Novel method for realizing visible light catalytic asymmetric oxidation by using micro reactor
A micro-reactor and micro-channel reactor technology, applied in the field of flow chemistry, can solve problems such as safety hazards, photocatalytic reaction amplification and production limitations, and achieve the effects of process safety, effective continuity and amplification, and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: Preparation of 2-hydroxyl-1-indanone-2-formic acid adamantyl
[0022]
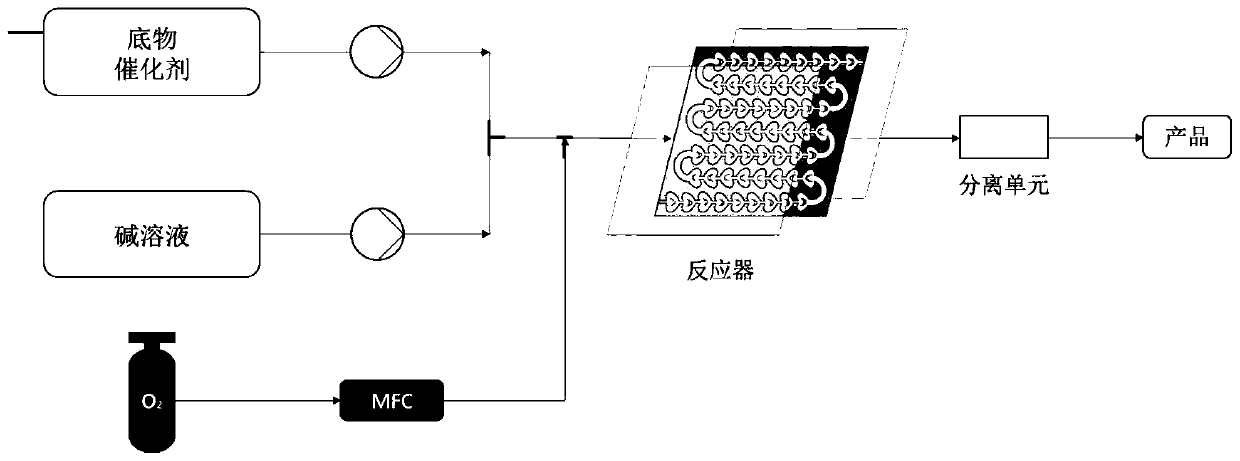
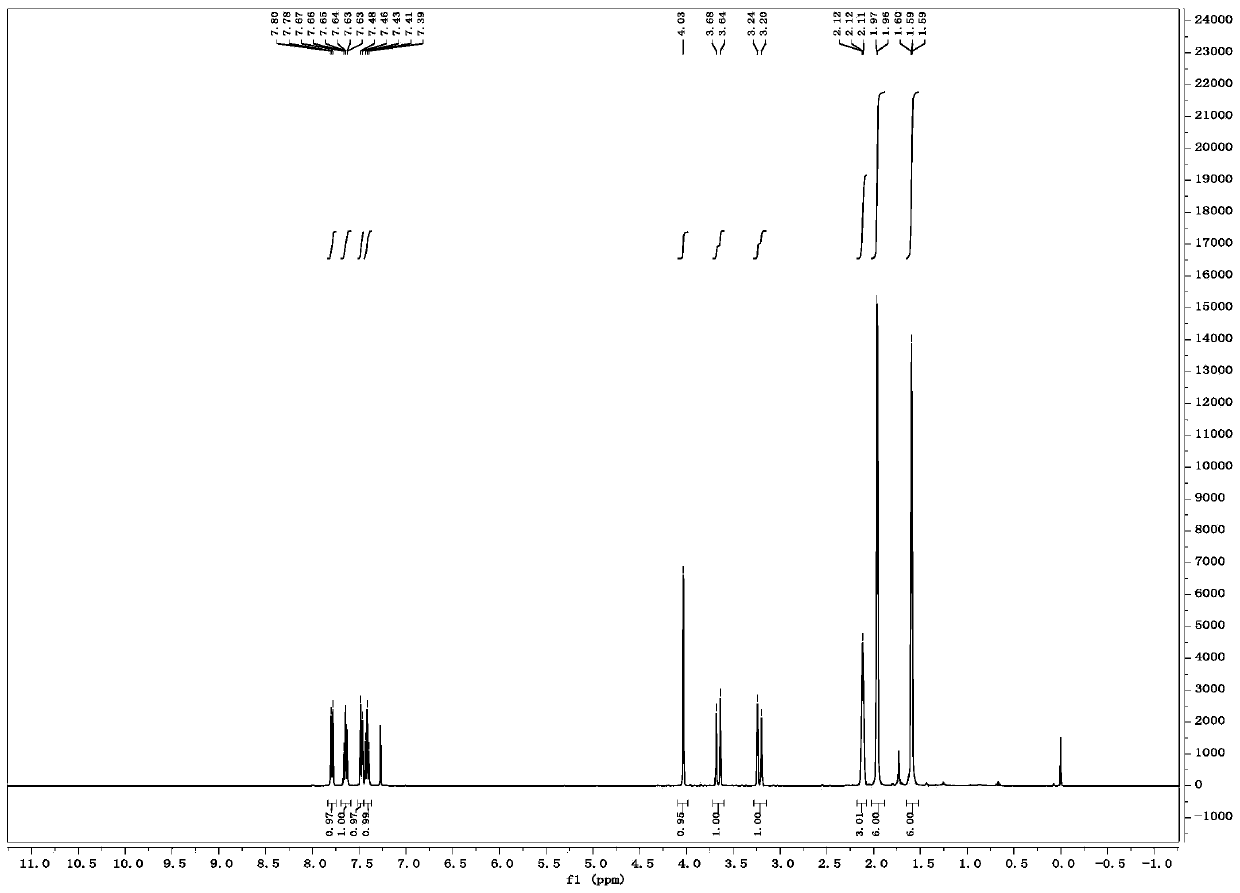
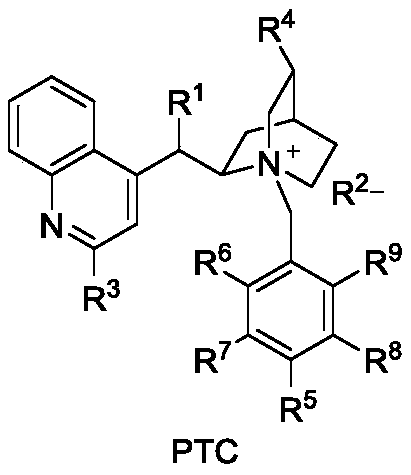
[0023] Weigh 0.031g (0.1mmol) 1-indanone-2-carboxylate adamantyl (Ia-1), 0.0077g (0.01mmol, 10mol%) catalyst PTC-1, in 20mL test tube, add 10mL toluene, solid dissolves completely , recorded as a bottle; another configuration of 50% K 2 HPO 4 The solution is denoted as bottle b. The temperature of the entire reaction system is controlled at 20°C. The organic phase in bottle a is pumped in at a rate of 1.5mL / min from pump A, and the alkaline solution in bottle b is pumped in at a rate of 1.5mL / min from pump B. The flow rate of oxygen (oxygen content 99.99%) was 3 mL / min, the reaction pressure was 1 bar, the light source was white light, and the residence time was 90 min. The starting material was completely converted and the organic phase was worked up to give the oxidation product IIa-1 (0.031 g, 95% yield, 82% ee). 1 H NMR (400MHz, Chloroform-d) δ7.79 (d, J = 7.7Hz, 1H), 7.65 (t...
Embodiment 2
[0024] Example 2: Preparation of 2-hydroxy-1-indanone-2-formic acid adamantyl process enlarged 10 times volume
[0025]
[0026] Weigh 0.31g (1mmol) 1-indanone-2-carboxylate adamantyl (Ia-1), 0.077g (0.1mmol, 10mol%) catalyst PTC-1, in 200mL test tube, add 100mL toluene, solid dissolves completely, Recorded as a bottle; another 50% K 2 HPO 4The solution is denoted as bottle b. The temperature of the entire reaction system is controlled at 20°C. The organic phase in bottle a is pumped in at a rate of 1.5mL / min from pump A, and the alkaline solution in bottle b is pumped in at a rate of 1.5mL / min from pump B. The flow rate of oxygen (oxygen content 99.99%) was 3 mL / min, the reaction pressure was 1 bar, the light source was white light, and the residence time was 90 min. The starting material was completely converted and the organic phase was worked up to give the oxidation product IIa-1 (0.31 g, 95% yield, 82% ee).
Embodiment 3
[0027] Example 3: Preparation of 5-chloro-2-hydroxyl-1-indanone-2-carboxylic acid adamantyl
[0028]
[0029] Weigh 0.0344g (0.1mmol) 5-chloro-1-indanone-2-formic acid adamantyl (Ia-2), 0.0008g (0.001mmol, 1mol%) catalyst PTC-2, in 200mL test tube, add 100mL adjacent Xylene, the solid is completely dissolved, recorded as a bottle; another 20% K 2 HPO 4 The solution is denoted as bottle b. The temperature of the entire reaction system is controlled at 10°C. The organic phase in bottle a is pumped in at a rate of 0.5mL / min from pump A, and the alkaline solution in bottle b is pumped in at a rate of 0.5mL / min from pump B. The air flow rate was 1mL / min, the reaction pressure was 0bar (atmospheric pressure), the light source was 300nm LEDs, and the residence time was 5min. The starting material was completely converted and the organic phase was worked up to give the oxidation product IIa-2 (0.0339 g, 94% yield, 80% ee).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com