A kind of tandem synthesis method of 2-(phenylmethylene) malononitrile or its derivatives
A technology of phenylmethylene and synthetic methods, applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylic acid nitrile, etc., can solve the problems of low yield, long reaction time, complicated separation of catalysts and products, etc. To achieve the effect of high reactivity, short reaction time and cheap catalyst
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
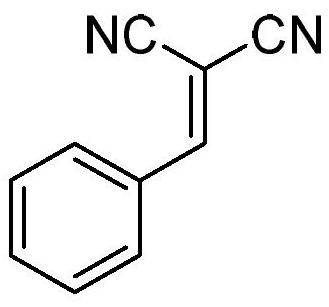
[0030] Preparation of 2- (phenylmethylene) malononitrile (structural formula below):
[0031]
[0032] The mixture 3.3mmol benzaldehydedimethylacetal 3.8mmol malononitrile and diluted with ethyl acetate to 10mL, and then the resultant solution at a flow rate 0.5mL / min pressure injected into the HPLC column (the PS-piperazine 0.8-1.0g piperazine-1-sulfonic acid and PS- 0.8-1.0g), while the HPLC column was immersed in an oil bath at 50 deg.] C. After completion of the reaction, HPLC column rinsed with ethyl acetate, the product was collected, the solvent was evaporated, recrystallized from ethanol, to give solid product, 94% yield.
[0033] Compared with the compound prepared by conventional routes, the presence of a catalyst the method cheap, highly reactive, short reaction time, can be obtained without separation of the intermediate product and the product yield advantages. Melting point of the compound, IR and NMR characterized as follows: m.p.84.0-84.4 ℃; FT-IR (KBr, cm -1 )...
Embodiment 2
[0035] (E) -2- cyano-3-phenyl acrylate (structural formula below):
[0036]
[0037] The diluted 3.3mmol benzaldehydedimethylacetal with a nitrile compound mixed with ethyl acetate to ethyl acetate 3.8mmol 10mL, then the resulting solution at a flow rate 0.5mL / min pressure injected into the HPLC column (the PS-piperazine 0.8 PS- -1.0g and piperazine-1-sulfonic acid 0.8-1.0g), while the HPLC column was immersed in an oil bath at 50 deg.] C. After completion of the reaction, HPLC column rinsed with ethyl acetate, the product was collected, the solvent was evaporated, recrystallized from ethanol, to give solid product, 86% yield.
[0038] Compared with the compound prepared by conventional routes, the presence of a catalyst the method cheap, highly reactive, short reaction time, can be obtained without separation of the intermediate product and the product yield advantages. Melting point of the compound, IR and NMR characterized as follows: m.p.49.0-50.2 ℃; FT-IR (KBr, cm -1 ): 3...
Embodiment 3
[0040] Preparation of 2- (4-methoxy-methylene) malononitrile (structural formula below):
[0041]
[0042] The mixture was methoxybenzaldehyde dimethyl acetal 3.3mmol 3.8mmol of malononitrile and diluted with ethyl acetate to 10mL, and then the resultant solution at a flow rate 0.5mL / min pressure injected into the HPLC column (the PS-piperazine and 0.8 to 1.0 g PS- piperazine-1-sulfonic acid 0.8-1.0g), while the HPLC column was immersed in an oil bath at 50 deg.] C. After completion of the reaction, HPLC column rinsed with ethyl acetate, the product was collected, the solvent was evaporated, recrystallized from ethanol, to give solid product, 95% yield.
[0043] Compared with the compound prepared by conventional routes, the presence of a catalyst the method cheap, highly reactive, short reaction time, can be obtained without separation of the intermediate product and the product yield advantages. Melting point of the compound, IR and NMR characterized as follows: m.p.115.1-11...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com