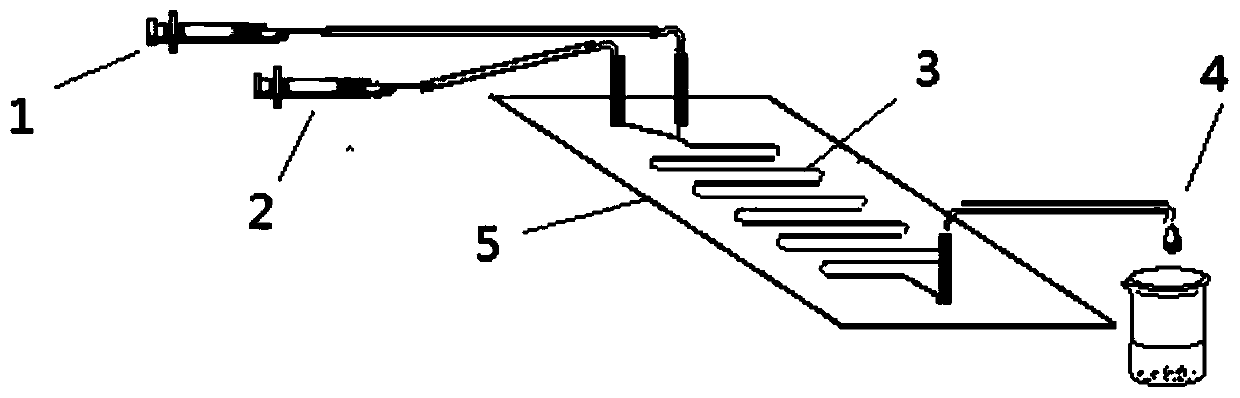
On-line phenylethanol beta-amino-alcohol derivative synthesis method on basis of enzymatic aminolysis by flow chemistry
ActiveCN109706194AShort reaction timeImprove conversion rateBioreactor/fermenter combinationsBiological substance pretreatmentsStyrene oxideSynthesis methods
The invention discloses an on-line phenylethanol beta-amino-alcohol derivative synthesis method on basis of enzymatic aminolysis by flow chemistry. The on-line phenylethanol beta-amino-alcohol derivative synthesis method on basis of enzymatic aminolysis by flow chemistry comprises the following steps: taking methanol as a reaction solvent, aniline compounds and styrene oxide as raw materials, wherein the molar ratio of the aniline compounds to the styrene oxide is 1 to (0.6-1.4), and lipase Lipozyme RM IM as a catalyst; putting the raw materials and the reaction solvent into a syringe; uniformly filling a reaction channel of a microfluidic channel reactor with the lipase Lipozyme RM IM and continuously introducing the raw materials and the reaction solvent into the reaction channel under push force of an injection pump so as to be subjected to ring-opening reaction, wherein the inner diameter of the reaction channel of the microfluidic channel reactor is 0.8-2.4 millimeters and the length of the reaction channel is 0.5-1.0m; carrying out ring-opening reaction at 30-50 DEG C for 10-30 minutes, and collecting reacted solution on line by using a product collector; and then, performingconventional post-processing on the reacted solution so as to obtain phenylethanol beta-amino-alcohol derivatives. The on-line nitroimidazole derivative synthesis method disclosed by the invention has the advantages of being short in reaction time, excellent in selectivity and high in yield.
Owner:ZHEJIANG UNIV OF TECH
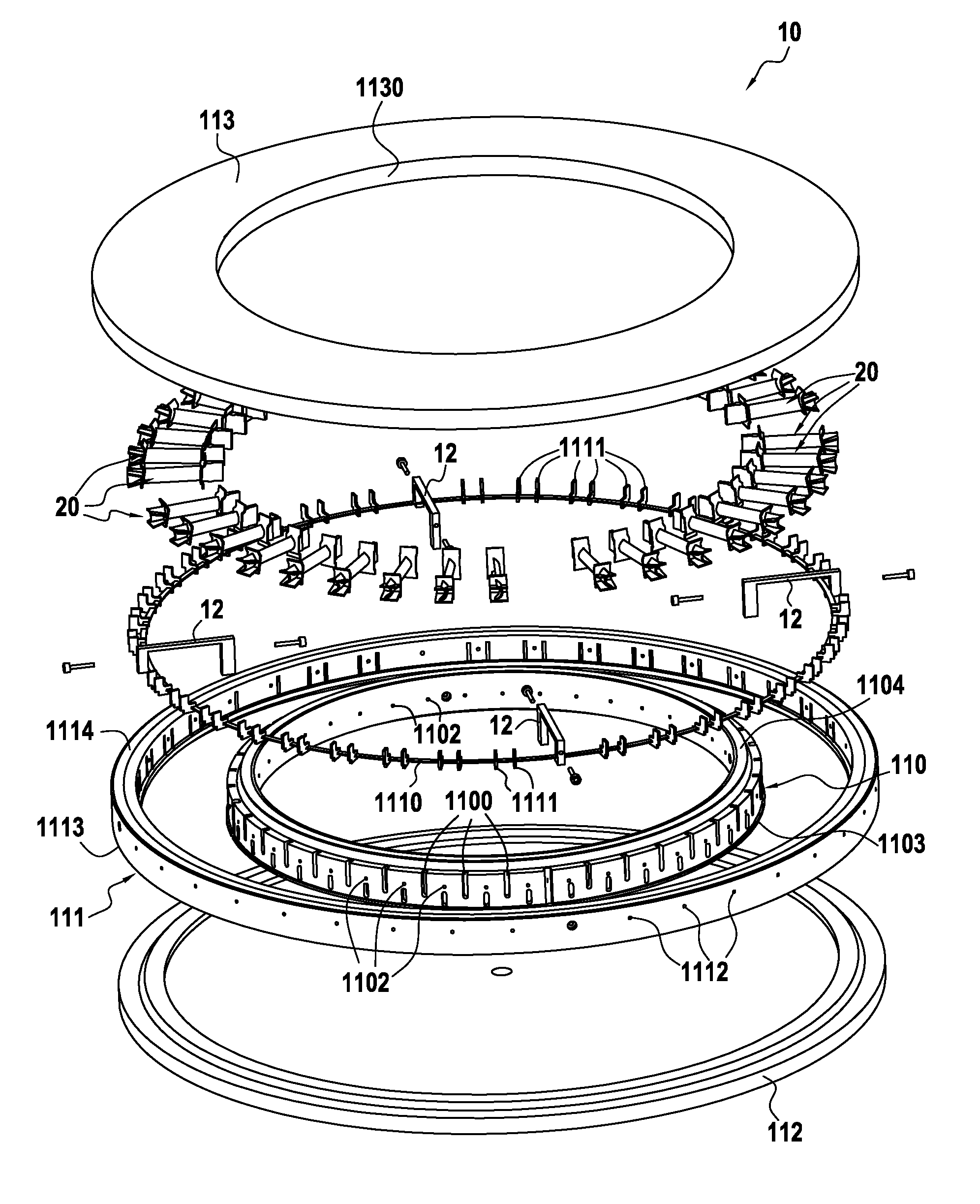
Device for Loading Porous Substrates of Three-Dimensional Shape in Order to be Densified by Directed Flow Chemical Vapor Infiltration
ActiveUS20150075428A1Increase load capacityMinimizing densification gradientChemical vapor deposition coatingPorous substrateGas phase
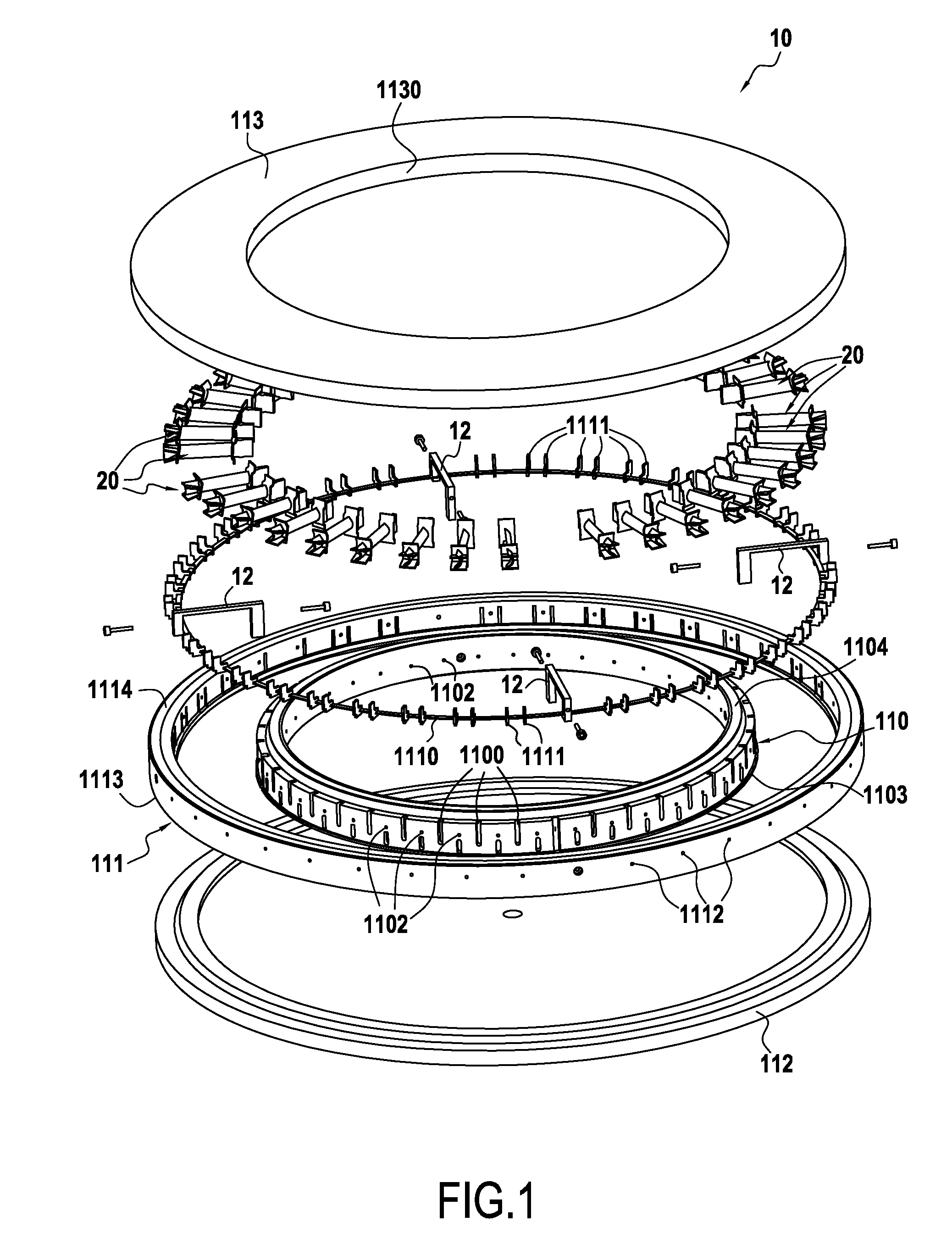
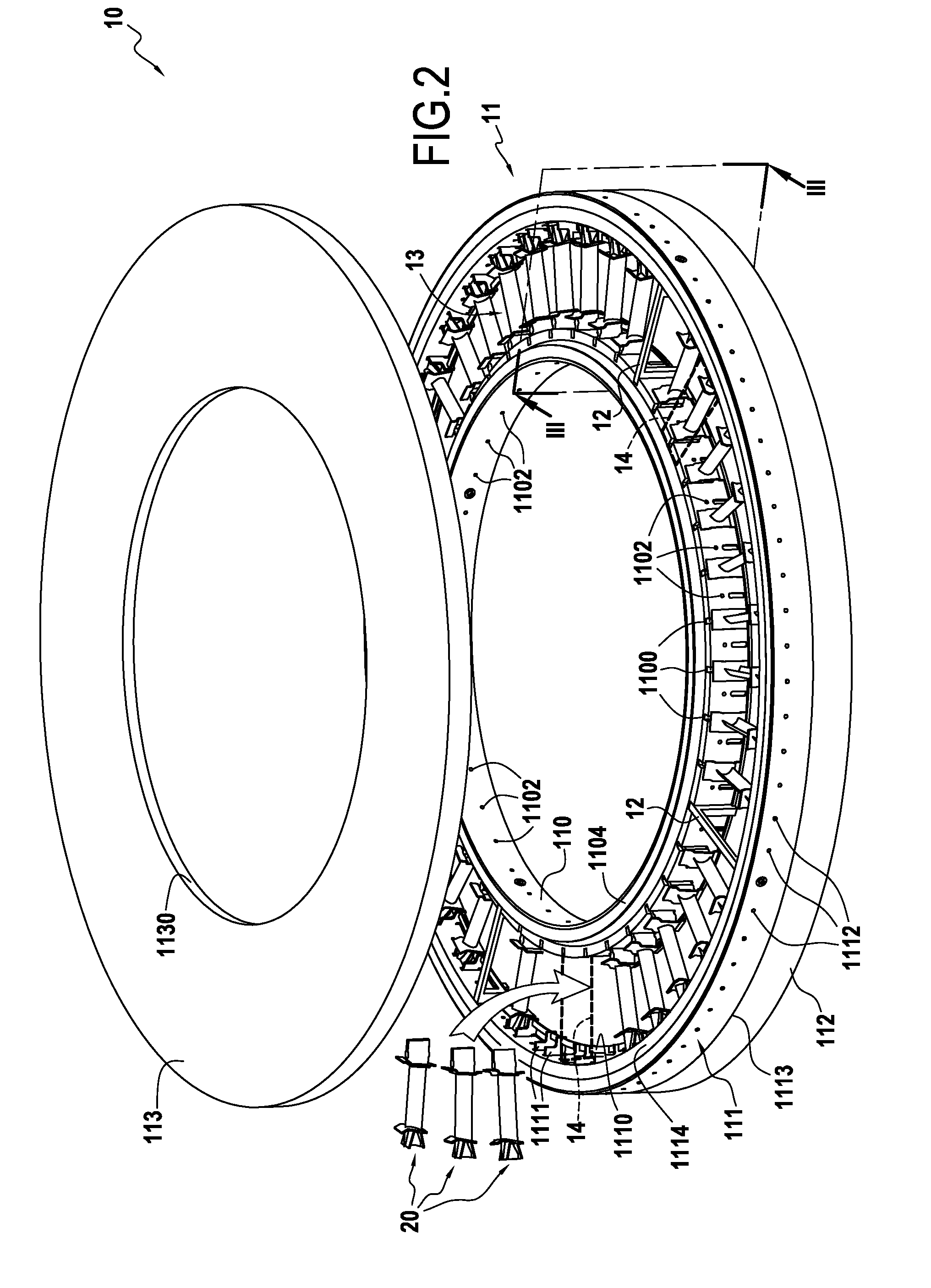
A loader device for loading porous substrates of three-dimensional shapes extending mainly in a longitudinal direction into a reaction chamber of an infiltration oven for densification of the preforms by directed flow chemical vapor infiltration. The device comprising at least one annular loader stage formed by first and second annular vertical walls arranged coaxially relative to each other and defining between them an annular loader space for the porous substrates to be densified. First and second plates respectively cover the bottom portion and the top portion of the annular loader space. The first and second annular vertical walls include support elements arranged in the annular loader space so as to define between them unit loader cells, each for receiving a respective substrate to be densified. The device also comprises gas feed orifices and gas exhaust orifices in the vicinity of each unit loader cell.
Owner:SAFRAN CERAMICS SA
Microfluid distributor and multi-channel parallel amplification fluid uniform distribution method
ActiveCN113145037ASolve the problem of flow differences caused by different pressure dropsWide range of operating conditionsTransportation and packagingChemical/physical/physico-chemical microreactorsEngineeringMechanical engineering
The invention belongs to the technical field of flow chemistry, and particularly relates to a microfluid distributor and a multi-channel parallel amplification fluid uniform distribution method. The microfluid distributor is composed of two fluid flow distribution modules and a fluid mixing module assembled between the two fluid flow distribution modules. The fluid flow distribution module comprises a fluid inlet channel, a fluid flow distribution chamber and a fluid distribution channel; the fluid mixing module comprises a fluid mixing channel, a mixed liquid collecting chamber, a mixed liquid outlet channel and a mixed liquid outlet; and the three modules are communicated through channels. Two fluids are simultaneously input into the two fluid flow distribution modules respectively, uniformly flow into the corresponding fluid distribution channels in the two fluid flow distribution chambers respectively, then flow into the fluid mixing module, then enter the mixed liquid collecting chamber and finally flow out through the mixed liquid outlet channel. The microfluid distributor has the advantages that the operating condition range is wide, the fluid flow among a plurality of parallel fluid channels can be strictly and uniformly distributed, the pressure drop is small, and the energy consumption is low.
Owner:FUDAN UNIV
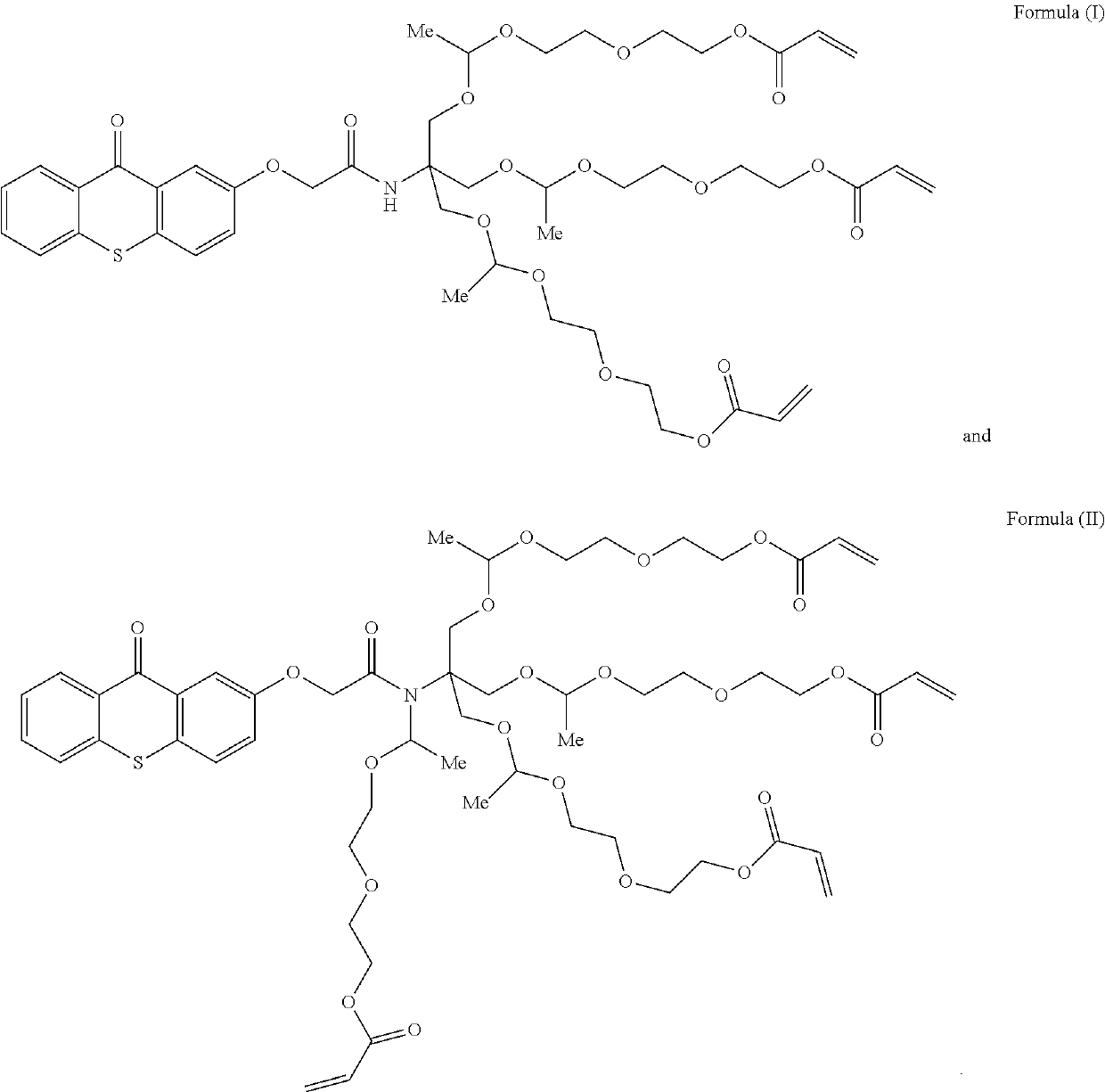
Polymerizable photoinitiators
ActiveUS20190185689A1Improve thermal stabilityLess migrateableOrganic chemistryDuplicating/marking methodsOrganic chemistryFlow chemistry
A specific mixture of polymerizable photoinitiators contains a thioxanthone group. A method for manufacturing the mixture of polymerizable photoinitiators uses flow chemistry.
Owner:AGFA NV
Application of flow chemistry in toluene nitrification
PendingCN112500294AHigh selectivityQuick responseSulfur compoundsBulk chemical productionNitriliruptorPtru catalyst
The invention discloses an application of flow chemistry in toluene nitrification. Toluene is used as a solvent, nitric acid is used as a nitrating agent, sulfuric acid is used as a catalyst, and a nitration reaction is carried out in a micro-channel reactor. The micro-channel reactor is applied to toluene nitration reaction, the advantages of high target product selectivity, high reaction speed and mild and easily-controlled reaction of the micro-channel reactor are utilized, and compared with a traditional method, the reaction temperature is lower than that of industrial toluene nitration reaction; meanwhile, the large specific surface area of the micro-channel reactor is utilized, heat exchange of a reaction system is fast, and the possibility of accidents caused by misoperation is reduced.
Owner:成都睿和德医药科技有限公司
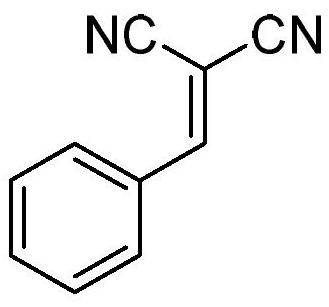
Tandem synthesis method of 2-(phenylmethylene)malononitrile or derivative thereof
ActiveCN110092734AHigh purityAvoid separationCarboxylic acid nitrile preparationOrganic compound preparationHydrogenGram
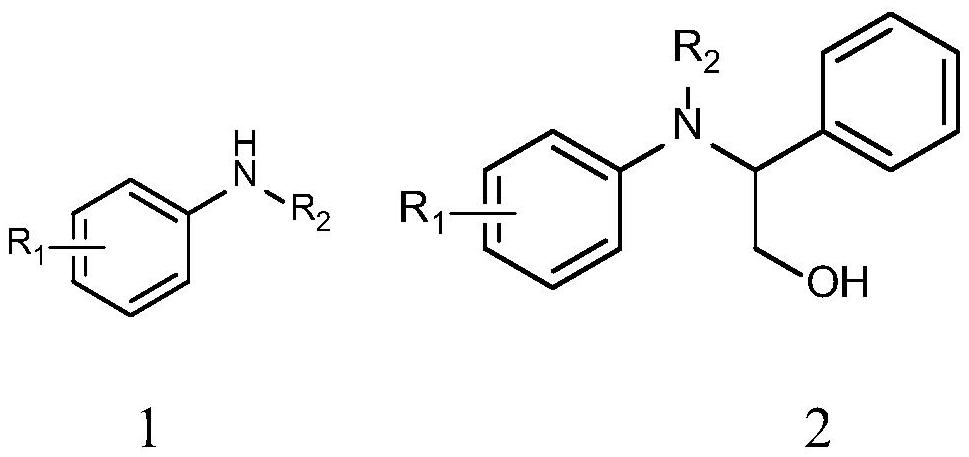
A tandem synthesis method of 2-(phenylmethylene)malononitrile or a derivative thereof is disclosed. The general structural formula of the 2-(phenylmethylene)malononitrile or a derivative thereof is shown in the description, wherein R1 is hydrogen, p-methoxy, 2-bromo, 3-bromo or 4-bromo, and R2 is a cyano or ester group. A chloromethylated polystyrene microsphere acid-base functionalized catalyst is synthesized, a tandem reaction is successfully applied to the flow chemistry, and the 2-(phenylmethylene)malononitrile or a derivative thereof is synthesized in a gram scale. Compared with traditional test tube reactions, the method greatly reduces the reaction time, improves the yield and product purity, and avoids a step of separating a product from the catalyst.
Owner:HUAQIAO UNIVERSITY
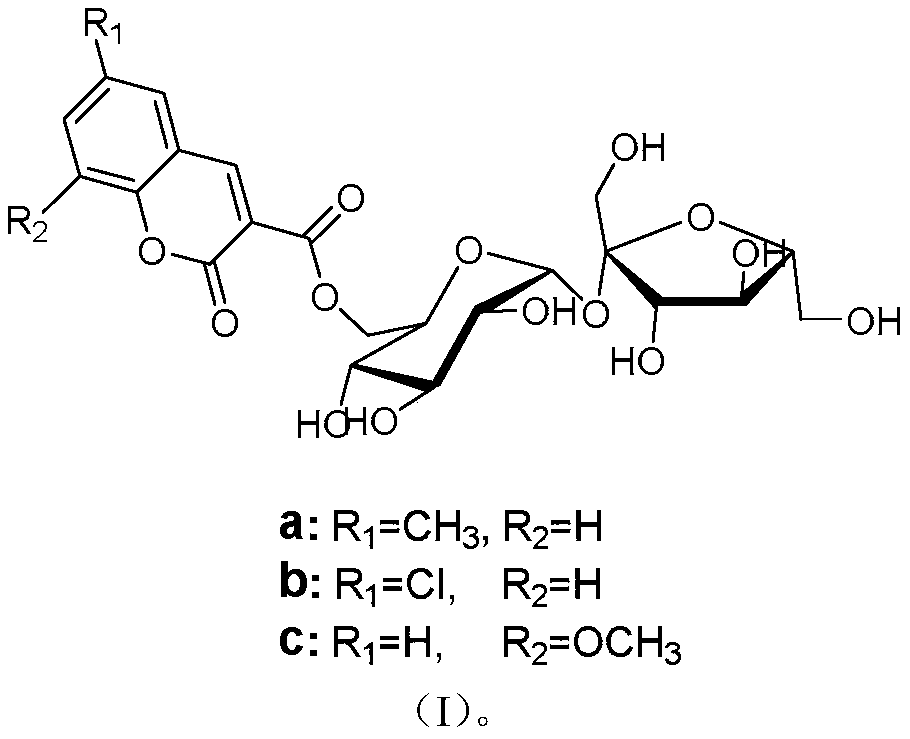
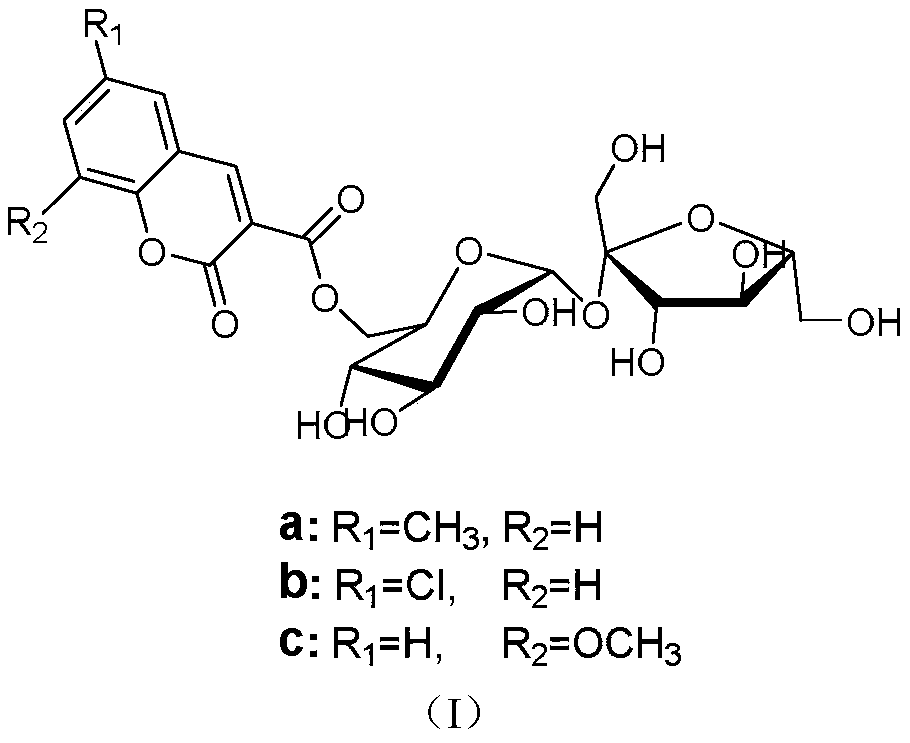
Method for on-line synthesis of coumarin-3-carboxylic acid sugar ester derivative based on flow chemistry enzymatic catalysis
ActiveCN111560408AShort reaction timeImprove conversion rateBioreactor/fermenter combinationsBiological substance pretreatmentsReaction temperaturePhysical chemistry
The invention relates to a method for on-line synthesis of coumarin-3-carboxylic acid sugar ester derivatives based on flow chemistry enzymatic catalysis. The method comprises the following steps: uniformly filling a reaction channel of a microfluidic channel reactor with lipase Lipozyme RM IM; respectively dissolving coumarin-3-carboxylic acid methyl ester and a carbohydrate compound by using a reaction solvent; injecting raw materials into a pipeline through a first injector and a second injector respectively and gathering; controlling the reaction temperature to be 30-60 DEG C and the continuous flowing reaction time of a mixed solution in the reaction channel to be 10-60 minutes, collecting a reaction solution flowing out of the reaction channel on line through a product collector, andperforming post-treatment to obtain a coumarin-3-carboxylic acid sugar ester derivative product, the method has the advantages of short reaction time, high yield and good selectivity.
Owner:ZHEJIANG UNIV OF TECH
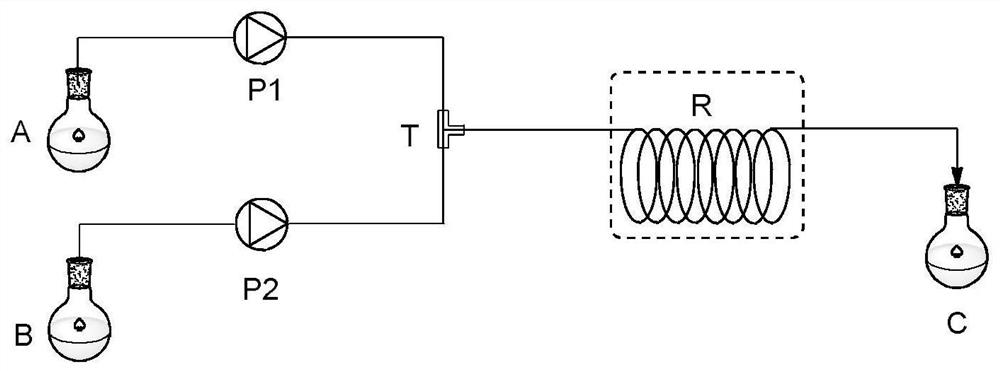
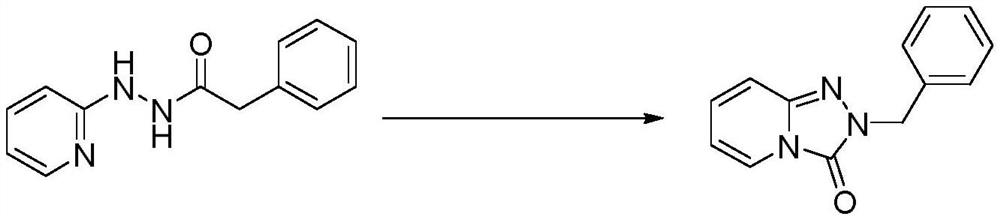
Method for preparing pyridino-triazolone compound through flow chemistry
ActiveCN113135911AReduce backmixingHigh mass and heat transfer efficiencyOrganic chemistryChemical industryOrganic solventPhysical chemistry
The invention relates to a method for preparing a pyridino- triazolone compound (II) through flow chemistry. The method comprises the following steps: a hydrazide compound (I) is dissolved in an organic solvent, the solution is placed in a first storage tank, an oxidantis dissolved in the organic solvent, and the solution is placed in a second storage tank; and aterials in the first storage tank and the second storage tank are conveyed through metering pumps respectively and enter a mixer to be mixed, reaction liquid obtained through mixing continuously enters a tubular reactor to be subjected to oxidation rearrangement reaction, the temperature of oxidation rearrangement is 0-70 DEG C, the retention time of the mixed reaction liquid in the tubular reactor is 0.1-10 h, material liquid obtained after reaction enters a receiving tank and is subjected to post-treatment, and after-treatment is conducted on the material liquid to obtain the product (II). In the tubular reaction, the material back mixing is less, the mass transfer and heat transfer efficiency is high, and the side reaction is obviously reduced; a reaction system is simple, raw materials, especially hydrazide compounds, are easily obtained, a substrate does not need to be prepared in multiple steps, and the total yield is relatively high; and the continuous tubular reaction can accurately control reaction parameters and has an automation prospect;.
Owner:ZHEJIANG UNIV OF TECH
A kind of preparation method of heterocyclic boronic acid compound
ActiveCN108250228BHigh yieldHigh purityGroup 3/13 element organic compoundsChemical reactionPhysical chemistry
Owner:上海泰坦科技股份有限公司
A method for the continuous preparation of chiral α-hydroxy-β-dicarbonyl compounds by visible light-catalyzed molecular oxygen oxidation via a microreactor
ActiveCN109293506BQuick responseMild reaction conditionsOrganic compound preparationCarboxylic acid esters preparationPtru catalystFlow chemistry
Belonging to the field of flow chemistry, the invention provides a method for continuous preparation of a chiral alpha-hydroxy-beta-dicarbonyl compound by visible light catalyzed molecular oxygen oxidation with a microreactor. The method utilizes a chiral quinine derivative quaternary ammonium salt as the chiral phase transfer catalyst, in the presence of an organic photosensitizer, by means of microreactor, preparation of the chiral alpha-hydroxy-beta-dicarbonyl compound by visible light activated gas molecular oxygen catalyzed continuous oxidation of a beta-dicarbonyl compound. The method provided by the invention can achieve a substrate conversion rate close to 100% in 1-10min reaction residence time, a product selectivity higher than 95%, and a product stereoselectivity ee value higherthan 80%. The reaction is green, the environmental burden is low, and the method realizes a continuous process, and has the advantages of large-scale production and low cost.
Owner:DALIAN UNIV OF TECH
A Method for Online Synthesis of Phenylalcohol β-Amino Alcohol Derivatives Based on Flow Chemistry Enzymatic Aminolysis Reaction
ActiveCN109706194BShort reaction timeImprove conversion rateBioreactor/fermenter combinationsBiological substance pretreatmentsReaction temperatureAminolysis
The invention discloses a method for on-line synthesis of phenylethanol β-amino alcohol derivatives based on flow chemical enzymatic aminolysis reaction: using methanol as a reaction solvent, aniline compounds and benzene oxide with a molar ratio of 1:0.6-1.4 Ethylene is used as a raw material, and lipase Lipozyme RM IM is used as a catalyst. The raw material and reaction solvent are placed in a syringe, and the lipase Lipozyme RM IM is evenly filled in the reaction channel of the microfluidic channel reactor, driven by a syringe pump. The raw materials and the reaction solvent are continuously fed into the reaction channel to carry out the ring-opening reaction. The inner diameter of the reaction channel of the microfluidic channel reactor is 0.8-2.4mm, and the length of the reaction channel is 0.5-1.0m; the ring-opening reaction temperature is controlled at 30 ~50°C, the ring-opening reaction time is 10-30min, the reaction solution is collected online by the product collector, and the reaction solution is subjected to conventional post-treatment to obtain phenylethyl alcohol β-amino alcohol derivatives. The invention has the advantages of short reaction time, high selectivity and high yield.
Owner:ZHEJIANG UNIV OF TECH
Assembled catalytic filler, preparation method thereof and application of assembled catalytic filler in flow chemical catalytic system
PendingCN114700063AThe synthesis process is simpleIncrease productivityOrganic compound preparationCatalyst activation/preparationFiberPtru catalyst
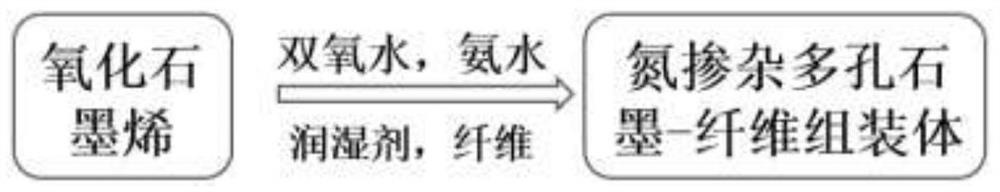
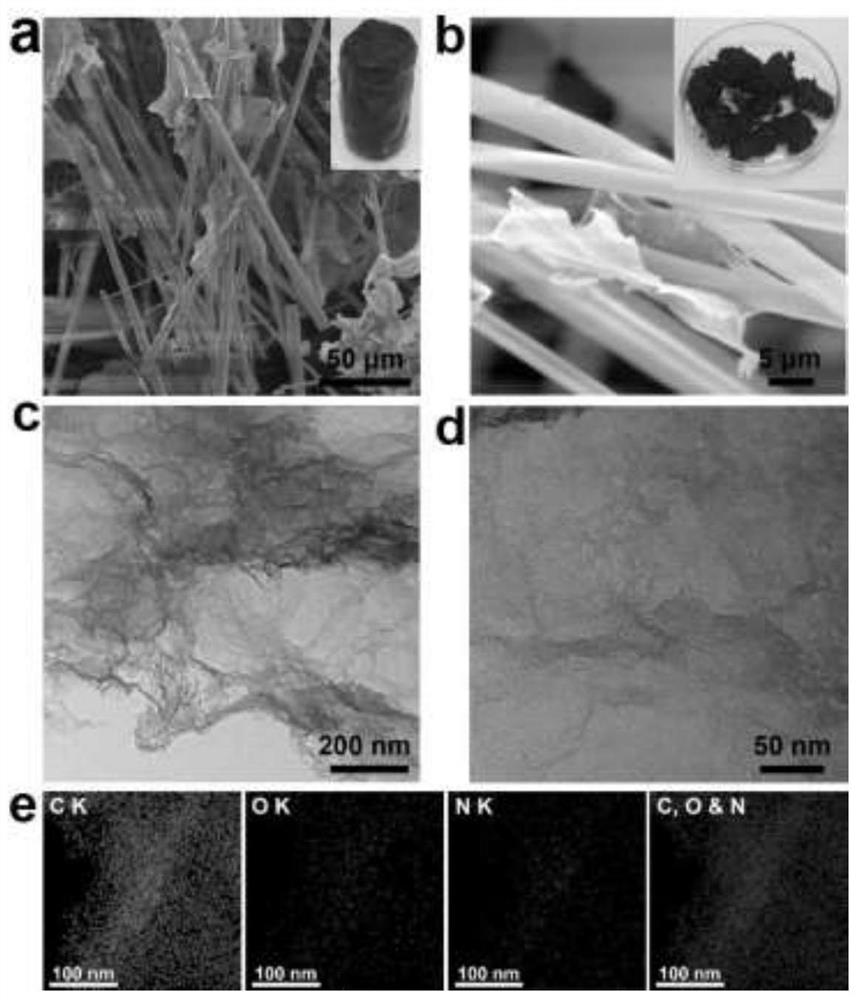
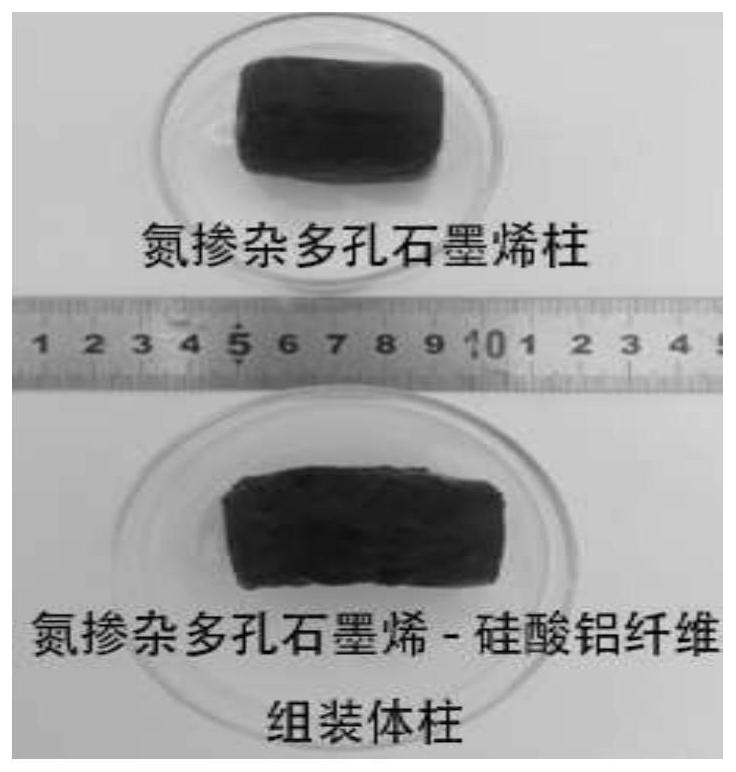
Graphene oxide and a fiber material are used as main assembly raw materials, a water-soluble small molecular organic matter is used as a wetting agent, ammonia water is used as a nitrogen source, hydrogen peroxide is used as a pore forming agent, and a composite hydrogel assembly catalyst with a multi-stage porous structure is prepared through a one-step hydrothermal method. The assembled catalytic filler obtained by the invention does not need to use traditional modification means such as introduction of active metal, can show excellent catalytic reaction efficiency, has good mechanical properties and cycling stability, and can provide a new idea for preparation of a high-performance flow chemical system catalyst; the preparation method is simple, raw materials are easy to obtain, equipment requirements are low, conditions are mild, production cost is low, and the preparation method is suitable for popularization and application.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
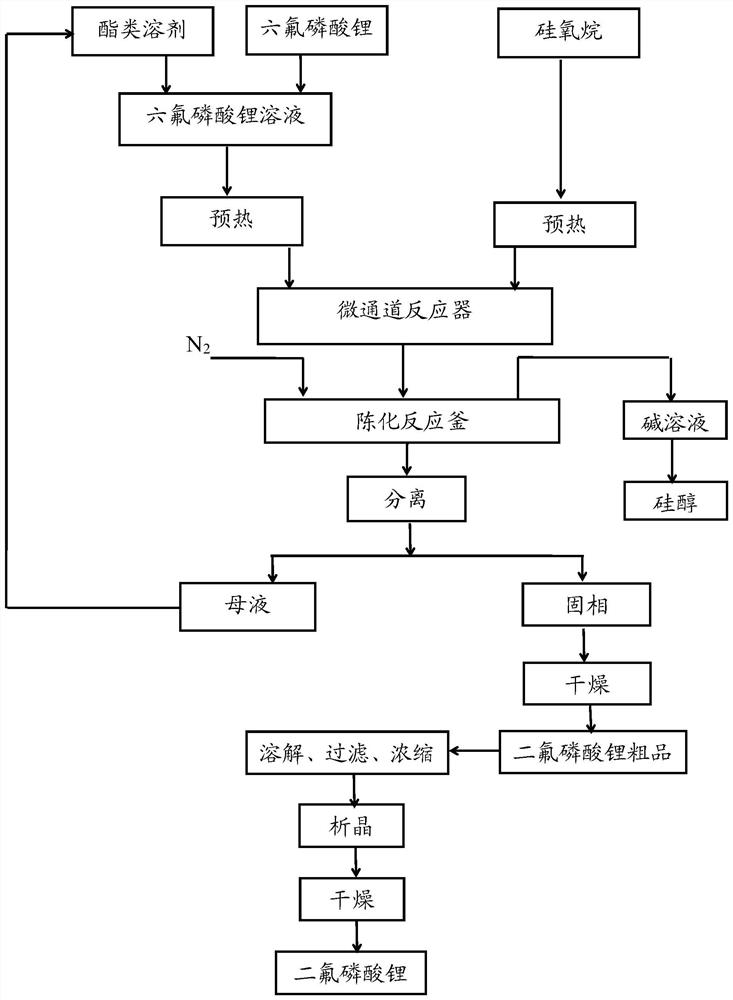
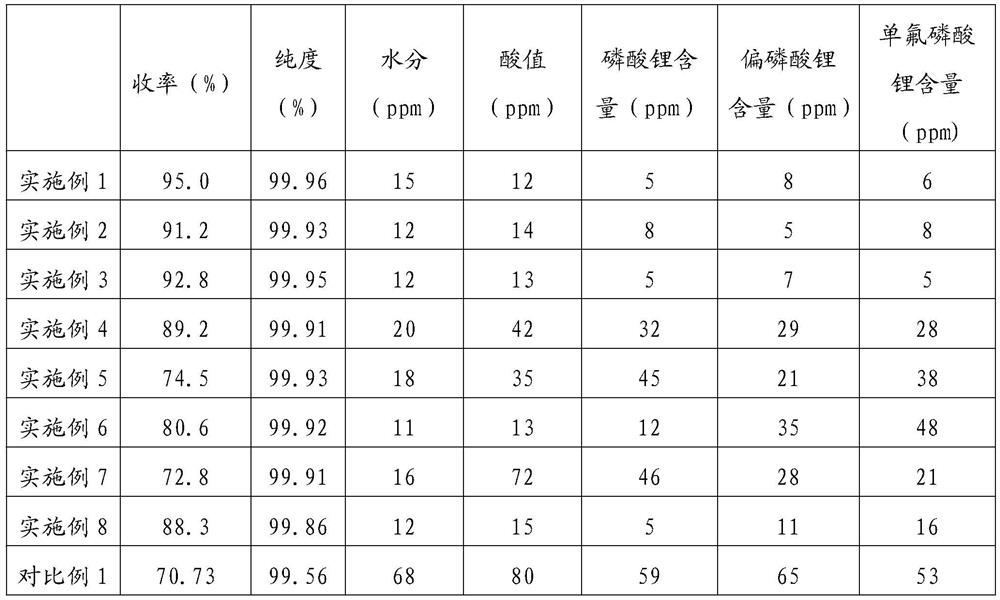
Method for preparing lithium difluorophosphate by flow chemical method
ActiveCN113880066AReduce contentTemperature controlAlkali metal fluoridesPhosphorus compoundsPhosphoric acidPhysical chemistry
The invention relates to the field of lithium ion secondary batteries, in particular to a method for preparing lithium difluorophosphate by a flow chemical method. The invention provides a method for preparing lithium difluorophosphate by a chemical flow method, which comprises the following steps of: respectively conveying a lithium hexafluorophosphate solution and siloxane into a pipeline of a micro-channel reactor to carry out continuous flow chemical reaction, and aging and separating the reaction liquid to take a solid phase, thereby obtaining lithium difluorophosphate. The purity of the prepared lithium difluorophosphate is 99.9% or above, and the content of lithium phosphate, the content of lithium metaphosphate and the content of lithium monofluorophosphate can be controlled to be 10 ppm or below. In the preparation process, air is isolated to control moisture, the micro-channel reaction is free of backmixing, the mass transfer effect is good, and sufficient reaction is ensured. Gas-liquid separation is carried out while aging reaction is carried out, so that the reaction efficiency is improved. The whole process is closed, siloxane and solvent closed-loop production is achieved, no obvious amplification effect exists, the production safety is high, and high-quality lithium difluorophosphate can be efficiently prepared with low cost.
Owner:SHENZHEN YANYI NEW MATERIALS CO LTD
A cloth chip gravity/capillary flow chemiluminescence method
ActiveCN105344391BEasy to useGood biocompatibilityChemiluminescene/bioluminescenceLaboratory glasswaresFiberPeristaltic pump
The invention discloses a cloth chip gravity / capillary flow chemiluminescence method, in particular discloses a cloth chip with gravity / capillary force coupling driving fluid and its application in detecting heavy metal ions. The cloth chip of the present invention is divided into a hydrophobic area and a hydrophilic area, and the hydrophilic area is further divided into three parts: a sampling area, a detection area and a fluid channel area; when in use, the cloth chip should be placed on a support closely, The inclined surface of the bracket forms an included angle with the horizontal plane. After placement, the sample loading area and fluid channel area should be on the inclined surface of the bracket, and the detection area should be on the horizontal part of the bracket. At the same time, a hydrophobic sheet should be placed under the detection area of the cloth chip. Compared with traditional flow chemiluminescence, the cloth chip and detection method of the present invention do not need any expensive pump device (precision syringe pump, peristaltic pump, etc.) to drive liquid flow. The invention only drives the liquid flow through the natural gravity of the liquid and the capillary force of the cloth fiber gap.
Owner:SOUTH CHINA NORMAL UNIVERSITY
A kind of preparation method of heterocyclic biphenyl boronic acid
ActiveCN108409767BHigh reaction yieldRaise the reaction temperatureGroup 3/13 element organic compoundsOrganolithium reagentBoronic acid
The invention provides a preparation method of heterocyclic biphenyl boric acid. The preparation method comprises the following steps: dissolving a compound shown in formula I into a solvent A, dissolving a boronizing reagent and an organolithium reagent into a solvent B, then performing continuous flowing feeding reaction on the reagent A and the reagent B, after the reaction is finished, using an alkaline reagent for performing hydrolysis reaction to obtain the heterocyclic biphenyl boric acid; according to the preparation method provided by the invention, a flowing chemical technique is used for synthesizing a heterocyclic biphenyl boric acid compound, compared with the existing conventional reaction method, the reaction yield is increased, the yield of part of the compound can reach upto 82% or above, the reaction time is shortened, the reaction can be rapidly finished within 1 hour, and the reaction temperature is raised, the reaction can be completed at around -20 DEG C to 10 DEG C, energy loss caused by reacting at the ultralow temperature environment can be avoided; the preparation method is an excellent synthetic method, and has a good application prospect.
Owner:上海泰坦科技股份有限公司
Method for preparing pyridotriazolone compounds by flow chemistry
ActiveCN113135911BReduce backmixingHigh mass and heat transfer efficiencyOrganic chemistryChemical industryChemical compoundOrganosolv
A method for preparing pyridotriazolone compound (II) by flow chemistry: dissolving hydrazide compound (I) in an organic solvent and placing it in a first storage tank, dissolving an oxidant in an organic solvent and placing it in a second storage tank In the storage tank; the materials in the first storage tank and the second storage tank are transported by metering pumps respectively, enter the mixer for mixing, and the mixed reaction liquid enters the tubular reactor continuously for oxidation rearrangement reaction, oxidation rearrangement The temperature is 0-70°C, the residence time of the mixed reaction solution in the tubular reactor is 0.1-10h, the reacted feed liquid enters the receiving tank, and after post-treatment, the product (II) is obtained; the tubular reaction of the present invention There is less material backmixing in the medium, high mass and heat transfer efficiency, and the occurrence of side reactions is significantly reduced; the reaction system is simple, the raw materials, especially hydrazide compounds, are easy to obtain, the substrate does not need to be prepared in multiple steps, and the total yield is high; continuous The tubular reaction can precisely control the parameters of the reaction, and has the prospect of automation;
Owner:ZHEJIANG UNIV OF TECH
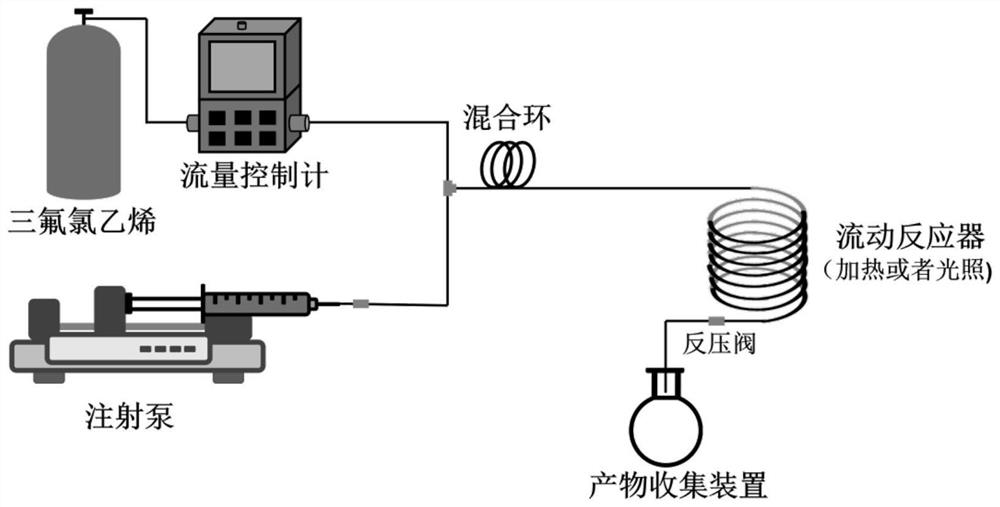
Flow chemical synthesis method of chlorotrifluoroethylene copolymer
The invention belongs to the technical field of polymer synthesis, and particularly relates to a flowing chemical synthesis method of a chlorotrifluoroethylene copolymer. The method comprises the following steps: forming gas-liquid two-phase flow by chlorotrifluoroethylene gas and reaction liquid dissolved with vinyl monomers and an initiator in a flowing chemical reaction device, and carrying out controllable free radical polymerization under the condition of illumination or heating to obtain the corresponding chlorotrifluoroethylene copolymer. A polymerization result shows that the method disclosed by the invention is safe and simple to operate and high in polymerization reaction speed, and polymer synthesis with different requirements can be realized by increasing time, increasing flow velocity and correspondingly using a larger reactor. The flow polymerization method is safe and simple to operate, high in polymerization reaction speed and beneficial to customized synthesis of the chlorotrifluoroethylene copolymer.
Owner:FUDAN UNIV +1
Method for online enzymatic synthesis of coumarin-3-carboxylic acid-6'-O-D-sucrose ester derivative based on flow chemistry
ActiveCN111455005AShort reaction timeCost-effectiveBioreactor/fermenter combinationsBiological substance pretreatmentsEnzymatic synthesisSucrose
A method for online enzymatic synthesis of a coumarin-3-carboxylic acid-6'-O-D-sucrose ester derivative based on the flow chemistry comprises the steps of: uniformly filling lipase Lipozyme RM IM in areaction channel of a micro-fluidic channel reactor, after respectively dissolving a coumarin-3-methyl carboxylate derivative and D-saccharose by using a reactive solvent, respectively injecting intoa pipeline to gather through a first injector and a second injector, performing reaction by entering the reaction channel, and controlling the reaction temperature at 30-60 DEG C, wherein the continuous flow reaction time of the mixed liquid in the reaction channel is 10-60 min; the reaction liquid flowing out of the reaction channel is collected online through a product collector; and thus, thecoumarin-3-carboxylic acid-6'-O-D-sucrose ester derivative is obtained through aftertreatment. The method has the advantages of being short in reaction time, high in productivity and good in selectivity.
Owner:ZHEJIANG FORESTRY UNIVERSITY
Measuring method for spreading rate in paper-making reconstituted tobacco production process
ActiveCN103439466BObjective accuracyMeet process technology control needsMaterial analysisFlow chemistryIn degree
Owner:FUJIAN JINMIN RECONSTITUTED TOBACCO DEV
Continuous chemical reaction system and method for arylboronic acid ester synthesis through micro-fluidic chip under flowing
PendingCN113444116AImprove continuityRealize automated monitoringChemical/physical/physico-chemical microreactorsGroup 3/13 element organic compoundsFluid phaseChemical reaction
The invention discloses a flowing chemical reaction system for preparing diazonium salt and synthesizing arylboronic acid ester through a micro-fluidic chip at room temperature as required under flowing. The system comprises a micro-fluidic chip diazonium salt preparation reaction module, a micro-fluidic chip arylboronic acid ester synthesis module and an online monitoring module; the micro-fluidic chip diazonium salt preparation reaction module comprises a sample bottle, a high-performance liquid phase infusion pump, a 3D mixed chip, a temperature-controllable reaction chip, a constant-temperature circulating water bath device and a fluid switching valve; the micro-fluidic chip arylboronic acid ester synthesis module comprises a sample bottle, a high-performance liquid phase infusion pump, a 3D mixed chip, a temperature-controllable reaction chip, a constant-temperature circulating water bath device and a fluid switching valve; and the online monitoring module comprises a nuclear magnetic spectrometer and a computer. The invention also discloses a method for preparing arylboronic acid ester based on the system and an application of the arylboronic acid ester.
Owner:EAST CHINA NORMAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com