Method for preparing lithium difluorophosphate by flow chemical method
A lithium difluorophosphate, flow chemistry technology, applied in chemical instruments and methods, inorganic chemistry, phosphorus compounds, etc., can solve the problems of reduced yield and purity, unfriendly environment, decomposition, etc., to reduce solvent consumption, The effect of reducing production costs and reducing emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
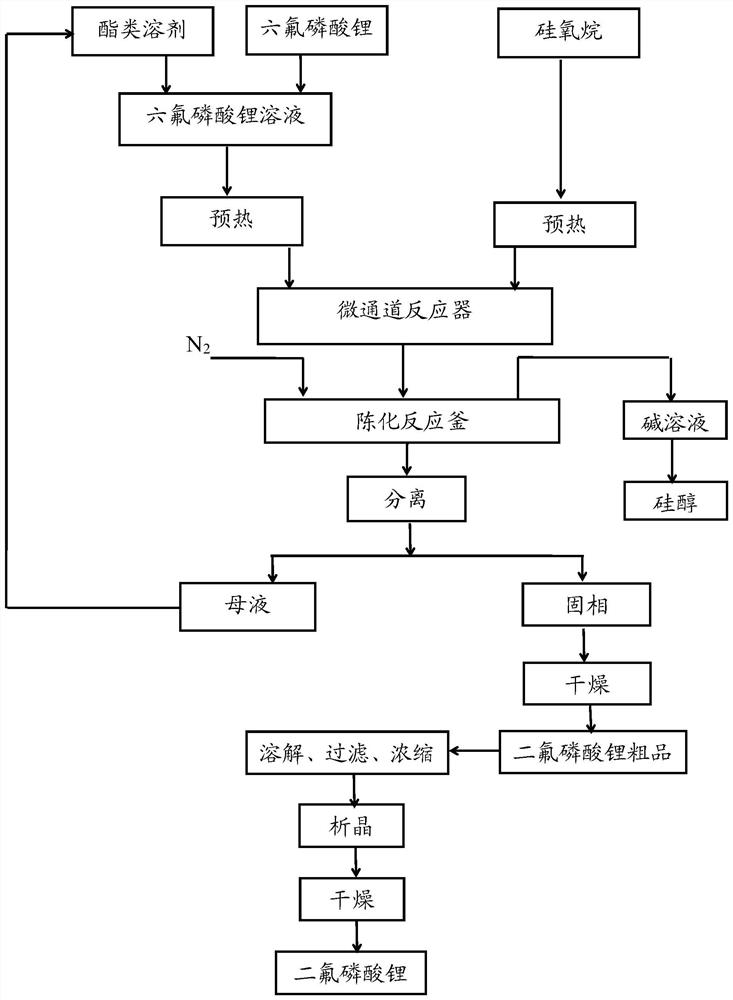
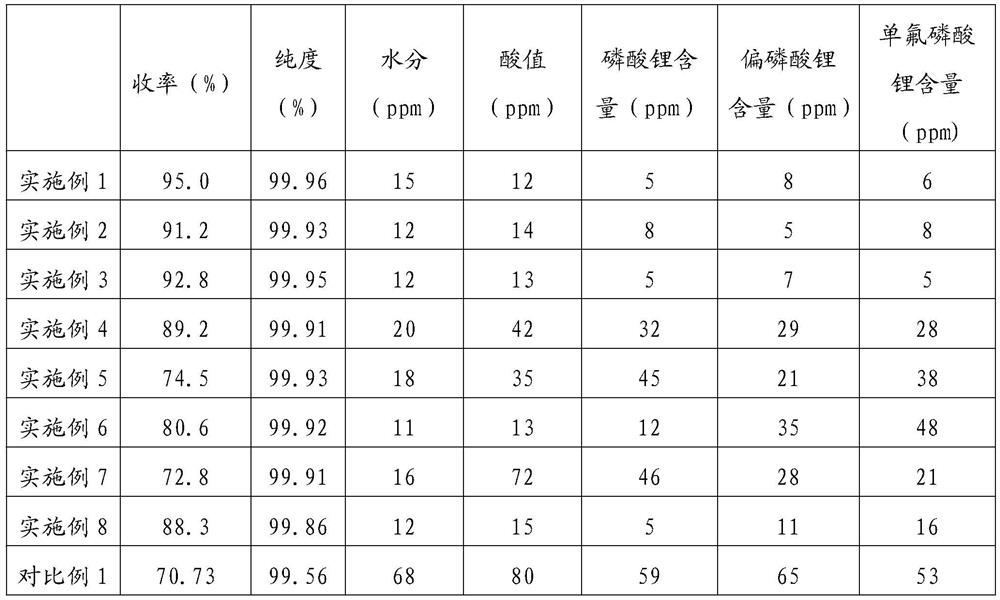
Image
Examples
Embodiment 1
[0062] The reaction equation involved in embodiment 1 is as follows:
[0063] LiPF 6 +2(CH 3 ) 3 -Si-O-Si-(CH 3 ) 3 = LiPO 2 f 2 +4(CH 3 ) 3 -Si-F↑
[0064] (1) Dissolve 30.00 g of lithium hexafluorophosphate in 70.00 g of dimethyl carbonate dehydrated by 4A molecular sieves in an ice-water bath at 0°C, stir for 0.5 h, and prepare a dimethyl carbonate solution of lithium hexafluorophosphate with a mass fraction of 30%. Wherein, the moisture content in the dimethyl carbonate detected by Metrohm 917 moisture tester is 15ppm.
[0065] (2) Use a peristaltic pump to preheat the dimethyl carbonate solution of lithium hexafluorophosphate and 70.55 g of hexamethyldisiloxane solution through a heat exchanger to 60 ° C respectively, wherein lithium hexafluorophosphate and hexamethyldisiloxane The molar ratio of Si-O units of alkane is 1:2.2.
[0066] (3) The dimethyl carbonate solution and the hexamethyldisiloxane solution of the preheated lithium hexafluorophosphate enter th...
Embodiment 2
[0072] The reaction equation involved in embodiment 2 is as follows:
[0073] LiPF 6 +0.5C 8 h 24 o 4 Si 4 = LiPO 2 f 2 +2(CH 3 ) 2 -Si-F2↑
[0074] (1) Dissolve 20.00 g of lithium hexafluorophosphate in 80.00 g of ethyl methyl carbonate dehydrated by 4A molecular sieves in a water bath at 5°C, stir for 0.5 h, and prepare a 20% lithium hexafluorophosphate solution in ethyl methyl carbonate. Wherein, the moisture content in ethyl methyl carbonate detected by Metrohm 917 moisture tester is 45ppm.
[0075] (2) Use a peristaltic pump to preheat the above-prepared lithium hexafluorophosphate ethyl methyl carbonate solution and 19.53 g of octamethylcyclotetrasiloxane solution to 50 ° C through a heat exchanger, wherein lithium hexafluorophosphate and octamethylcyclotetrasiloxane The molar ratio of Si-O units of siloxane is 1:2.0.
[0076] (3) The ethyl methyl carbonate solution of the preheated lithium hexafluorophosphate and the octamethylcyclotetrasiloxane solution ente...
Embodiment 3
[0082] The reaction equation involved in embodiment 3 is as follows:
[0083] LiPF 6 +2(C 2 h 5 ) 3 -Si-O-Si-(C 2 h 5 ) 3 = LiPO 2 f 2 +4(C 2 h 5 ) 3 -Si-F↑
[0084] (1) Dissolve 10.00 g of lithium hexafluorophosphate in 90.00 g of diethyl carbonate dehydrated by 4A molecular sieves in a water bath at 10°C, stir for 0.5 h, and prepare a diethyl carbonate solution of lithium hexafluorophosphate with a mass fraction of 10%. Wherein, the moisture content in the diethyl carbonate detected by Metrohm 917 moisture tester is 35ppm.
[0085] (2) Use a peristaltic pump to preheat the diethyl carbonate solution of lithium hexafluorophosphate and 40.58 g of hexaethyldisiloxane solution respectively through a heat exchanger to 70° C., wherein lithium hexafluorophosphate and hexaethyldisiloxane The molar ratio of Si-O units of alkane is 1:2.5.
[0086] (3) the diethyl carbonate solution of lithium hexafluorophosphate after preheating and hexaethyldisiloxane solution enter in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com